Characterization of a Human Neuronal Culture System for the Study of Cofilin–Actin Rod Pathology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture and Differentiation of Cells
2.2.1. SH-SY5Y and hES Cells
2.2.2. Human iPSC WTC-11
2.3. Cell Treatments
2.3.1. Viral Infection
2.3.2. Rod Inducers and Inhibitors
2.3.3. Fixation and Immunolabeling
2.3.4. Immunoblotting
2.4. Microscopy and Image Analysis
2.4.1. Imaging at 4 to 20×
2.4.2. Confocal Microscopy
2.4.3. Image Analysis
2.4.4. Electrophysiology
2.5. Statistics
3. Results
3.1. Formation of Cofilactin Rods in Human Neurons
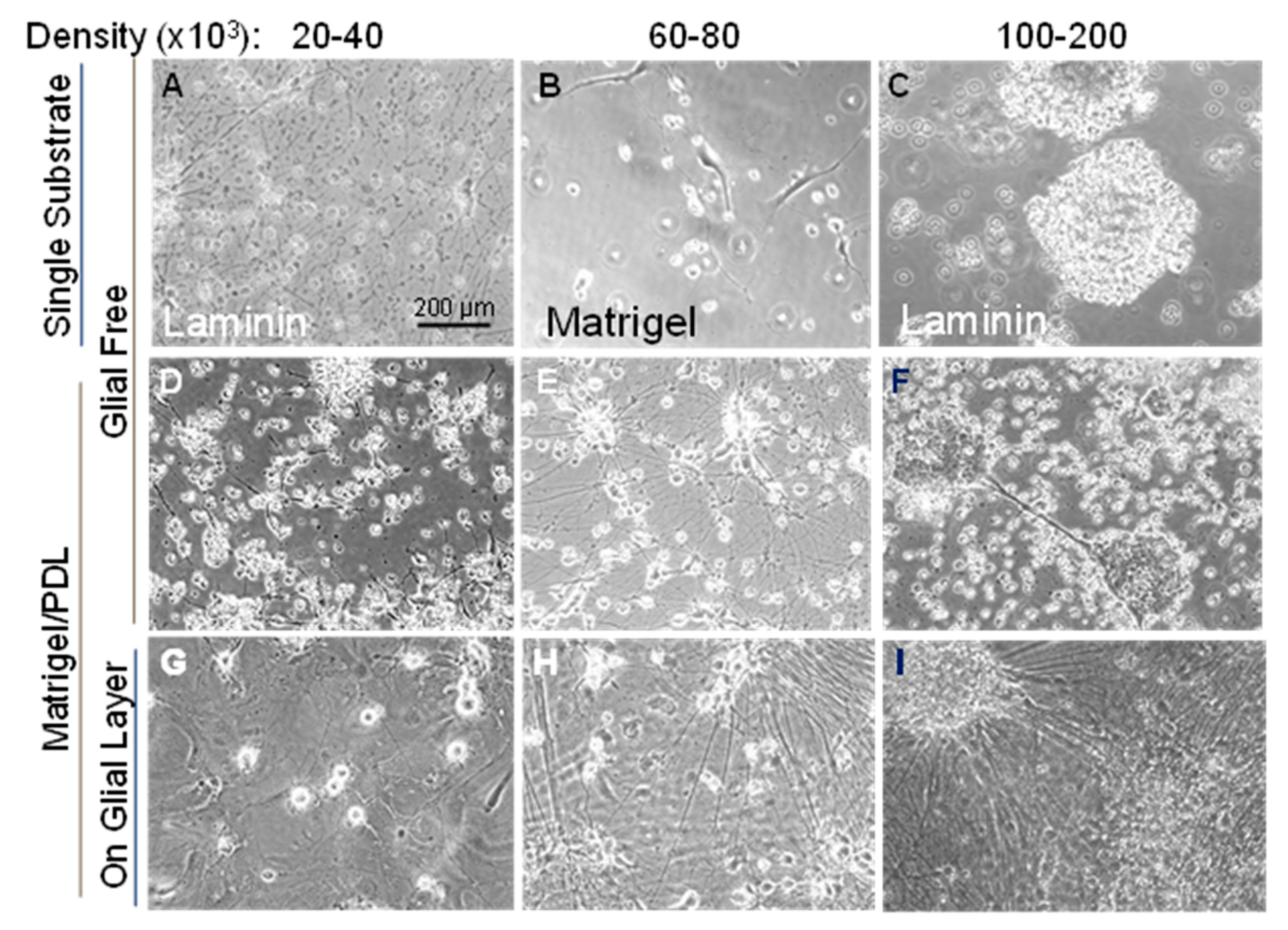
3.2. Optimizing Substrate and Cell Numbers for Differentiation of Human WTC-11 Cells
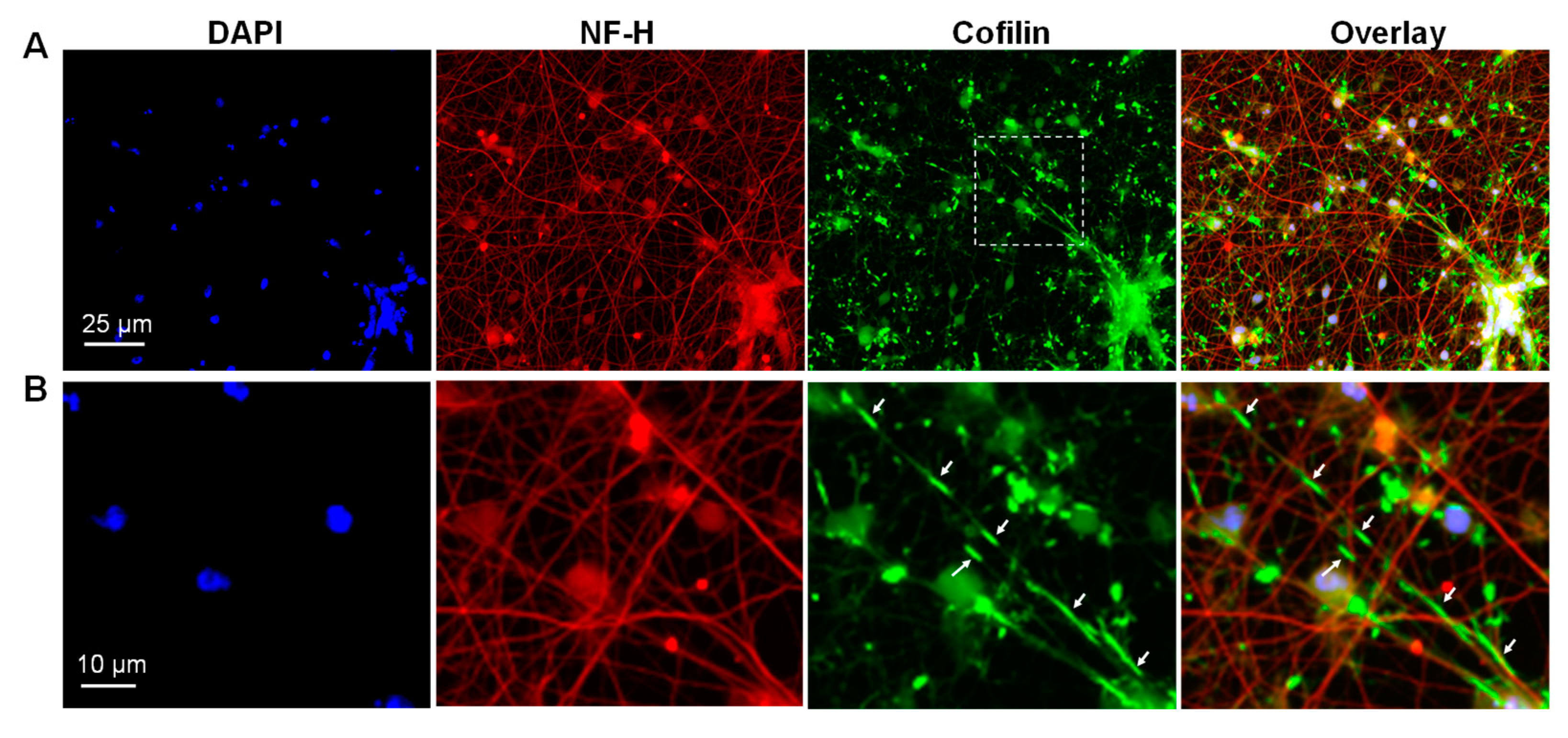
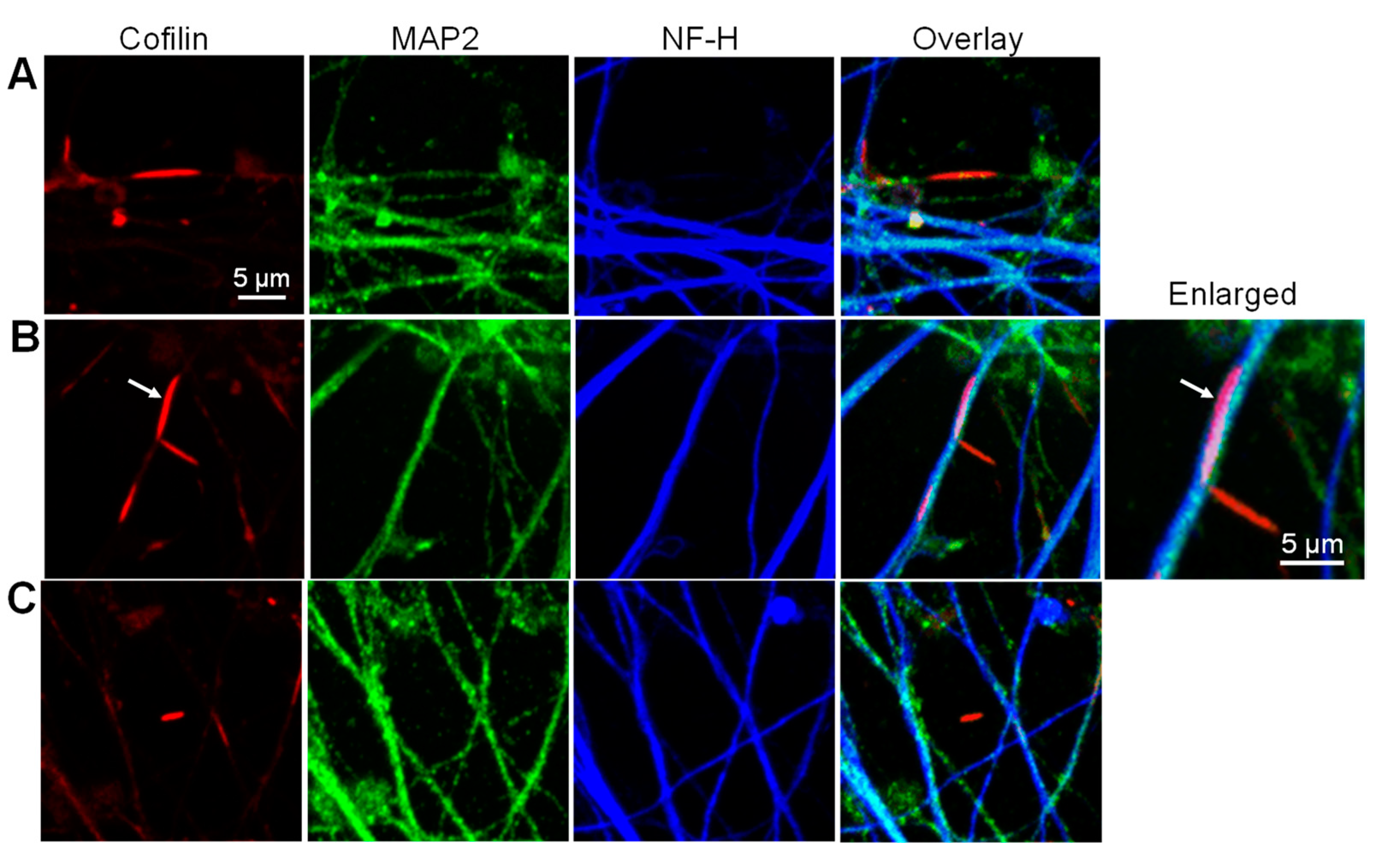
3.3. Cofilactin Bundles in Filopodia of Human i3Neuronal Growth Cones
3.4. i3Neuronal Development
3.4.1. Neurite Differentiation
3.4.2. Neuronal Survival
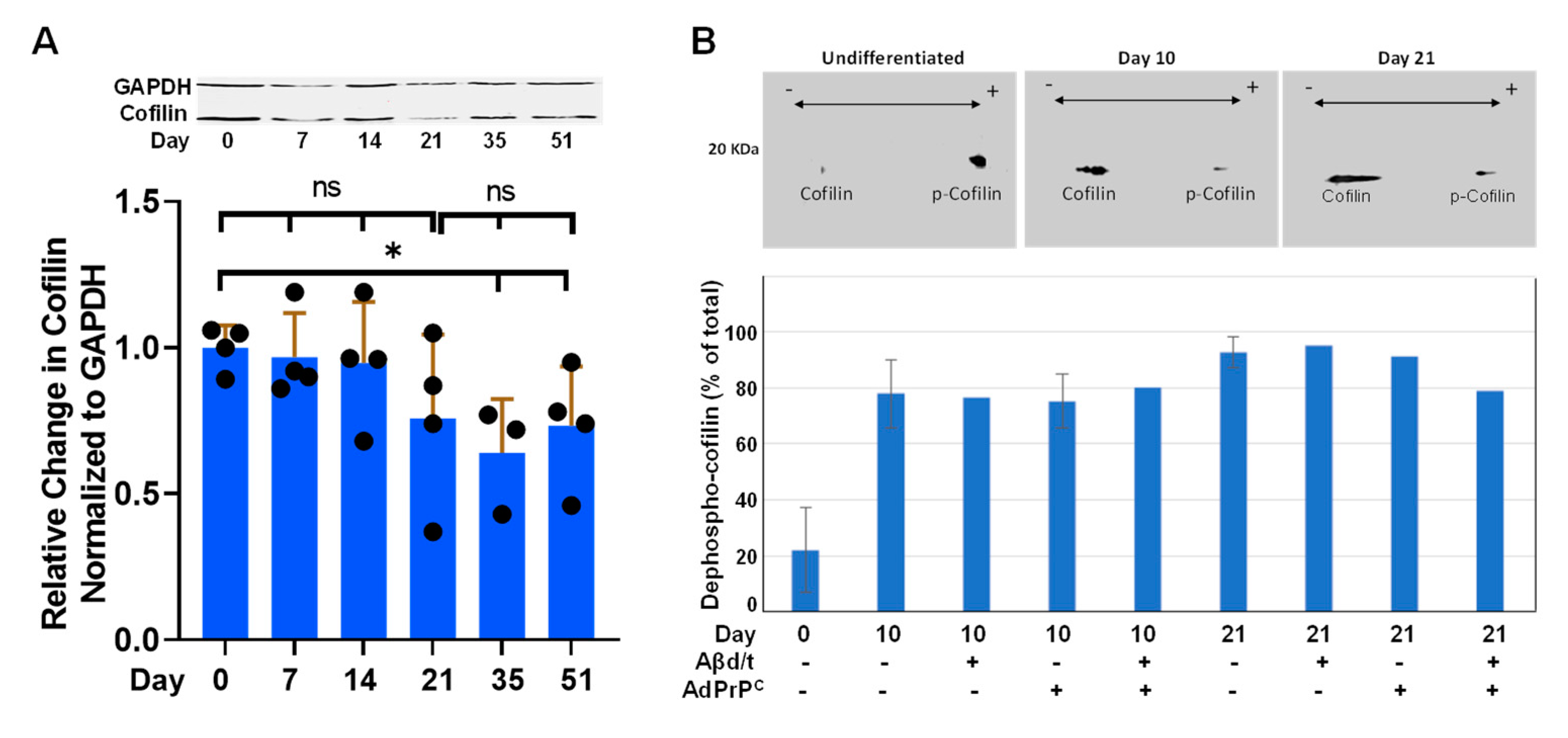
3.5. Cofilactin Rod Formation
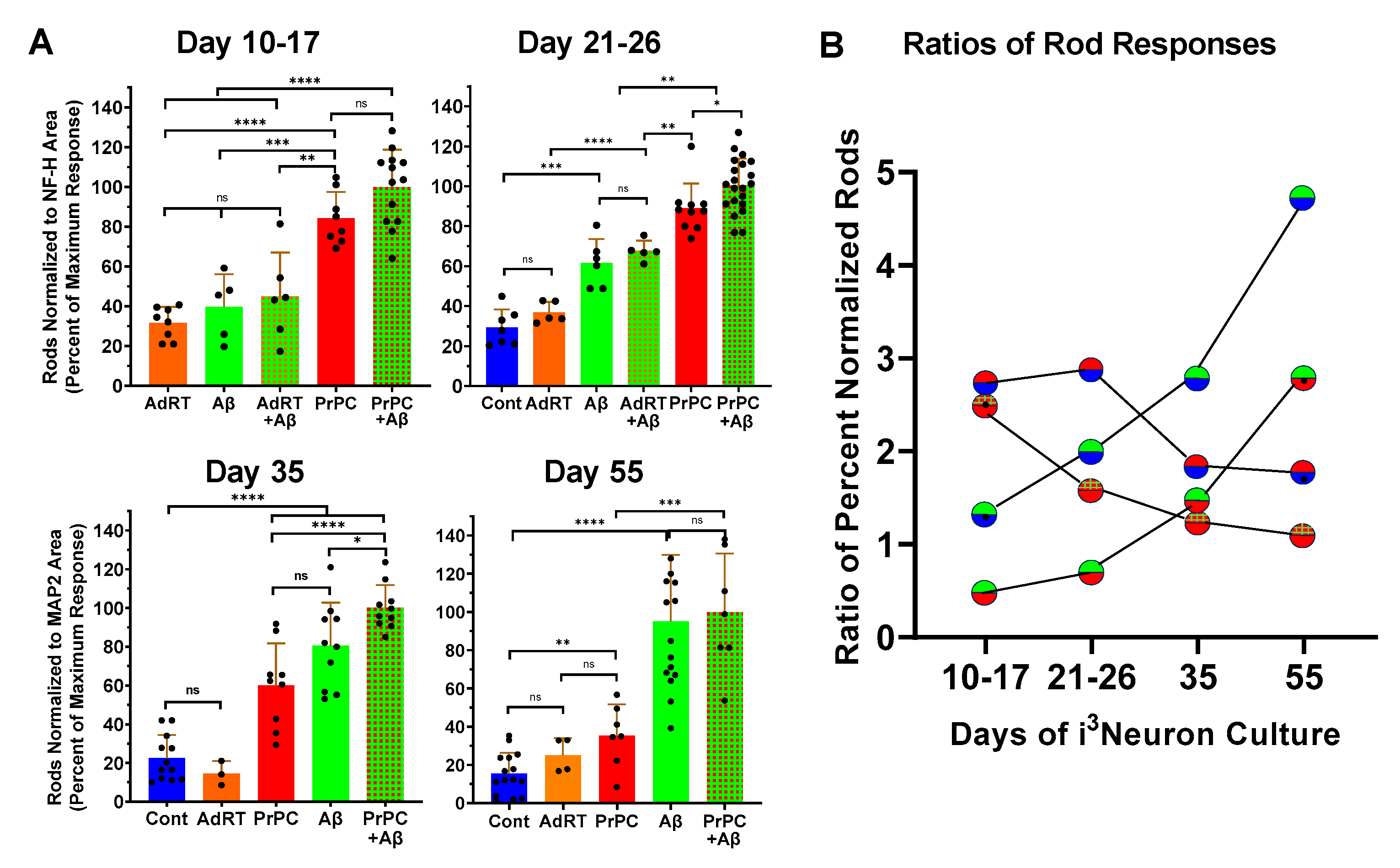
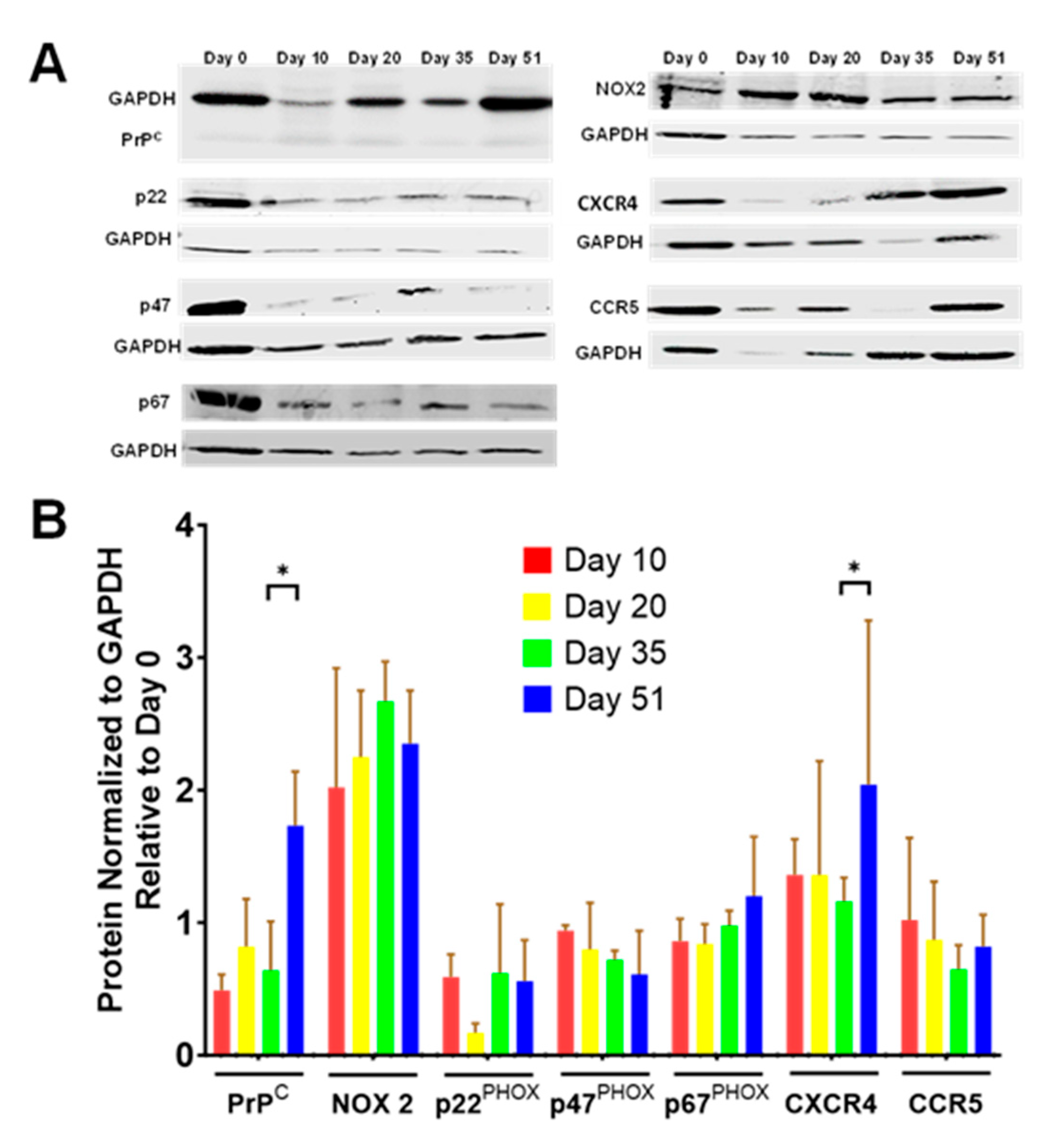
3.6. Developmental Changes in Expression of Rod Response Proteins in i3Neurons
3.7. Effects of CXCR4, CCR5, and NOX Inhibitors on Rod Formation
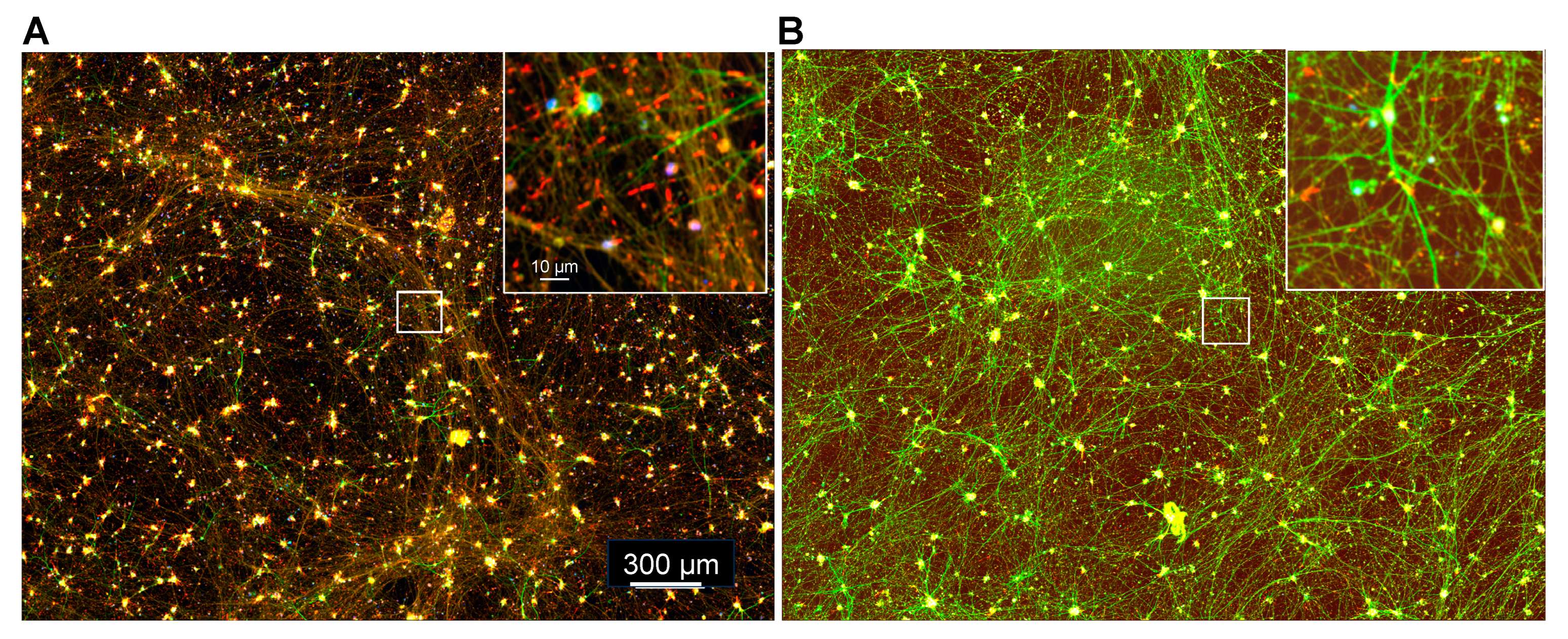
3.8. Localization of Aβd/t-Induced Rods to Neurites
3.9. Maturation of i3Neurons without Astrocytes: Development of Synaptic Specification and Functional Neuronal Properties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | beta-amyloid peptides derived from β- and γ-secretase cleavage of APP |
| Aβd/t | dimer/trimer fraction of secreted Aβ from the 7PA2 cell line |
| AD | Alzheimer’s disease |
| AdPrPC | adenovirus for expressing wild-type mouse PrPC |
| AdRT | Red Track adenovirus expressing mRFP used as infection control |
| Aip1 | actin-interacting protein 1 (aka WDR1) |
| APP | amyloid precursor protein |
| ATCC | American Type Culture Collection |
| BASE | β-secretase, a cleavage enzyme for APP |
| BSA | bovine serum albumin |
| Cofilactin | cofilin-saturated F-actin (1:1) |
| DMSO | dimethyl sulfoxide |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HAND | HIV-associated neurocognitive disorder |
| hESCs | human embryonic stem cells |
| HG-DMEM | high-glucose-containing Dulbecco’s Modified Eagle’s Medium |
| HIV gp120MN | Human Immunodeficiency Virus envelope protein derived from gp160 |
| hNB | homemade neurobasal medium |
| i3N | integrated, inducible, isogenic neurons derived from the WTC-11 cell line |
| iPSCs | induced pluripotent stem cells |
| moi | multiplicity of infection (virus particles added per cell) |
| NOX | NADPH oxidase |
