Hydrodynamic and Hemodynamic Interactions in Chronic Hydrocephalus
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. MRI Acquisition
2.3. Data Analysis
- -
- SVCSF: corresponds to the stroke volume of CSF, which oscillates through the upper cervical SAS at the level of the C2C3 intervertebral disc during one cardiac cycle. This reflects variations in intracranial CSF volume during a cardiac cycle.
- -
- SVAV: corresponds to the volume of blood, which oscillates in the cranial compartment during one cardiac cycle. This reflects variations in intracranial vascular volume during a cardiac cycle.
- -
- Hemohydrodynamic ratio: ratio between SVCSF and SVAV. SVCSF is the passive response of CSF to a pressure gradient between intracranial and spinal SAS (). SVAV is the main factor in intracranial volume variation during a cardiac cycle (). The combination of these two parameters gives the ratio of a change in volume to a change in pressure.
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Comparison of Hemodynamic and Hydrodynamic Parameters in Probable and Unlikely Hydrocephalus Populations
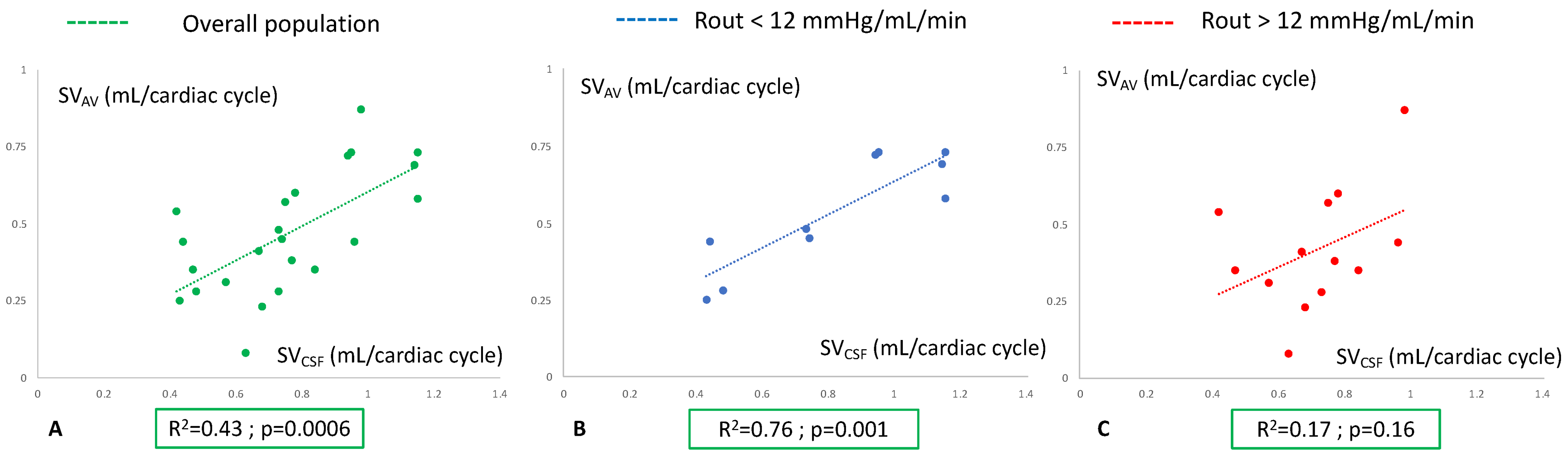
3.2. Analysis of Hemohydrodynamic Interactions in Probable and Unlikely Hydrocephalus Populations
4. Discussion
4.1. Comparison of Hemodynamics in Probable and Unlikely Hydrocephalus Populations
4.2. Comparison of Hydrodynamics in Probable and Unlikely Hydrocephalus Populations
4.3. Analysis of Hemohydrodynamic Interactions in the Two Patient Groups
4.4. Compliance in Chronic Hydrocephalus
4.5. Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hakim, S.; Adams, R.D. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J. Neurol. Sci. 1965, 2, 307–327. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Timofeev, I.; Lavinio, A.; Guazzo, E.; Hutchinson, P.; Pickard, J.D. Intracranial pressure: More than a number. Neurosurg. Focus 2007, 22, E10. [Google Scholar] [CrossRef]
- Weerakkody, R.A.; Czosnyka, M.; Schuhmann, M.U.; Schmidt, E.; Keong, N.; Santarius, T.; Pickard, J.D.; Czosnyka, Z. Clinical assessment of cerebrospinal fluid dynamics in hydrocephalus. Guide to interpretation based on observational study. Acta Neurol. Scand. 2011, 124, 85–98. [Google Scholar] [CrossRef]
