Atheroprotective Effect of Fucoidan in THP-1 Macrophages by Potential Upregulation of ABCA1
Abstract
:1. Introduction
2. Methods
2.1. Identification of Atherosclerosis-Related Proteins
2.2. Protein—Ligand Docking
2.3. Protein—Protein Docking
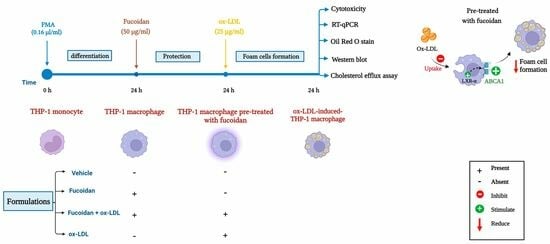
2.4. Monocyte Culture and Treatment
2.5. Cell Viability and Proliferation Assay
2.6. Quantitative Real Time PCR (qRT-PCR)
2.7. Oil Red O Staining
2.8. Western Blot Technique
2.9. Cholesterol Efflux Assay
2.10. Statistical Analysis
3. Results
3.1. Identification of Target Proteins
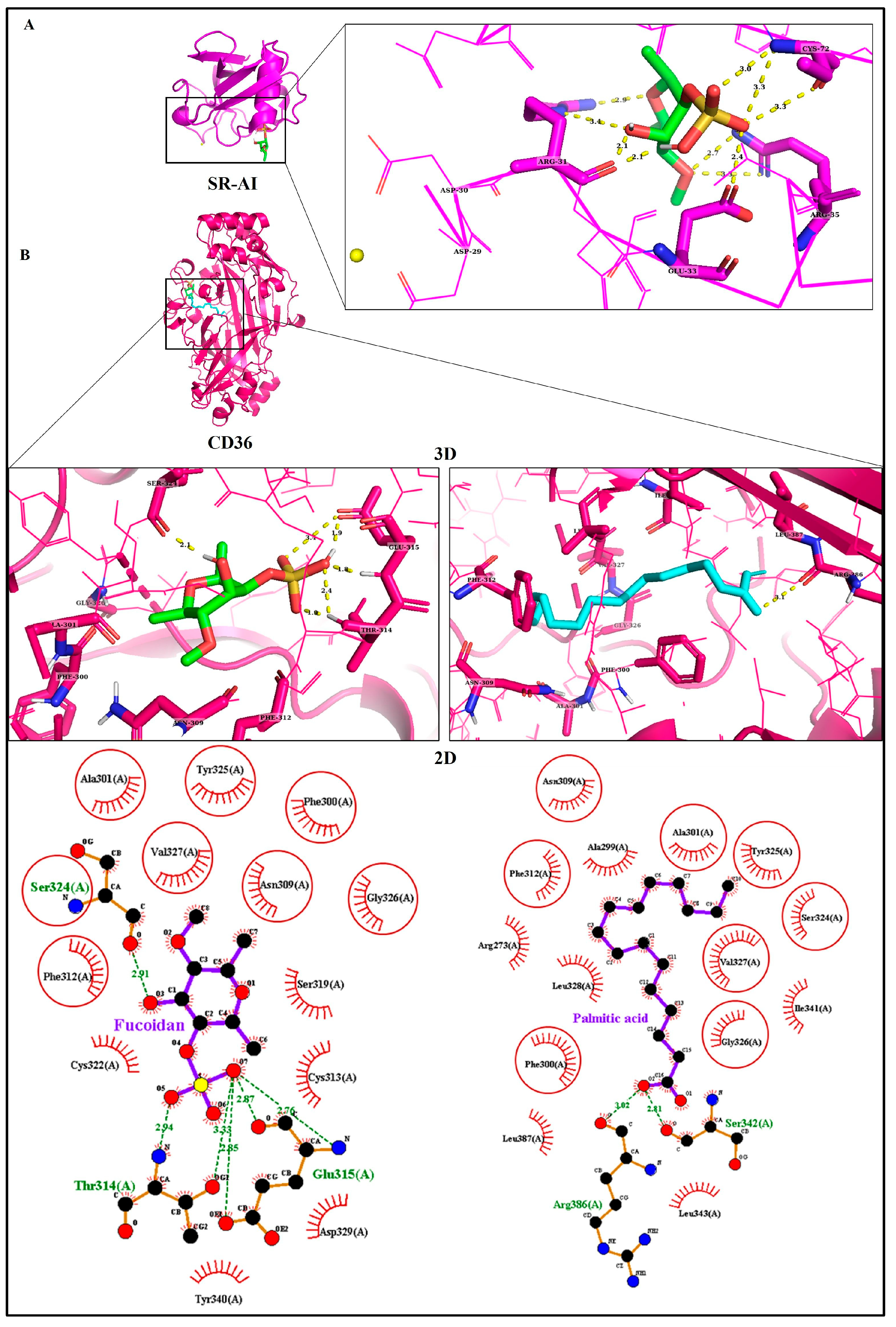
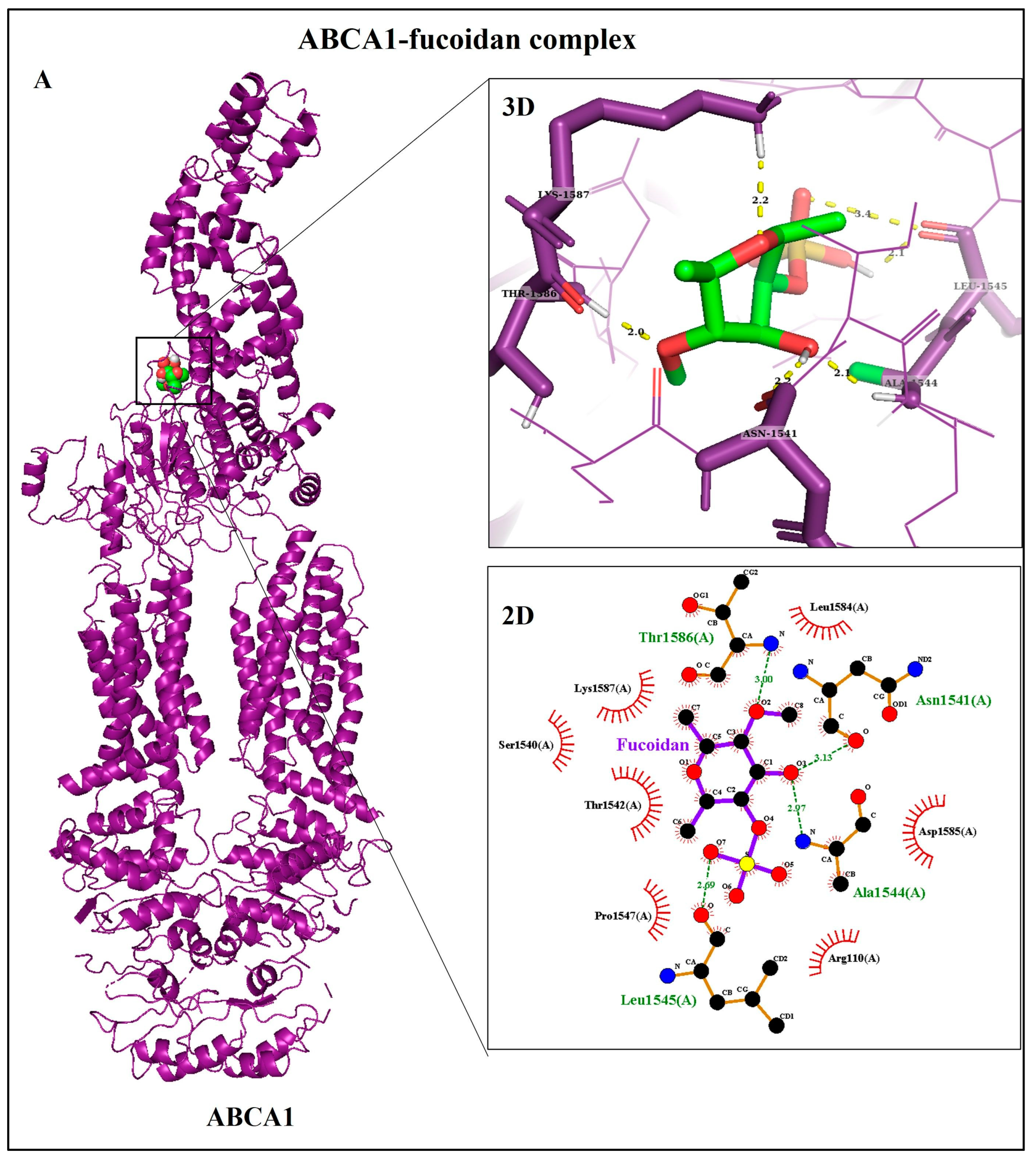
3.2. Molecular Docking Analyses
3.3. The Effect of Fucoidan on Cell Viability and Proliferation of THP-1 Macrophages
3.4. Fucoidan Modulates mRNA Expression of Genes Involved in ox-LDL Uptake
3.5. Fucoidan Reduces Lipid Accumulation in THP-1 Macrophages
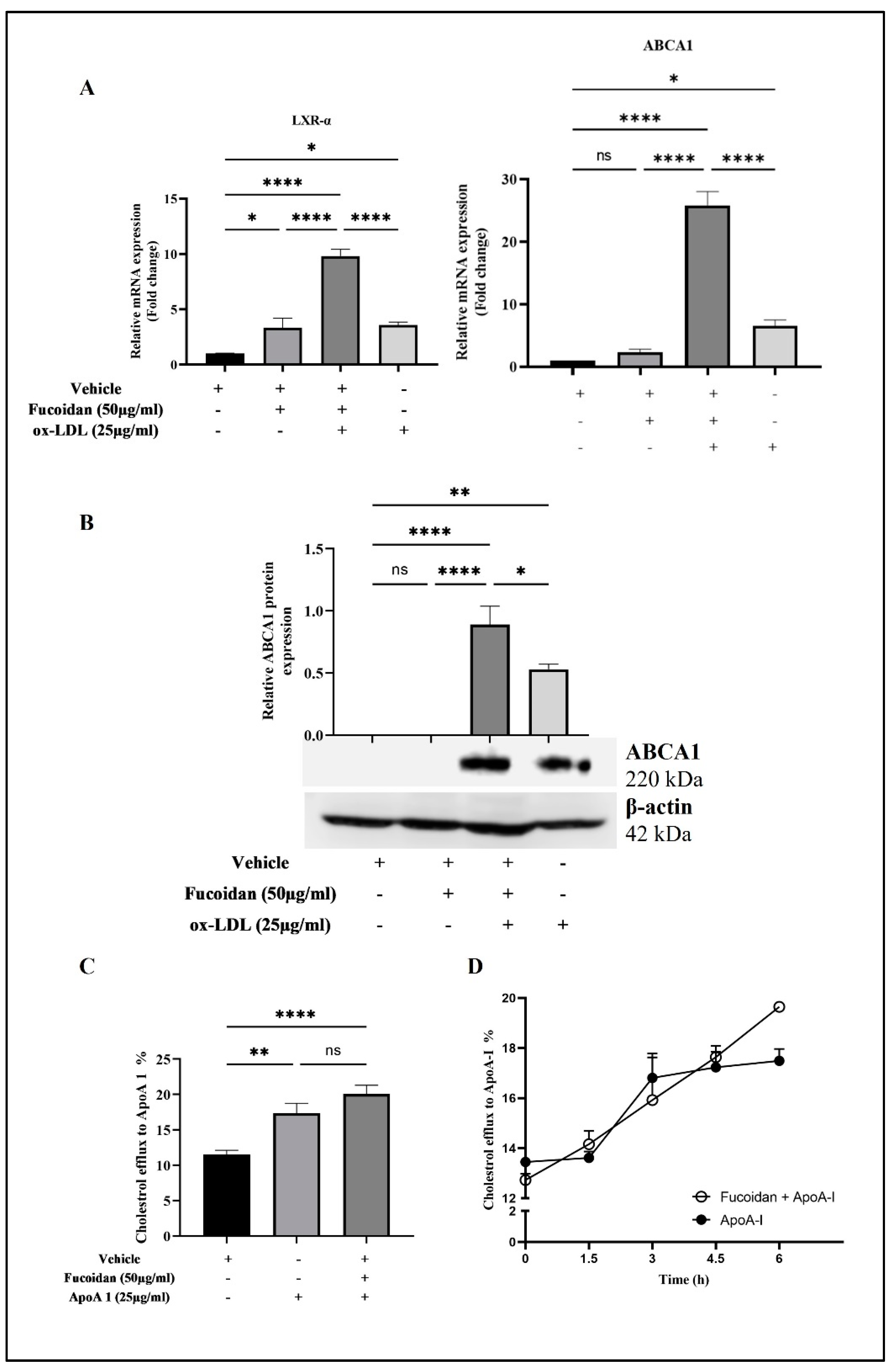
3.6. Upregulation of LXR-α and ABCA1 Expression by Fucoidan
3.7. Fucoidan Regulates Cholesterol Efflux from THP-1 Macrophages
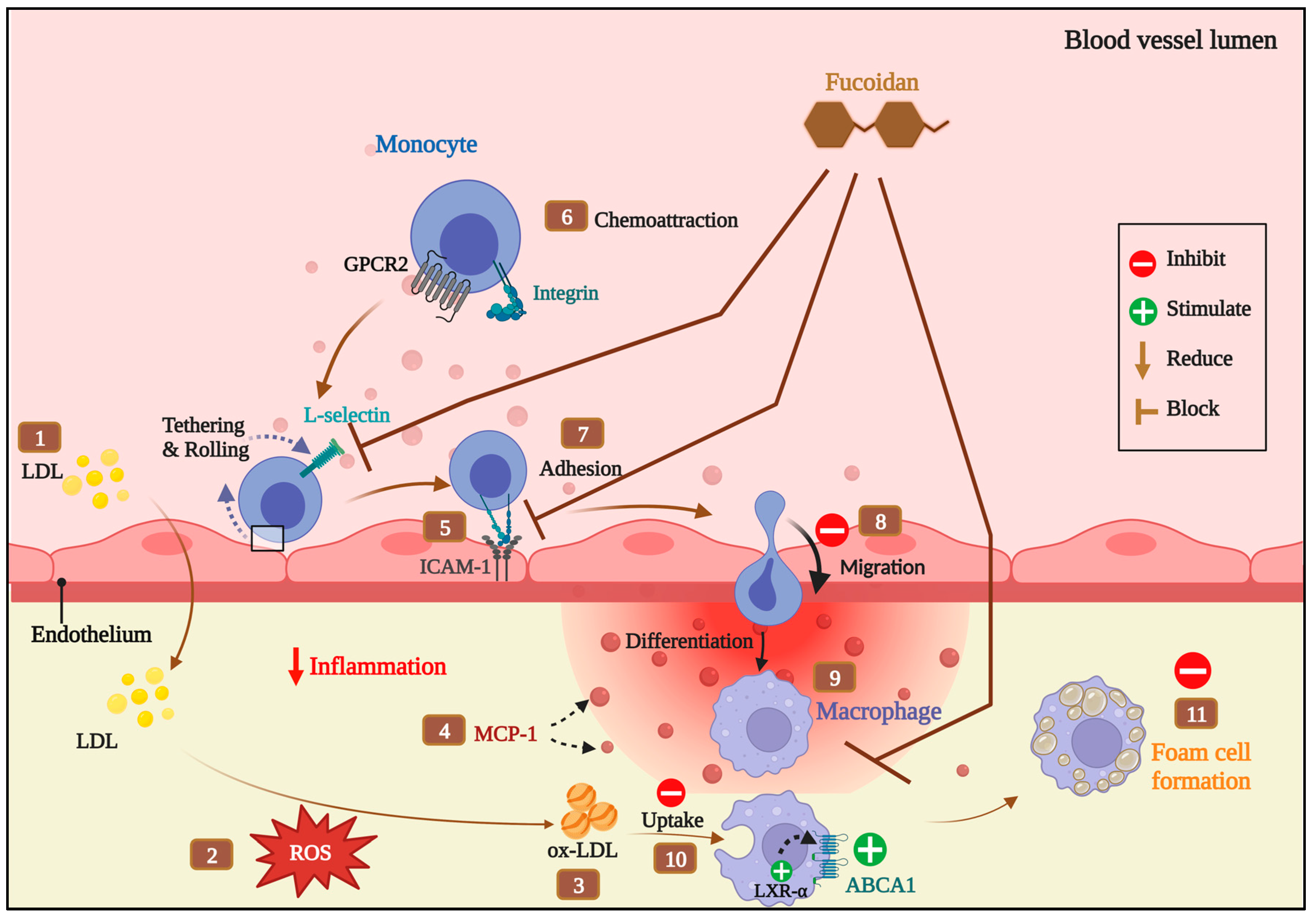
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Flynn, M.C.; Pernes, G.; Lee, M.K.S.; Nagareddy, P.R.; Murphy, A.J. Monocytes, Macrophages, and Metabolic Disease in Atherosclerosis. Front. Pharmacol. 2019, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Aldons, J.L. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An Endocytic Pathway for Internalising Large Gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef]