| PBS | phosphate-buffered saline |
| PDL | poly-D-lysine |
| PrPC | cellular prion protein (WT) |
| PSD95 | post-synaptic density protein 95 |
| TBS | Tris-buffered saline |
| TBST | TBS containing non-ionic detergent Tween 20 |
| VGLUT | vesicular glutamate transporter 1 |
References
- Minamide, L.S.; Striegl, A.M.; Boyle, J.A.; Meberg, P.J.; Bamburg, J.R. Neurodegenerative Stimuli Induce Persistent ADF/Cofilin-Actin Rods That Disrupt Distal Neurite Function. Nat. Cell Biol. 2000, 2, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Davies, D.S.; Tannenberg, R.K.; Fok, S.; Shepherd, C.; Dodd, P.R.; Cullen, K.M.; Goldsbury, C. Cofilin Rods and Aggregates Concur with Tau Pathology and the Development of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2014, 42, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bernstein, B.W. Actin Dynamics and Cofilin-Actin Rods in Alzheimer Disease. Cytoskeleton 2016, 73, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Maloney, M.T.; Minamide, L.S.; Kinley, A.W.; Boyle, J.A.; Bamburg, J.R. Beta-Secretase-Cleaved Amyloid Precursor Protein Accumulates at Actin Inclusions Induced in Neurons by Stress or Amyloid Beta: A Feedforward Mechanism for Alzheimer’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 11313–11321. [Google Scholar] [CrossRef]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Rowan, M.J.; Selkoe, D.J. Amyloid-Beta Oligomers: Their Production, Toxicity and Therapeutic Inhibition. Biochem. Soc. Trans. 2002, 30, 552–557. [Google Scholar] [CrossRef]
- Davis, R.C.; Marsden, I.T.; Maloney, M.T.; Minamide, L.S.; Podlisny, M.; Selkoe, D.J.; Bamburg, J.R. Amyloid Beta Dimers/Trimers Potently Induce Cofilin-Actin Rods That Are Inhibited by Maintaining Cofilin-Phosphorylation. Mol. Neurodegener. 2011, 6, 10. [Google Scholar] [CrossRef]
- Woo, J.A.; Zhao, X.; Khan, H.; Penn, C.; Wang, X.; Joly-Amado, A.; Weeber, E.; Morgan, D.; Kang, D.E. Slingshot-Cofilin Activation Mediates Mitochondrial and Synaptic Dysfunction via Aβ Ligation to Β1-Integrin Conformers. Cell Death Differ. 2015, 22, 1069–1070. [Google Scholar] [CrossRef]
- Woo, J.A.; Boggess, T.; Uhlar, C.; Wang, X.; Khan, H.; Cappos, G.; Joly-Amado, A.; De Narvaez, E.; Majid, S.; Minamide, L.S.; et al. RanBP9 at the Intersection between Cofilin and Aβ Pathologies: Rescue of Neurodegenerative Changes by RanBP9 Reduction. Cell Death Dis. 2015, 6, 1676. [Google Scholar] [CrossRef]
- Deng, Y.; Wei, J.; Cheng, J.; Zhong, P.; Xiong, Z.; Liu, A.; Lin, L.; Chen, S.; Yan, Z. Partial Amelioration of Synaptic and Cognitive Deficits by Inhibiting Cofilin Dephosphorylation in an Animal Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2016, 53, 1419–1432. [Google Scholar] [CrossRef]