- Kahlon, B.; Sundbärg, G.; Rehncrona, S. Comparison between the lumbar infusion and CSF tap tests to predict outcome after shunt surgery in suspected normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2002, 73, 721–726. [Google Scholar] [CrossRef]
- Boon, A.J.; Tans, J.T.; Delwel, E.J.; Egeler-Peerdeman, S.M.; Hanlo, P.W.; Wurzer, H.A.; Avezaat, C.J.; de Jong, D.A.; Gooskens, R.H.; Hermans, J. Dutch normal-pressure hydrocephalus study: Prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J. Neurosurg. 1997, 87, 687–693. [Google Scholar] [CrossRef]
- Cushing, H. Studies in Intracranial Physiology & Surgery: The Third Circulation, the Hypophysics, the Gliomas; Milford, H., Ed.; Oxford University Press: Oxford, UK, 1926. [Google Scholar]
- Cushing, H. Studies on the Cerebro-Spinal Fluid: I. Introduction. J. Med. Res. 1914, 31, 1–19. [Google Scholar]
- Wilson, M.H. Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 1338–1350. [Google Scholar] [CrossRef]
- Guillaume, J.; Janny, P. Continuous intracranial manometry; importance of the method and first results. Rev. Neurol. 1951, 84, 131–142. [Google Scholar]
- Kirkness, C.J.; Mitchell, P.H.; Burr, R.L.; March, K.S.; Newell, D.W. Intracranial pressure waveform analysis: Clinical and research implications. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses 2000, 32, 271–277. [Google Scholar] [CrossRef]
- Balédent, O.; Henry-Feugeas, M.C.; Idy-Peretti, I. Cerebrospinal fluid dynamics and relation with blood flow: A magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Investig. Radiol. 2001, 36, 368–377. [Google Scholar] [CrossRef]
- Alperin, N.J.; Lee, S.H.; Loth, F.; Raksin, P.B.; Lichtor, T. MR-Intracranial pressure (ICP): A method to measure intracranial elastance and pressure noninvasively by means of MR imaging: Baboon and human study. Radiology 2000, 217, 877–885. [Google Scholar] [CrossRef]
- Bateman, G.A. Vascular compliance in normal pressure hydrocephalus. AJNR Am. J. Neuroradiol. 2000, 21, 1574–1585. [Google Scholar]
- Lokossou, A.; Metanbou, S.; Gondry-Jouet, C.; Balédent, O. Extracranial versus intracranial hydro-hemodynamics during aging: A PC-MRI pilot cross-sectional study. Fluids Barriers CNS 2020, 17, 1–11. [Google Scholar] [CrossRef]
- Greitz, D.; Greitz, T. The pathogenesis and hemodynamics of hydrocephalus. A proposal for a new understanding. Int. J. Neuroradiol. 1997, 3, 367–375. [Google Scholar]