- Lin, X.P.; Mintern, J.D.; Gleeson, P.A. Macropinocytosis in Different Cell Types: Similarities and Differences. Membranes 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated Cholesterol Handling in Atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, I.; von Eckardstein, A.; Cavelier, C.; Radosavljevic, S.; Rohrer, L. Apolipoprotein A-I but Not High-Density Lipoproteins Are Internalised by RAW Macrophages: Roles of ATP-Binding Cassette Transporter A1 and Scavenger Receptor BI. J. Mol. Med. 2008, 86, 171–183. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Gelissen, I.C.; Ammit, A.J. Regulation of ATP Binding Cassette Transporter A1 (ABCA1) Expression: Cholesterol-Dependent and—Independent Signaling Pathways with Relevance to Inflammatory Lung Disease. Respir. Res. 2020, 21, 250. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, I.C.; Harris, M.; Rye, K.-A.; Quinn, C.; Brown, A.J.; Kockx, M.; Cartland, S.; Packianathan, M.; Kritharides, L.; Jessup, W. ABCA1 and ABCG1 Synergize to Mediate Cholesterol Export to ApoA-I. Arter. Thromb. Vasc. Biol. 2006, 26, 534–540. [Google Scholar] [CrossRef]
- Shapiro, M.D.; Fazio, S. From Lipids to Inflammation: New Approaches to Reducing Atherosclerotic Risk. Circ. Res. 2016, 118, 732–749. [Google Scholar] [CrossRef]
- Toth, P.P.; Patti, A.M.; Giglio, R.V.; Nikolic, D.; Castellino, G.; Rizzo, M.; Banach, M. Management of Statin Intolerance in 2018: Still More Questions Than Answers. Am. J. Cardiovasc. Drugs 2018, 18, 157–173. [Google Scholar] [CrossRef]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Huwait, E.; Al-Saedi, D.A.; Mirza, Z. Anti-Inflammatory Potential of Fucoidan for Atherosclerosis: In Silico and in Vitro Studies in THP-1 Cells. Molecules 2022, 27, 3197. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Barshir, R.; Fishilevich, S.; Iny-Stein, T.; Zelig, O.; Mazor, Y.; Guan-Golan, Y.; Safran, M.; Lancet, D. GeneCaRNA: A Comprehensive Gene-Centric Database of Human Non-Coding RNAs in the geneCards Suite. J. Mol. Biol. 2021, 433, 166913. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An Interactive Venn Diagram Viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Cheng, C.; Zheng, E.; Yu, B.; Zhang, Z.; Wang, Y.; Liu, Y.; He, Y. Recognition of Lipoproteins by Scavenger Receptor Class A Members. J. Biol. Chem. 2021, 297, 100948. [Google Scholar] [CrossRef]
- Hsieh, F.-L.; Turner, L.; Bolla, J.R.; Robinson, C.V.; Lavstsen, T.; Higgins, M.K. The Structural Basis for CD36 Binding by the Malaria Parasite. Nat. Commun. 2016, 7, 12837. [Google Scholar] [CrossRef]
- Qian, H.; Zhao, X.; Cao, P.; Lei, J.; Yan, N.; Gong, X. Structure of the Human Lipid Exporter ABCA1. Cell 2017, 169, 1228–1239.e10. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Atkinson, D. Crystal Structure of C-Terminal Truncated Apolipoprotein A-I Reveals the Assembly of High Density Lipoprotein (HDL) by Dimerization. J. Biol. Chem. 2011, 286, 38570–38582. [Google Scholar] [CrossRef] [PubMed]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro Web Server for Protein-Protein Docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK Server for Integrated Protein–Protein Docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Laskowski, R.A. PDBsum: Summaries and Analyses of PDB Structures. Nucleic Acids Res. 2001, 29, 221–222. [Google Scholar] [CrossRef]
- Moss, J.W.E.; Davies, T.S.; Garaiova, I.; Plummer, S.F.; Michael, D.R.; Ramji, D.P. A Unique Combination of Nutritionally Active Ingredients Can Prevent Several Key Processes Associated with Atherosclerosis in Vitro. PLoS ONE 2016, 11, e0151057. [Google Scholar] [CrossRef]
- Xu, W.; Yu, L.; Zhou, W.; Luo, M. Resistin Increases Lipid Accumulation and CD36 Expression in Human Macrophages. Biochem. Biophys. Res. Commun. 2006, 351, 376–382. [Google Scholar] [CrossRef]