- Laurén, J.; Gimbel, D.A.; Nygaard, H.B.; Gilbert, J.W.; Strittmatter, S.M. Cellular Prion Protein Mediates Impairment of Synaptic Plasticity by Amyloid-Beta Oligomers. Nature 2009, 457, 1128–1132. [Google Scholar] [CrossRef]
- Walsh, K.P.; Minamide, L.S.; Kane, S.J.; Shaw, A.E.; Brown, D.R.; Pulford, B.; Zabel, M.D.; Lambeth, J.D.; Kuhn, T.B.; Bamburg, J.R. Amyloid-β and Proinflammatory Cytokines Utilize a Prion Protein-Dependent Pathway to Activate NADPH Oxidase and Induce Cofilin-Actin Rods in Hippocampal Neurons. PLoS ONE 2014, 9, e95995. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Kuhn, T.B.; Chen, J.; Bamburg, J.R. HIV Associated Neurodegenerative Disorders: A New Perspective on the Role of Lipid Rafts in Gp120-Mediated Neurotoxicity. Curr. HIV Res. 2018, 16, 258–269. [Google Scholar] [CrossRef]
- Smith, L.K.; Babcock, I.W.; Minamide, L.S.; Shaw, A.E.; Bamburg, J.R.; Kuhn, T.B. Direct Interaction of HIV Gp120 with Neuronal CXCR4 and CCR5 Receptors Induces Cofilin-Actin Rod Pathology via a Cellular Prion Protein- and NOX-Dependent Mechanism. PLoS ONE 2021, 16, e0248309. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.O.; Santejo, M.; Babcock, I.W.; Magalhães, A.; Minamide, L.S.; Castillo, E.; Gerhardt, E.; Fahlbusch, C.; Swanson, R.A.; Outeiro, T.F.; et al. Cofilin Pathology Is a New Player on α-Synuclein-Induced Spine Impairment in Models of Hippocampal Synucleinopathy. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Pak, C.; Han, Y.; Ahlenius, H.; Zhang, Z.; Chanda, S.; Marro, S.; Patzke, C.; Acuna, C.; Covy, J.; et al. Rapid Single-Step Induction of Functional Neurons from Human Pluripotent Stem Cells. Neuron 2013, 78, 785–798. [Google Scholar] [CrossRef]
- Wang, C.; Ward, M.E.; Chen, R.; Liu, K.; Tracy, T.E.; Chen, X.; Xie, M.; Sohn, P.D.; Ludwig, C.; Meyer-Franke, A.; et al. Scalable Production of iPSC-Derived Human Neurons to Identify Tau-Lowering Compounds by High-Content Screening. Stem Cell Rep. 2017, 9, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Ang, C.E.; Lee, Q.Y.; Ghebrial, M.; Haag, D.; Shibuya, Y.; Wernig, M.; Südhof, T.C. Direct Reprogramming of Human Neurons Identifies MARCKSL1 as a Pathogenic Mediator of Valproic Acid-Induced Teratogenicity. Cell Stem Cell 2019, 25, 103–119.e6. [Google Scholar] [CrossRef] [PubMed]
- Fernandopulle, M.S.; Prestil, R.; Grunseich, C.; Wang, C.; Gan, L.; Ward, M.E. Transcription Factor-Mediated Differentiation of Human iPSCs into Neurons. Curr. Protoc. Cell Biol. 2018, 79, e51. [Google Scholar] [CrossRef]
