Effects of Schlemm’s Canal Suture Implantation Surgery and Pilocarpine Eye Drops on Trabecular Meshwork Pulsatile Motion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Surgical Technique
2.4. Assessments
2.5. IOP Examinations
2.6. PhS-OCT Examinations
2.7. Data Analysis
3. Results
3.1. Demographics and Baseline Characteristics
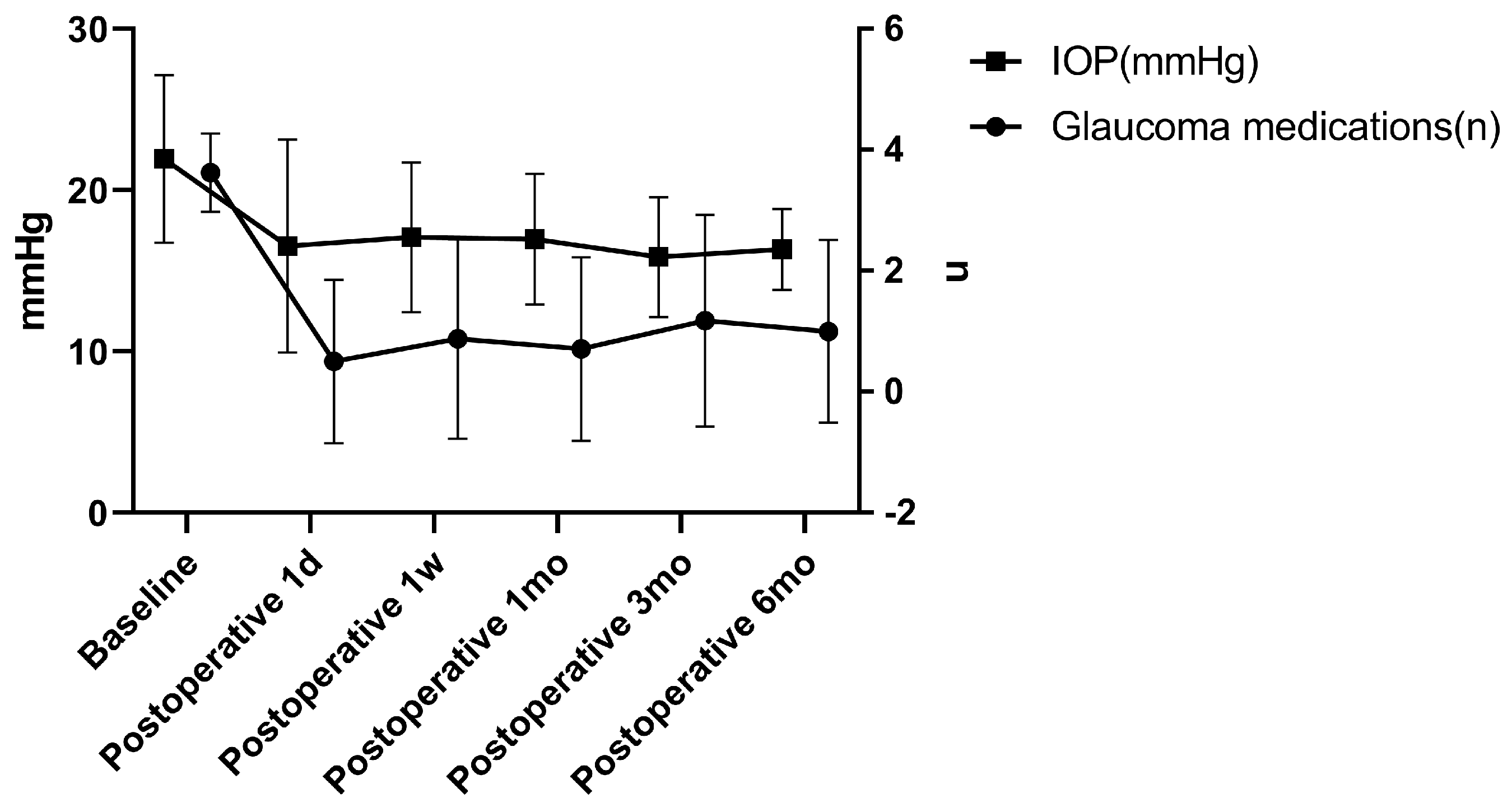
3.2. IOP and Medications
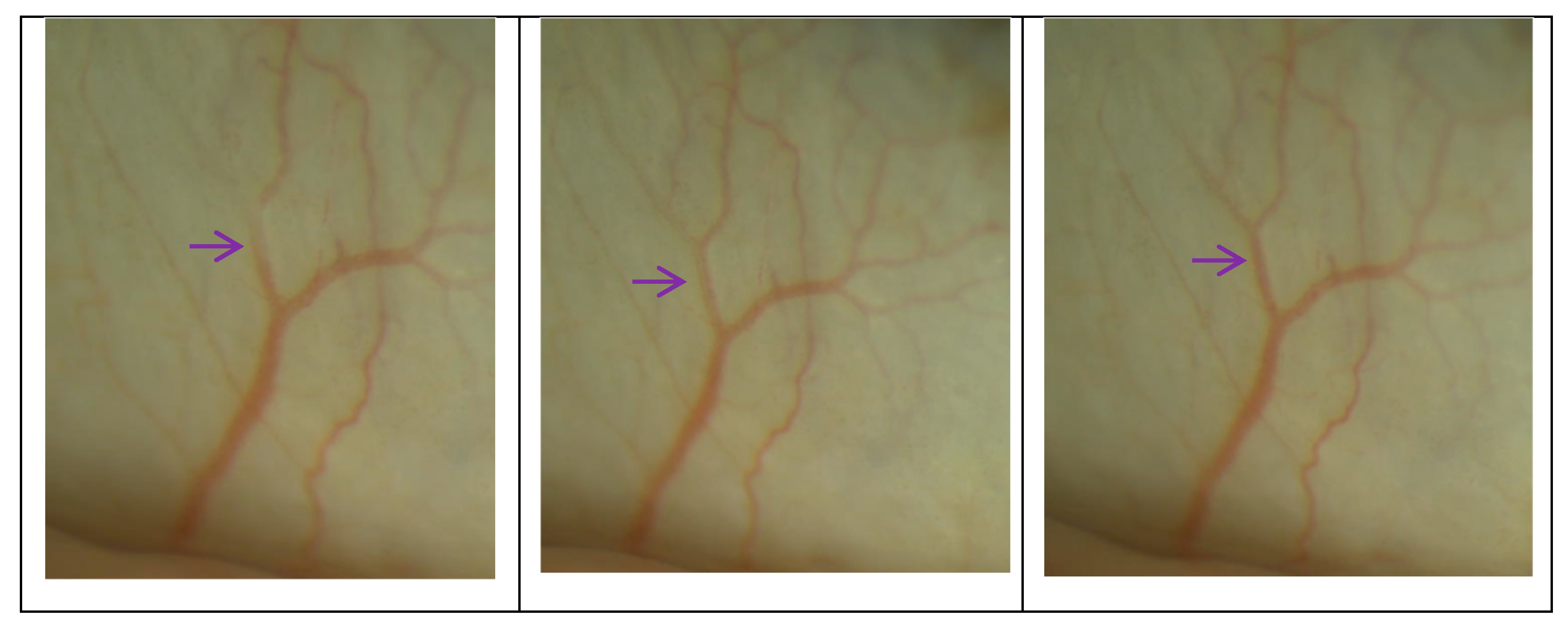
3.3. The MV and CDisp of the TM after Surgery
3.4. The MV and CDisp of the TM before and after Pilocarpine Eye Drops
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ethier, C.R.; Yoo, P.; Berdahl, J.P. The effects of negative periocular pressure on intraocular pressure. Exp. Eye Res. 2020, 191, 107928. [Google Scholar] [CrossRef]
- Konopińska, J.; Lewczuk, K.; Jabłońska, J.; Mariak, Z.; Rękas, M. Microinvasive Glaucoma Surgery: A Review of Schlemm’s Canal-Based Procedures. Clin. Ophthalmol. 2021, 15, 1109–1118. [Google Scholar] [CrossRef]
- Soundararajan, A.; Wang, T.; Sundararajan, R.; Wijeratne, A.; Mosley, A.; Harvey, F.C.; Bhattacharya, S.; Pattabiraman, P.P. Multiomics analysis reveals the mechanical stress-dependent changes in trabecular meshwork cytoskeletal-extracellular matrix interactions. Front. Cell Dev. Biol. 2022, 10, 874828. [Google Scholar] [CrossRef]
- Andrew, N.H.; Akkach, S.; Casson, R.J. A review of aqueous outflow resistance and its relevance to microinvasive glaucoma surgery. Surv. Ophthalmol. 2020, 65, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Nakakura, S.; Tabuchi, H. Heart rhythm-synchronized fibrin flap in a glaucoma tube shunt: The heartbeat acts as a drainage pump for the aqueous humor: A case report. Medicine 2021, 100, e26603. [Google Scholar] [CrossRef]
- Xin, C.; Wang, R.K.; Song, S.; Shen, T.; Wen, J.; Martin, E.; Jiang, Y.; Padilla, S.; Johnstone, M. Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Exp. Eye Res. 2017, 158, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Yu-Wai-Man, C. Glaucoma: Novel antifibrotic therapeutics for the trabecular meshwork. Eur. J. Pharmacol. 2023, 954, 175882. [Google Scholar] [CrossRef]
- Karimi, A.; Rahmati, S.M.; Razaghi, R.; Crawford Downs, J.; Acott, T.S.; Wang, R.K.; Johnstone, M. Biomechanics of human trabecular meshwork in healthy and glaucoma eyes via dynamic Schlemm’s canal pressurization. Comput. Methods Programs Biomed. 2022, 221, 106921. [Google Scholar] [CrossRef]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef]