- Balédent, O.; Gondry-Jouet, C.; Meyer, M.-E.; De Marco, G.; Le Gars, D.; Henry-Feugeas, M.-C.; Idy-Peretti, I. Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Investig. Radiol. 2004, 39, 45–55. [Google Scholar] [CrossRef]
- Bateman, G.A. The pathophysiology of idiopathic normal pressure hydrocephalus: Cerebral ischemia or altered venous hemodynamics? AJNR Am. J. Neuroradiol. 2008, 29, 198–203. [Google Scholar] [CrossRef]
- Bradley, W.G. Cerebrospinal fluid dynamics and shunt responsiveness in patients with normal-pressure hydrocephalus. Mayo Clin. Proc. Mayo Clin. 2002, 77, 507–508. [Google Scholar] [CrossRef]
- Bradley, W.G.; Kortman, K.E.; Burgoyne, B. Flowing cerebrospinal fluid in normal and hydrocephalic states: Appearance on MR images. Radiology 1986, 159, 611–616. [Google Scholar] [CrossRef]
- Bradley, W.G.; Scalzo, D.; Queralt, J.; Nitz, W.N.; Atkinson, D.J.; Wong, P. Normal-pressure hydrocephalus: Evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996, 198, 523–529. [Google Scholar] [CrossRef]
- Greitz, D.; Hannerz, J.; Rähn, T.; Bolander, H.; Ericsson, A. MR imaging of cerebrospinal fluid dynamics in health and disease. On the vascular pathogenesis of communicating hydrocephalus and benign intracranial hypertension. Acta Radiol. Stockh. Swed. 1987 1994, 35, 204–211. [Google Scholar]
- Luetmer, P.H.; Huston, J.; Friedman, J.A.; Dixon, G.R.; Petersen, R.C.; Jack, C.R.; McClelland, R.L.; Ebersold, M.J. Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: Technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 2002, 50, 534–543, discussion 543–544. [Google Scholar]
- Alperin, N.; Sivaramakrishnan, A.; Lichtor, T. Magnetic resonance imaging-based measurements of cerebrospinal fluid and blood flow as indicators of intracranial compliance in patients with Chiari malformation. J. Neurosurg. 2005, 103, 46–52. [Google Scholar] [CrossRef]
- Ringstad, G.; Emblem, K.E.; Geier, O.; Alperin, N.; Eide, P.K. Aqueductal Stroke Volume: Comparisons with Intracranial Pressure Scores in Idiopathic Normal Pressure Hydrocephalus. AJNR Am. J. Neuroradiol. 2015, 36, 1623–1630. [Google Scholar] [CrossRef]
- Bateman, G.A. Toward a better understanding of normal pressure hydrocephalus. AJNR Am. J. Neuroradiol. 2001, 22, 596. [Google Scholar]