- Saenz, J.; Santa-María, C.; Reyes-Quiroz, M.E.; Geniz, I.; Jiménez, J.; Sobrino, F.; Alba, G. Grapefruit Flavonoid Naringenin Regulates the Expression of LXRα in THP-1 Macrophages by Modulating AMP-Activated Protein Kinase. Mol. Pharm. 2018, 15, 1735–1745. [Google Scholar] [CrossRef]
- O’Morain, V.L.; Chan, Y.; Williams, J.O.; Alotibi, R.; Alahmadi, A.; Rodrigues, N.P.; Plummer, S.F.; Hughes, T.R.; Michael, D.R.; Ramji, D.P. The Lab4P Consortium of Probiotics Attenuates Atherosclerosis in LDL Receptor Deficient Mice Fed a High Fat Diet and Causes Plaque Stabilization by Inhibiting Inflammation and Several Pro-Atherogenic Processes. Mol. Nutr. Food Res. 2021, 65, 2100214. [Google Scholar] [CrossRef]
- Aldridge, A.; Kouroupis, D.; Churchman, S.; English, A.; Ingham, E.; Jones, E. Assay Validation for the Assessment of Adipogenesis of Multipotential Stromal Cells—A Direct Comparison of Four Different Methods. Cytotherapy 2013, 15, 89–101. [Google Scholar] [CrossRef]
- Lin, Z.; Tan, X.; Zhang, Y.; Li, F.; Luo, P.; Liu, H. Molecular Targets and Related Biologic Activities of Fucoidan: A Review. Mar. Drugs 2020, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-α and PPAR-γ Activators Induce Cholesterol Removal from Human Macrophage Foam Cells through Stimulation of the ABCA1 Pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, Y.; Lei, Y.; Tzvetkov, N.T.; Liu, X.; Yeung, A.W.K.; Xu, S.; Atanasov, A.G.; Wai, A.; Yeung, K.; et al. Targeting Foam Cell Formation in Atherosclerosis: Therapeutic Potential of Natural Products. Pharmacol. Rev. 2019, 71, 596–670. [Google Scholar] [CrossRef]
- Pluddemann, A.; Neyen, C.; Gordon, S. Macrophage Scavenger Receptors and Host-Derived Ligands. Methods 2007, 43, 207–217. [Google Scholar] [CrossRef]