- Mallick, S.; Sharma, M.; Kumar, A.; Du, Y. Cell-Based Therapies for Trabecular Meshwork Regeneration to Treat Glaucoma. Biomolecules 2021, 11, 1258. [Google Scholar] [CrossRef]
- Karimi, A.; Crouch, D.J.; Razaghi, R.; Crawford Downs, J.; Acott, T.S.; Kelley, M.J.; Behnsen, J.G.; Bosworth, L.A.; Sheridan, C.M. Morphological and biomechanical analyses of the human healthy and glaucomatous aqueous outflow pathway: Imaging-to-modeling. Comput. Methods Programs Biomed. 2023, 236, 107485. [Google Scholar] [CrossRef]
- Vahabikashi, A.; Gelman, A.; Dong, B.; Gong, L.; Cha, E.D.K.; Schimmel, M.; Tamm, E.R.; Perkumas, K.; Stamer, W.D.; Sun, C.; et al. Increased stiffness and flow resistance of the inner wall of Schlemm’s canal in glaucomatous human eyes. Proc. Natl. Acad. Sci. USA 2019, 116, 26555–26563. [Google Scholar] [CrossRef]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Morgan, J.T.; Raghunathan, V.K.; Chang, Y.R.; Murphy, C.J.; Russell, P. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget 2015, 6, 15362–15374. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Park, S.A.; Weber, D.; Phinney, B.S.; Murphy, C.J.; Russell, P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Song, S.; Johnstone, M.; Wang, N.; Wang, R.K. Quantification of Pulse-Dependent Trabecular Meshwork Motion in Normal Humans Using Phase-Sensitive OCT. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.F.; Koh, S.H.; McLaughlin, R.A.; Kennedy, K.M.; Munro, P.R.; Sampson, D.D. Strain estimation in phase-sensitive optical coherence elastography. Biomed. Opt. Express. 2012, 3, 1865–1879. [Google Scholar] [CrossRef]
- Wang, K.; Johnstone, M.A.; Xin, C.; Song, S.; Padilla, S.; Vranka, J.A.; Acott, T.S.; Zhou, K.; Schwaner, S.A.; Wang, R.K.; et al. Estimating Human Trabecular Meshwork Stiffness by Numerical Modeling and Advanced OCT Imaging. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4809–4817. [Google Scholar] [CrossRef]
- Johnstone, M.; Xin, C.; Tan, J.; Martin, E.; Wen, J.; Wang, R.K. Aqueous outflow regulation—21st century concepts. Prog. Retin. Eye Res. 2020, 83, 100917. [Google Scholar] [PubMed]