- Enzmann, D.R.; Pelc, N.J. Cerebrospinal fluid flow measured by phase-contrast cine MR. AJNR Am. J. Neuroradiol. 1993, 14, 1301–1307, discussion 1309–1310. [Google Scholar]
- Corkill, R.G.; Garnett, M.R.; Blamire, A.M.; Rajagopalan, B.; Cadoux-Hudson, T.A.D.; Styles, P. Multi-modal MRI in normal pressure hydrocephalus identifies pre-operative haemodynamic and diffusion coefficient changes in normal appearing white matter correlating with surgical outcome. Clin. Neurol. Neurosurg. 2003, 105, 193–202. [Google Scholar] [CrossRef]
- Kitagaki, H.; Mori, E.; Ishii, K.; Yamaji, S.; Hirono, N.; Imamura, T. CSF spaces in idiopathic normal pressure hydrocephalus: Morphology and volumetry. AJNR Am. J. Neuroradiol. 1998, 19, 1277–1284. [Google Scholar]
- Mase, M.; Yamada, K.; Banno, T.; Miyachi, T.; Ohara, S.; Matsumoto, T. Quantitative analysis of CSF flow dynamics using MRI in normal pressure hydrocephalus. Acta Neurochir. Suppl. 1998, 71, 350–353. [Google Scholar]
- Miyati, T.; Mase, M.; Banno, T.; Kasuga, T.; Yamada, K.; Fujita, H.; Koshida, K.; Sanada, S.; Onoguchi, M. Frequency analyses of CSF flow on cine MRI in normal pressure hydrocephalus. Eur. Radiol. 2003, 13, 1019–1024. [Google Scholar] [CrossRef]
- Lokossou, A.; Balédent, O.; Garnotel, S.; Page, G.; Balardy, L.; Czosnyka, Z.; Payoux, P.; Schmidt, E.A. ICP Monitoring and Phase-Contrast MRI to Investigate Intracranial Compliance. Acta Neurochir. Suppl. 2018, 126, 247–253. [Google Scholar] [CrossRef]
- Brasil, S.; Solla, D.J.F.; Nogueira, R.d.C.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.d.S. Novel Noninvasive Technique for Intracranial Pressure Waveform Monitoring in Critical Care. J. Pers. Med. 2021, 11, 1302. [Google Scholar] [CrossRef]
- van Wageningen, T.A.; Antonovaite, N.; Paardekam, E.; Brevé, J.J.P.; Iannuzzi, D.; van Dam, A.-M. Viscoelastic properties of white and gray matter-derived microglia differentiate upon treatment with lipopolysaccharide but not upon treatment with myelin. J. Neuroinflammation 2021, 18, 83. [Google Scholar] [CrossRef]
- Vakili, S.; Moran, D.; Hung, A.; Elder, B.D.; Jeon, L.; Fialho, H.; Sankey, E.W.; Jusué-Torres, I.; Goodwin, C.R.; Lu, J.; et al. Timing of surgical treatment for idiopathic normal pressure hydrocephalus: Association between treatment delay and reduced short-term benefit. Neurosurg. Focus 2016, 41, E2. [Google Scholar] [CrossRef]