- Peppercorn, K.; Kleffmann, T.; Jones, O.; Hughes, S.; Tate, W. Secreted Amyloid Precursor Protein Alpha, a Neuroprotective Protein in the Brain Has Widespread Effects on the Transcriptome and Proteome of Human Inducible Pluripotent Stem Cell-Derived Glutamatergic Neurons Related to Memory Mechanisms. Front. Neurosci. 2022, 16, 858524. [Google Scholar] [CrossRef]
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Kuskowski, M.A.; Selkoe, D.J.; Ashe, K.H. Natural Oligomers of the Amyloid-Beta Protein Specifically Disrupt Cognitive Function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef]
- Shankar, G.M.; Bloodgood, B.L.; Townsend, M.; Walsh, D.M.; Selkoe, D.J.; Sabatini, B.L. Natural Oligomers of the Alzheimer Amyloid-Beta Protein Induce Reversible Synapse Loss by Modulating an NMDA-Type Glutamate Receptor-Dependent Signaling Pathway. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef]
- Abe, H.; Ohshima, S.; Obinata, T. A Cofilin-like Protein Is Involved in the Regulation of Actin Assembly in Developing Skeletal Muscle. J. Biochem. 1989, 106, 696–702. [Google Scholar] [CrossRef]
- Shaw, A.E.; Minamide, L.S.; Bill, C.L.; Funk, J.D.; Maiti, S.; Bamburg, J.R. Cross-Reactivity of Antibodies to Actin- Depolymerizing Factor/Cofilin Family Proteins and Identification of the Major Epitope Recognized by a Mammalian Actin-Depolymerizing Factor/Cofilin Antibody. Electrophoresis 2004, 25, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Bardy, C.; van den Hurk, M.; Eames, T.; Marchand, C.; Hernandez, R.V.; Kellogg, M.; Gorris, M.; Galet, B.; Palomares, V.; Brown, J.; et al. Neuronal Medium That Supports Basic Synaptic Functions and Activity of Human Neurons in Vitro. Proc. Natl. Acad. Sci. USA 2015, 112, E2725–E2734. [Google Scholar] [CrossRef]
- Goslin, K.; Amussen, H.; Banker, G. Rat Hippocampal Neurons in Low Density Cultures. In Culturing Nerve Cells; MIT Press: Cambridge, MA, USA, 1998; pp. 339–370. [Google Scholar]
- Meberg, P.J.; Miller, M.W. Culturing Hippocampal and Cortical Neurons. Methods Cell Biol. 2003, 71, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Minamide, L.S.; Shaw, A.E.; Sarmiere, P.D.; Wiggan, O.; Maloney, M.T.; Bernstein, B.W.; Sneider, J.M.; Gonzalez, J.A.; Bamburg, J.R. Production and Use of Replication-Deficient Adenovirus for Transgene Expression in Neurons. Methods Cell Biol. 2003, 71, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Symons, M.H.; Mitchison, T.J. Control of Actin Polymerization in Live and Permeabilized Fibroblasts. J. Cell Biol. 1991, 114, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Minamide, L.S.; Hylton, R.K.; Swulius, M.T.; Bamburg, J.R. Visualizing Cofilin-Actin Filaments by Immunofluorescence and cryoEM. Essential Steps for Observing Cofilactin in Cells. In Signal Transduction Immunocytochemistry: Methods and Protocols; Methods in Molecular Biology; Springer Nature: New York, NY, USA, 2022; Volume 2593, pp. 265–281. [Google Scholar]