- Salimi, A.; Nithianandan, H.; Al Farsi, H.; Harasymowycz, P.; Saheb, H. Gonioscopy-Assisted Transluminal Trabeculotomy in Younger to Middle-Aged Adults: One-Year Outcomes. Ophthalmol. Glaucoma. 2021, 4, 162–172. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Han, Y.; Shi, Y.; Xin, C.; Yin, P.; Li, M.; Cao, K.; Wang, N. Outcomes of gonioscopy-assisted transluminal trabeculotomy in juvenile-onset primary open-angle glaucoma. Eye 2021, 35, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.I.K.; Fea, A.; Au, L.; Ang, R.E.; Harasymowycz, P.; Jampel, H.D.; Samuelson, T.W.; Chang, D.F.; Rhee, D.J.; COMPARE Investigators. A Prospective Randomized Trial Comparing Hydrus and iStent Microinvasive Glaucoma Surgery Implants for Standalone Treatment of Open-Angle Glaucoma: The COMPARE Study. Ophthalmology 2020, 127, 52–61. [Google Scholar] [CrossRef]
- Kaufman, P.L. Deconstructing aqueous humor outflow—The last 50 years. Exp. Eye Res. 2020, 197, 108105. [Google Scholar] [CrossRef]
- Liang, X.; Adie, S.G.; John, R.; Boppart, S.A. Dynamic spectral-domain optical coherence elastography for tissue characterization. Opt. Express 2010, 18, 14183–14190. [Google Scholar] [CrossRef]
- Shin, J.W.; Sung, K.R.; Park, S.W. Patterns of Progressive Ganglion Cell-Inner Plexiform Layer Thinning in Glaucoma Detected by OCT. Ophthalmology 2018, 125, 1515–1525. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Song, S.; Arnal, B.; Wong, E.Y.; Huang, Z.; Wang, R.K.; O’Donnell, M. Shear wave pulse compression for dynamic elastography using phase-sensitive optical coherence tomography. J. Biomed. Opt. 2014, 19, 16013. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wei, W.; Hsieh, B.Y.; Pelivanov, I.; Shen, T.T.; O’Donnell, M.; Wang, R.K. Strategies to improve phase-stability of ultrafast swept source optical coherence tomography for single shot imaging of transient mechanical waves at 16 kHz frame rate. Appl. Phys. Lett. 2016, 108, 191104. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Xin, C.; Xu, J.; Hu, J.; Wang, H.; Wang, N.; Johnstone, M. Pulsatile Trabecular Meshwork Motion: An Indicator of IOP Control in Primary Open-Angle Glaucoma. J. Clin. Med. 2022, 11, 2696. [Google Scholar] [CrossRef]
- Rashidi, N.; Pant, A.D.; Salinas, S.D.; Shah, M.; Thomas, V.S.; Zhang, G.; Dorairaj, S.; Amini, R. Iris stromal cell nuclei deform to more elongated shapes during pharmacologically-induced miosis and mydriasis. Exp. Eye Res. 2021, 202, 108373. [Google Scholar] [CrossRef]
- Kaufman, P.L. Aqueous humor dynamics following total iridectomy in the cynomolgus monkey. Investig. Ophthalmol. Vis. Sci. 1979, 18, 870–874. [Google Scholar]