- Huwait, E.; Almowallad, S.; Al-Massabi, R.; Saddeek, S.; Gauthaman, K.; Prola, A. Punicalagin Targets Atherosclerosis: Gene Expression Profiling of THP-1 Macrophages Treated with Punicalagin and Molecular Docking. Curr. Issues Mol. Biol. 2022, 44, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.L.; Ramji, D.P. The Influence of Dysfunctional Signaling and Lipid Homeostasis in Mediating the Inflammatory Responses during Atherosclerosis. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1498–1510. [Google Scholar] [CrossRef]
- McLaren, J.E.; Michael, D.R.; Guschina, I.A.; Harwood, J.L.; Ramji, D.P. Eicosapentaenoic Acid and Docosahexaenoic Acid Regulate Modified LDL Uptake and Macropinocytosis in Human Macrophages. Lipids 2011, 46, 1053–1061. [Google Scholar] [CrossRef]
- Gallagher, H.; Williams, J.O.; Ferekidis, N.; Ismail, A.; Chan, Y.-H.; Michael, D.R.; Guschina, I.A.; Tyrrell, V.J.; O’Donnell, V.B.; Harwood, J.L.; et al. Dihomo-γ-Linolenic Acid Inhibits Several Key Cellular Processes Associated with Atherosclerosis. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 2538–2550. [Google Scholar] [CrossRef]
- Huwait, E.A.; Saddeek, S.Y.; Al-Massabi, R.F.; Almowallad, S.J.; Pushparaj, P.N.; Kalamegam, G. Antiatherogenic Effects of Quercetin in the THP-1 Macrophage Model In Vitro, with Insights into Its Signaling Mechanisms Using in Silico Analysis. Front. Pharmacol. 2021, 12, 698138. [Google Scholar] [CrossRef]
- Huwait, E.; Almassabi, R.; Almowallad, S.; Saddeek, S.; Karim, S.; Kalamegam, G.; Mirza, Z. Microarray Expression Profile of Myricetin-Treated THP-1 Macrophages Exhibits Alterations in Atherosclerosis-Related Regulator Molecules and LXR/RXR Pathway. Int. J. Mol. Sci. 2022, 24, 278. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Haller, V.; Ritsch, A. A Novel Candidate for Prevention and Treatment of Atherosclerosis: Urolithin b Decreases Lipid Plaque Deposition in apoE−/− Mice and Increases Early Stages of Reverse Cholesterol Transport in Ox-LDL Treated Macrophages Cells. Mol. Nutr. Food Res. 2019, 63, 1800887. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Seshadri, S.; Anwar, K.; Littlefield, M.J.; Belilos, E.; Carsons, S.E.; Reiss, A.B. Infliximab Reverses Suppression of Cholesterol Efflux Proteins by TNF-α: A Possible Mechanism for Modulation of Atherogenesis. BioMed Res. Int. 2014, 2014, 312647. [Google Scholar] [CrossRef]
- Cheng, Y.; Pan, X.; Wang, J.; Li, X.; Yang, S.; Yin, R.; Ma, A.; Zhu, X. Fucoidan Inhibits NLRP3 Inflammasome Activation by Enhancing P62/SQSTM1-Dependent Selective Autophagy to Alleviate Atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 3186306. [Google Scholar] [CrossRef] [PubMed]
- Takala, R.; Ramji, D.P.; Andrews, R.; Zhou, Y.; Burston, J.; Choy, E. Anti-Inflammatory and Immunoregulatory Effects of Pinolenic Acid in Rheumatoid Arthritis. Rheumatology 2022, 61, 992–1004. [Google Scholar] [CrossRef]
- Ozasa, H.; Ayaori, M.; Iizuka, M.; Terao, Y.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Uehara, Y.; et al. Pioglitazone Enhances Cholesterol Efflux from Macrophages by Increasing ABCA1/ABCG1 Expressions via PPARγ/LXRα Pathway: Findings from in Vitro and Ex Vivo Studies. Atherosclerosis 2011, 219, 141–150. [Google Scholar] [CrossRef]
- Fernandes-Braga, W.; Aguilar, E.C.; Navia-Pelaez, J.M.; Ávila, D.L.; Rezende, L.; de Oliveira Andrade, L.; Miranda, S.E.M.; Barros, A.L.B.d.; Capettini, L.d.S.A.; Alvarez-Leite, J.I. The Atheroprotective Role of Fucoidan Involves the Reduction of Foam Cell Formation by Altering Cholesterol Flux-Associated Factors in Macrophages. Biochem. Biophys. Res. Commun. 