- Wessel, D.; Flügge, U.I. A Method for the Quantitative Recovery of Protein in Dilute Solution in the Presence of Detergents and Lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Barth, B.M.; Stewart-Smeets, S.; Kuhn, T.B. Proinflammatory Cytokines Provoke Oxidative Damage to Actin in Neuronal Cells Mediated by Rac1 and NADPH Oxidase. Mol. Cell. Neurosci. 2009, 41, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, J.V.; Griffiths, H.H.; Watt, N.T.; Hooper, N.M. Prion Protein-Mediated Toxicity of Amyloid-β Oligomers Requires Lipid Rafts and the Transmembrane LRP1. J. Biol. Chem. 2013, 288, 8935–8951. [Google Scholar] [CrossRef]
- Santillo, S.; Schiano Moriello, A.; Di Maio, V. Electrophysiological Variability in the SH-SY5Y Cellular Line. Gen. Physiol. Biophys. 2014, 33, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. JoVE 2016, 108, 53193. [Google Scholar] [CrossRef]
- Flynn, K.C.; Hellal, F.; Neukirchen, D.; Jacob, S.; Tahirovic, S.; Dupraz, S.; Stern, S.; Garvalov, B.K.; Gurniak, C.; Shaw, A.E.; et al. ADF/Cofilin-Mediated Actin Retrograde Flow Directs Neurite Formation in the Developing Brain. Neuron 2012, 76, 1091–1107. [Google Scholar] [CrossRef]
- Hylton, R.K.; Heebner, J.E.; Grillo, M.A.; Swulius, M.T. Cofilactin Filaments Regulate Filopodial Structure and Dynamics in Neuronal Growth Cones. Nat. Commun. 2022, 13, 2439. [Google Scholar] [CrossRef] [PubMed]
- Stettler, O.; Bush, M.S.; Kasper, M.; Schlosshauer, B.; Gordon-Weeks, P.R. Monoclonal Antibody 2G13, a New Axonal Growth Cone Marker. J. Neurocytol. 1999, 28, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.; Banker, G.; Steward, O.; Binder, L.; Payne, M. MAP2 Is Localized to the Dendrites of Hippocampal Neurons Which Develop in Culture. Brain Res. 1984, 315, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Pennypacker, K.; Fischer, I.; Levitt, P. Early in Vitro Genesis and Differentiation of Axons and Dendrites by Hippocampal Neurons Analyzed Quantitatively with Neurofilament-H and Microtubule-Associated Protein 2 Antibodies. Exp. Neurol. 1991, 111, 25–35. [Google Scholar] [CrossRef]
- Minamide, L.S.; Maiti, S.; Boyle, J.A.; Davis, R.C.; Coppinger, J.A.; Bao, Y.; Huang, T.Y.; Yates, J.; Bokoch, G.M.; Bamburg, J.R. Isolation and Characterization of Cytoplasmic Cofilin-Actin Rods. J. Biol. Chem. 2010, 285, 5450–5460. [Google Scholar] [CrossRef]