- Tektas, O.Y.; Lütjen-Drecoll, E.; Scholz, M. Qualitative and quantitative morphologic changes in the vasculature and extracellular matrix of the prelaminar optic nerve head in eyes with POAG. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5083–5091. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.A.; Lütjen-Drecoll, E.; Kaufman, P.L. Age-related posterior ciliary muscle restriction—A link between trabecular meshwork and optic nerve head pathophysiology. Exp. Eye Res. 2017, 158, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Li, Y.; Rakymzhan, A.; Xie, Z.; Wang, R.K. Measurement and visualization of stimulus-evoked tissue dynamics in mouse barrel cortex using phase-sensitive optical coherence tomography. Biomed. Opt. Express. 2020, 11, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Song, S.; Johnstone, M.A.; Zhang, Q.; Xu, J.; Zhang, X.; Wang, R.K.; Wen, J.C. Reduced Pulsatile Trabecular Meshwork Motion in Eyes With Primary Open Angle Glaucoma Using Phase-Sensitive Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 21. [Google Scholar] [CrossRef]
- Jiang, X.; Torres, M.; Varma, R.; Los Angeles Latino Eye Study Group. Variation in IOP and the Risk of Developing Open-Angle Glaucoma: The Los Angeles Latino Eye Study. Am. J. Ophthalmol. 2018, 188, 51–59. [Google Scholar] [CrossRef]
- Sultan, M.B.; Mansberger, S.L.; Lee, P.P. Understanding the importance of IOP variables in glaucoma: A systematic review. Surv. Ophthalmol. 2009, 54, 643–662. [Google Scholar] [CrossRef]
- Huang, A.S.; Li, M.; Yang, D.; Wang, H.; Wang, N.; Weinreb, R.N. Aqueous Angiography in Living Nonhuman Primates Shows Segmental, Pulsatile, and Dynamic Angiographic Aqueous Humor Outflow. Ophthalmology 2017, 124, 793–803. [Google Scholar] [CrossRef]
- Mansouri, K.; Shaarawy, T. Update on Schlemm’s canal based procedures. Middle East. Afr. J. Ophthalmol. 2015, 22, 38–44. [Google Scholar] [CrossRef]
- Cagini, C.; Peruzzi, C.; Fiore, T.; Spadea, L.; Lippera, M.; Lippera, S. Canaloplasty: Current Value in the Management of Glaucoma. J. Ophthalmol. 2016, 2016, 7080475. [Google Scholar] [CrossRef]





| All Participants | |
|---|---|
| Age (years) | 43.29 ± 10.73 |
| Sex (F:M) | 7/15 |
| Eyes (n) | 24 |
| POAG | |
| Mild (n) | 4 |
| Moderate (n) | 17 |
| Severe (n) | 3 |
| Central corneal thickness (μm) | 544.96 ± 47.84 |
| Axial length (mm) | 24.57 ± 1.50 |
| Number of corneal endothelial cells (mm2) | 2584.8 ± 290.53 |
| Intraocular pressure (mmHg) | 22.16 ± 5.23 |
| Mean deviation (dB) | 9.63 ± 3.22 |
| Anterior chamber depth (mm) | 3.08 ± 0.33 |
| Glaucoma medications (n) | 3.63 ± 0.65 |
| Heart rate (times/minute) | 72.25 ± 6.07 |
| Mean arterial pressure (mmHg) | 122.04 ± 15.08 |