2023, 650, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alves, V.C.; Shiga, T.M.; do Nascimento, J.R.O. Polysaccharides from Chayote Enhance Lipid Efflux and Regulate NLRP3 Inflammasome Priming in Macrophage-like THP-1 Cells Exposed to Cholesterol Crystals. Int. J. Biol. Macromol. 2019, 127, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Cheng, Z.; Cai, X.; Wang, H. The Effects of Phellinus Linteus Polysaccharide Extracts on Cholesterol Efflux in Oxidized Low-Density Lipoprotein–Loaded THP-1 Macrophages. J. Investig. Med. 2015, 63, 752–757. [Google Scholar] [CrossRef]
- Hansen, J.E.; Lund, O.; Engelbrecht, J.; Bohr, H.; Nielsen, J.O.; Hansen, J.E.S.; Brunak, S. Prediction of O-Glycosylation of Mammalian Proteins: Specificity Patterns of UDP-GalNAc:Polypeptide N-Acetylgalactosaminyltransferase. Biochem. J. 1995, 308, 801–813. [Google Scholar] [CrossRef]
- Steen, P.V.d.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Concepts and Principles of O-Linked Glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. [Google Scholar] [CrossRef]
- Almassabi, R.F.; Huwait, E.A.; Almowallad, S.J.; Saddeek, S.Y.; Gauthaman, K. In Vitro: The Modulating Effect of Myricetin on the Atherosclerosis Related Processes in THP1 Macrophages. J. Pharm. Res. Int. 2021, 32, 62–73. [Google Scholar] [CrossRef]
- Tan, C.; Zhou, L.; Wen, W.; Xiao, N. Curcumin Promotes Cholesterol Efflux by Regulating ABCA1 Expression through miR-125a-5p/SIRT6 Axis in THP-1 Macrophage to Prevent Atherosclerosis. J. Toxicol. Sci. 2021, 46, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Q.; Pang, L.; Huang, G.; Huang, J.; Shi, M.; Sun, X.; Wang, Y. Arctigenin Promotes Cholesterol Efflux from THP-1 Macrophages through PPAR-γ/LXR-α Signaling Pathway. Biochem. Biophys. Res. Commun. 2013, 441, 321–326. [Google Scholar] [CrossRef] [PubMed]



| Target Gene | Primer Sequence (5′-3′) | Reference |