- Tahtamouni, L.H.; Shaw, A.E.; Hasan, M.H.; Yasin, S.R.; Bamburg, J.R. Non-Overlapping Activities of ADF and Cofilin-1 during the Migration of Metastatic Breast Tumor Cells. BMC Cell Biol. 2013, 14, 45. [Google Scholar] [CrossRef]
- De Clercq, E. Mozobil® (Plerixafor, AMD3100), 10 Years after Its Approval by the US Food and Drug Administration. Antivir. Chem. Chemother. 2019, 27. [Google Scholar] [CrossRef]
- Gavriel, Y.; Rabinovich-Nikitin, I.; Ezra, A.; Barbiro, B.; Solomon, B. Subcutaneous Administration of AMD3100 into Mice Models of Alzheimer’s Disease Ameliorated Cognitive Impairment, Reduced Neuroinflammation, and Improved Pathophysiological Markers. J. Alzheimer’s Dis. JAD 2020, 78, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a Potent, Orally Bioavailable, and Selective Small-Molecule Inhibitor of Chemokine Receptor CCR5 with Broad-Spectrum Anti-Human Immunodeficiency Virus Type 1 Activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef] [PubMed]
- Burlingham, S.R.; Wong, N.F.; Peterkin, L.; Lubow, L.; Dos Santos Passos, C.; Benner, O.; Ghebrial, M.; Cast, T.P.; Xu-Friedman, M.A.; Südhof, T.C.; et al. Induction of Synapse Formation by de Novo Neurotransmitter Synthesis. Nat. Commun. 2022, 13, 3060. [Google Scholar] [CrossRef]
- Yahara, I.; Aizawa, H.; Moriyama, K.; Iida, K.; Yonezawa, N.; Nishida, E.; Hatanaka, H.; Inagaki, F. A Role of Cofilin/Destrin in Reorganization of Actin Cytoskeleton in Response to Stresses and Cell Stimuli. Cell Struct. Funct. 1996, 21, 421–424. [Google Scholar] [CrossRef]
- Aizawa, H.; Fukui, Y.; Yahara, I. Live Dynamics of Dictyostelium Cofilin Suggests a Role in Remodeling Actin Latticework into Bundles. J. Cell Sci. 1997, 110 Pt 19, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.C.; Maloney, M.T.; Minamide, L.S.; Flynn, K.C.; Stonebraker, M.A.; Bamburg, J.R. Mapping Cofilin-Actin Rods in Stressed Hippocampal Slices and the Role of Cdc42 in Amyloid-Beta-Induced Rods. J. Alzheimer’s Dis. JAD 2009, 18, 35–50. [Google Scholar] [CrossRef]
- Won, S.J.; Minnella, A.M.; Wu, L.; Eun, C.H.; Rome, E.; Herson, P.S.; Shaw, A.E.; Bamburg, J.R.; Swanson, R.A. Cofilin-Actin Rod Formation in Neuronal Processes after Brain Ischemia. PLoS ONE 2018, 13, e0198709. [Google Scholar] [CrossRef]
- Shu, L.; Chen, B.; Chen, B.; Xu, H.; Wang, G.; Huang, Y.; Zhao, Y.; Gong, H.; Jiang, M.; Chen, L.; et al. Brain Ischemic Insult Induces Cofilin Rod Formation Leading to Synaptic Dysfunction in Neurons. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 2181–2195. [Google Scholar] [CrossRef]
- Cichon, J.; Sun, C.; Chen, B.; Jiang, M.; Chen, X.A.; Sun, Y.; Wang, Y.; Chen, G. Cofilin Aggregation Blocks Intracellular Trafficking and Induces Synaptic Loss in Hippocampal Neurons. J. Biol. Chem. 2012, 287, 3919–3929. [Google Scholar] [CrossRef]