| Baseline (n) Mean ± SD | Post-Surgery (Time) (n) | Mean ± SD | Mean Difference | p-Value | |
|---|---|---|---|---|---|
| Intraocular pressure (mmHg) | 22.16 ± 5.23 (24) | 1 day (24) | 16.55 ± 6.60 | 5.61 ± 7.16 | 0.002 |
| 1 week (24) | 17.08 ± 4.63 | 4.83 ± 7.35 | 0.005 | ||
| 1 month (24) | 16.97 ± 4.05 | 6.30 ± 6.14 | 0.001 | ||
| 3 months (23) | 15.85 ± 3.71 | 6.61 ± 4.78 | <0.001 | ||
| 6 months (22) | 16.33 ± 2.51 | 6.03 ± 4.78 | <0.001 | ||
| Glaucoma medications (n) | 3.63 ± 0.65 (24) | 1 day (24) | 0.50 ± 1.35 | 3.13 ± 1.36 | <0.001 |
| 1 week (24) | 0.88 ± 1.65 | 2.75 ± 1.57 | <0.001 | ||
| 1 month (24) | 0.71 ± 1.51 | 2.92 ± 1.47 | <0.001 | ||
| 3 months (23) | 1.17 ± 1.75 | 2.44 ± 1.59 | <0.001 | ||
| 6 months (22) | 1.00 ± 1.51 | 2.59 ± 1.37 | <0.001 |
| Baseline (n) Mean ± SD | Post-Surgery (Months) (n) | Mean ± SD | Mean Difference | p-Value | |
|---|---|---|---|---|---|
| MV (μm/s) | 21.32 ± 2.63 (24) | 3 months (24) | 21.85 ± 2.28 | −0.520 ± 3.004 | 0.404 |
| 6 months (22) | 22.38 ± 2.38 | −1.050 ± 3.360 | 0.139 | ||
| CDisp (μm) | 0.204 ± 0.034 (24) | 3 months (24) | 0.199 ± 0.041 | 0.005 ± 0.043 | 0.560 |
| 6 months (22) | 0.209 ± 0.037 | −0.004 ± 0.042 | 0.576 |
| Eyes, n | Baseline Mean ± SD | After Pilocarpine | Mean Difference | p-Value | |
|---|---|---|---|---|---|
| MV (μm/s) | 24 | 21.32 ± 2.63 | 17.00 ± 2.43 | 4.32 ± 2.68 | <0.001 |
| CDisp (μm) | 24 | 0.204 ± 0.034 | 0.184 ± 0.035 | 0.020 ± 0.036 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, Q.; Du, R.; Xin, C.; Wang, N. Effects of Schlemm’s Canal Suture Implantation Surgery and Pilocarpine Eye Drops on Trabecular Meshwork Pulsatile Motion. Biomedicines 2023, 11, 2932. https://doi.org/10.3390/biomedicines11112932
Sang Q, Du R, Xin C, Wang N. Effects of Schlemm’s Canal Suture Implantation Surgery and Pilocarpine Eye Drops on Trabecular Meshwork Pulsatile Motion. Biomedicines. 2023; 11(11):2932. https://doi.org/10.3390/biomedicines11112932
Chicago/Turabian StyleSang, Qing, Rong Du, Chen Xin, and Ningli Wang. 2023. "Effects of Schlemm’s Canal Suture Implantation Surgery and Pilocarpine Eye Drops on Trabecular Meshwork Pulsatile Motion" Biomedicines 11, no. 11: 2932. https://doi.org/10.3390/biomedicines11112932
APA StyleSang, Q., Du, R., Xin, C., & Wang, N. (2023). Effects of Schlemm’s Canal Suture Implantation Surgery and Pilocarpine Eye Drops on Trabecular Meshwork Pulsatile Motion. Biomedicines, 11(11), 2932. https://doi.org/10.3390/biomedicines11112932