- Krahulik, D.; Vaverka, M.; Hrabalek, L.; Hampl, M.; Halaj, M.; Jablonsky, J.; Langova, K. Ventriculoperitoneal shunt in treating of idiopathic normal pressure hydrocephalus-single-center study. Acta Neurochir. 2020, 162, 1–7. [Google Scholar] [CrossRef]
- Scollato, A.; Gallina, P.; Gautam, B.; Pellicanò, G.; Cavallini, C.; Tenenbaum, R.; Di Lorenzo, N. Changes in aqueductal CSF stroke volume in shunted patients with idiopathic normal-pressure hydrocephalus. AJNR Am. J. Neuroradiol. 2009, 30, 1580–1586. [Google Scholar] [CrossRef]
- Czosnyka, Z.; Owler, B.; Keong, N.; Santarius, T.; Baledent, O.; Pickard, J.D.; Czosnyka, M. Impact of duration of symptoms on CSF dynamics in idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2011, 123, 414–418. [Google Scholar] [CrossRef]
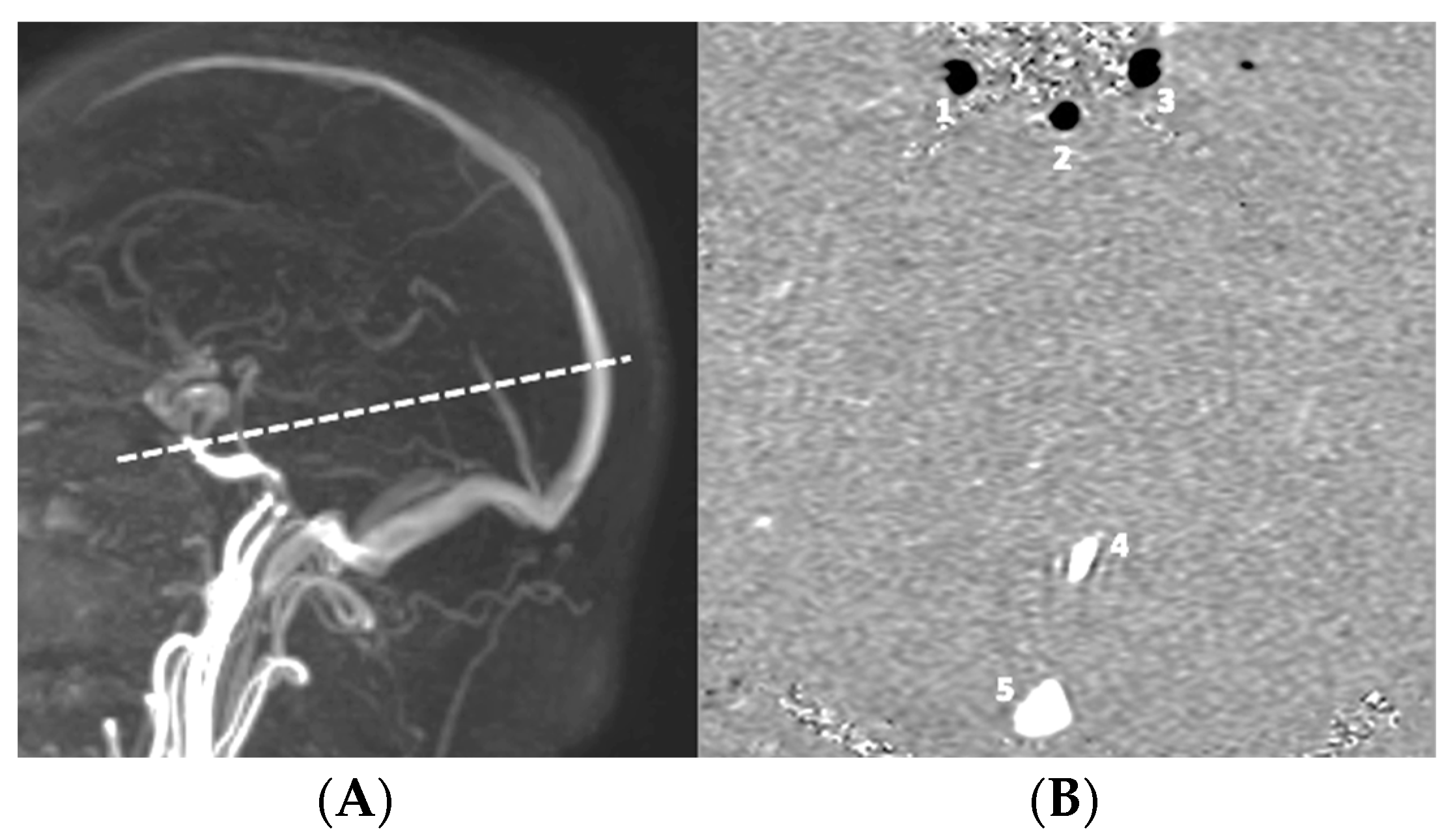


| CINE-PC | |
|---|---|
| Venc (cm/s) | 60 (blood)/5 (CSF) |
| FOV (cm2) | 14 × 14 |
| Resolution (mm2) | 1 × 1 |
| Thickness (mm2) | 2 |
| Flip Angle (degree) | 30 |
| EPI-factor | NA |
| SENSE | 1.5 |
| TE (ms) | 6.6 |
| TR (ms) | 10.9 |
| Readout time (ms) | 5.3 |
| Number of images | 32 |
| Acquisition time (s) | 50–115 |
| Number of images per cycle | 32 |
| Unlikely Hydrocephalus | Probable Hydrocephalus | p-Value | |
|---|---|---|---|
| Age (years) | 75 ± 7 | 73 ± 8 | 0.63 |
| Sex ratio (F/M) | 2/3 | 6/7 | 0.82 |
| SVAV (mL/cc) | 0.82 ± 0.20 | 0.71 ± 0.17 | 0.44 |
| SVCSF (mL/cc) | 0.54 ± 0.18 | 0.42 ± 0.20 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capel, C.; Owashi, K.; Peltier, J.; Balédent, O. Hydrodynamic and Hemodynamic Interactions in Chronic Hydrocephalus. Biomedicines 2023, 11, 2931. https://doi.org/10.3390/biomedicines11112931
Capel C, Owashi K, Peltier J, Balédent O. Hydrodynamic and Hemodynamic Interactions in Chronic Hydrocephalus. Biomedicines. 2023; 11(11):2931. https://doi.org/10.3390/biomedicines11112931
Chicago/Turabian StyleCapel, Cyrille, Kimi Owashi, Johann Peltier, and Olivier Balédent. 2023. "Hydrodynamic and Hemodynamic Interactions in Chronic Hydrocephalus" Biomedicines 11, no. 11: 2931. https://doi.org/10.3390/biomedicines11112931
APA StyleCapel, C., Owashi, K., Peltier, J., & Balédent, O. (2023). Hydrodynamic and Hemodynamic Interactions in Chronic Hydrocephalus. Biomedicines, 11(11), 2931. https://doi.org/10.3390/biomedicines11112931