- Takayama, K.; Matsuda, K.; Abe, H. Formation of Actin-Cofilin Rods by Depletion Forces. Biochem. Biophys. Res. Commun. 2022, 626, 200–204. [Google Scholar] [CrossRef]
- Nagasaki, A.; Katoh, K.; Hoshi, M.; Doi, M.; Nakamura, C.; Uyeda, T.Q.P. Characterization of Phalloidin-Negative Nuclear Actin Filaments in U2OS Cells Expressing Cytoplasmic Actin-EGFP. Genes Cells Devoted Mol. Cell. Mech. 2022, 27, 317–330. [Google Scholar] [CrossRef]
- Cast, T.P.; Boesch, D.J.; Smyth, K.; Shaw, A.E.; Ghebrial, M.; Chanda, S. An Autism-Associated Mutation Impairs Neuroligin-4 Glycosylation and Enhances Excitatory Synaptic Transmission in Human Neurons. J. Neurosci. 2021, 41, 392–407. [Google Scholar] [CrossRef]
- Mi, J.; Shaw, A.E.; Pak, C.W.; Walsh, K.P.; Minamide, L.S.; Bernstein, B.W.; Kuhn, T.B.; Bamburg, J.R. A Genetically Encoded Reporter for Real-Time Imaging of Cofilin-Actin Rods in Living Neurons. PLoS ONE 2013, 8, e83609. [Google Scholar] [CrossRef]
- Kommaddi, R.P.; Das, D.; Karunakaran, S.; Nanguneri, S.; Bapat, D.; Ray, A.; Shaw, E.; Bennett, D.A.; Nair, D.; Ravindranath, V. Aβ Mediates F-Actin Disassembly in Dendritic Spines Leading to Cognitive Deficits in Alzheimer’s Disease. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Lee, C.W.; Fan, Y.; Komlos, D.; Tang, X.; Sun, C.; Yu, K.; Hartzell, H.C.; Chen, G.; Bamburg, J.R.; et al. ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during Synaptic Plasticity. Nat. Neurosci. 2010, 13, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.B.; Gurniak, C.B.; Renner, M.; Vara, H.; Morando, L.; Görlich, A.; Sassoè-Pognetto, M.; Banchaabouchi, M.A.; Giustetto, M.; Triller, A.; et al. Learning, AMPA Receptor Mobility and Synaptic Plasticity Depend on n-Cofilin-Mediated Actin Dynamics. EMBO J. 2010, 29, 1889–1902. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, B.; Saffin, J.-M.; Halpain, S. Activity-Dependent Dendritic Spine Shrinkage and Growth Involve Downregulation of Cofilin via Distinct Mechanisms. PLoS ONE 2014, 9, e94787. [Google Scholar] [CrossRef]
- Wennagel, D.; Braz, B.Y.; Capizzi, M.; Barnat, M.; Humbert, S. Huntingtin Coordinates Dendritic Spine Morphology and Function through Cofilin-Mediated Control of the Actin Cytoskeleton. Cell Rep. 2022, 40, 111261. [Google Scholar] [CrossRef]
- Salem, F.B.; Bunner, W.P.; Prabhu, V.V.; Kuyateh, A.-B.; O’Bryant, C.T.; Murashov, A.K.; Szatmari, E.M.; Hughes, R.M. CofActor: A Light- and Stress-Gated Optogenetic Clustering Tool to Study Disease-Associated Cytoskeletal Dynamics in Living Cells. J. Biol. Chem. 2020, 295, 11231–11245. [Google Scholar] [CrossRef]
- Wurz, A.I.; Schulz, A.M.; O’Bryant, C.T.; Sharp, J.F.; Hughes, R.M. Cytoskeletal Dysregulation and Neurodegenerative Disease: Formation, Monitoring, and Inhibition of Cofilin-Actin Rods. Front. Cell. Neurosci. 2022, 16, 982074. [Google Scholar] [CrossRef]
- Ashraf, T.; Jiang, W.; Hoque, M.T.; Henderson, J.; Wu, C.; Bendayan, R. Role of Anti-Inflammatory Compounds in Human Immunodeficiency Virus-1 Glycoprotein120-Mediated Brain Inflammation. J. Neuroinflamm. 2014, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.-W.; Feng, R.; Lau, W.K.-W.; Law, A.C.-K.; Yeung, P.K.-K.; Chung, S.K. Endothelin Type B Receptor Promotes Cofilin Rod Formation and Dendritic Loss in Neurons by Inducing Oxidative Stress and Cofilin Activation. J. Biol. Chem. 2019, 294, 12495–12506. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.B.; Tahtamouni, L.H.; Alderfer, S.A.; Minamide, L.S.; Walsh, K.P.; Shaw, A.E.; Yanouri, O.; Haigler, H.N.; Ruff, M.R.; Bamburg, J.R. Chemokine Receptor Antagonists Prevent and Reverse Prion-Dependent Cofilactin Rod Pathology and Protect Developing Synapses in Cultured Rodent and Human Neurons. Biomedicines. (In preparation)
- Saxton, M.N.; Morisaki, T.; Krapf, D.; Kimura, H.; Stasevich, T.J. Live-Cell Imaging Uncovers the Relationship between Histone Acetylation, Transcription Initiation, and Nucleosome Mobility. Sci. Adv. 2023, 9, eadh4819. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahtamouni, L.H.; Alderfer, S.A.; Kuhn, T.B.; Minamide, L.S.; Chanda, S.; Ruff, M.R.; Bamburg, J.R. Characterization of a Human Neuronal Culture System for the Study of Cofilin–Actin Rod Pathology. Biomedicines 2023, 11, 2942. https://doi.org/10.3390/biomedicines11112942
Tahtamouni LH, Alderfer SA, Kuhn TB, Minamide LS, Chanda S, Ruff MR, Bamburg JR. Characterization of a Human Neuronal Culture System for the Study of Cofilin–Actin Rod Pathology. Biomedicines. 2023; 11(11):2942. https://doi.org/10.3390/biomedicines11112942
Chicago/Turabian StyleTahtamouni, Lubna H., Sydney A. Alderfer, Thomas B. Kuhn, Laurie S. Minamide, Soham Chanda, Michael R. Ruff, and James R. Bamburg. 2023. "Characterization of a Human Neuronal Culture System for the Study of Cofilin–Actin Rod Pathology" Biomedicines 11, no. 11: 2942. https://doi.org/10.3390/biomedicines11112942
APA StyleTahtamouni, L. H., Alderfer, S. A., Kuhn, T. B., Minamide, L. S., Chanda, S., Ruff, M. R., & Bamburg, J. R. (2023). Characterization of a Human Neuronal Culture System for the Study of Cofilin–Actin Rod Pathology. Biomedicines, 11(11), 2942. https://doi.org/10.3390/biomedicines11112942