|---|---|---|
| GAPDH | F: CTTTTGCGTCGCCAGCCGAG | [26] |
| R: GCCCAATACGACCAAATCCGTTGACT | ||
| CD36 | F: ATT GCCCTTTACCTCGT | [27] |
| R: GCC TTG GAT GGA AGA ACA AA | ||
| SR-AI | F: ATTGCCCTTTACCTCGT | |
| R: TCATTTCCTTTT CCC GTGAG | ||
| LXR-α | F: AAGCCCTGCATGCCTACGT | [28] |
| R: TGCAGACGCAGTGCAAACA | ||
| ABCA1 | F: AGTGGAAACAGTTAATGACCAG | [29] |
| R: GCAGCTGACATGTTTGTCTTC |
| A | (Protein–Ligand Docking) | |||||
|---|---|---|---|---|---|---|
| Receptor (PDB ID) | Ligand | ΔG (Kcal/mol) | Ki | Type of Interactions | ΔSASA (Å2) | |
| H-Bonds | Hydrophobic | |||||
| SR-AI (7DPX) | Fucoidan | −7.84 | 1.80 µM | Arg31 (2.1 Å), Glu33 (2.4 Å), Arg35 (2.2 Å), Cys72 (3.0 Å) | - | 36.02 |
| CD36 (5LGD) | Fucoidan | −11.37 | 4.66 nM | Thr314 (1.9 Å), Glu315 (1.8 Å), Ser324 (2.1 Å) | Phe300, Ala301, Asn309, Phe312, Cys313, Ser319, Cys322, Tyr325, Gly326, Val327, Asp329, Tyr340 | 138.6 |
| Palmitic acid | −8.30 | 821.7 nM | Arg386 (3.1 Å) | Ala299, Arg273, Phe300, Ala301, Asn309, Phe312, Ser324, Tyr325, Gly326, Val327, Leu328, Ile341, Leu343, Leu387 | 207.8 | |
| ABCA1 (5XJY) | Fucoidan | −9.75 | 71.68 nM | Asn1541 (2.2 Å), Ala1544 (2.1 Å), Leu1545 (2.1 Å), Thr1586 (2.0 Å), Lys1587(2.2 Å) | Arg110, Ser1540, Thr1542, Pro1547, Leu1584, Asp1585, Lys1587 | 142 |
| B | (Protein–protein docking) | |||||
| Webserver | Parameters | ABCA1–ApoA1 complex | ||||
| ClusPro server | Lowest energy | Cluster | 28 | |||
| embers | 172 | |||||
| Balanced | −4001 | |||||
| HDOCK server | Summary of Rank 1 | Docking score | −427.11 | |||
| Confidence score | 0.9961 | |||||
| Ligand RMSD (Å) | 0.93 | |||||
| PDBsum | Interface statistics | No. of salt bridges | 3 | |||
| No. of hydrogen bonds | 9 | |||||
| No. of non-bonded contacts | 322 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirza, Z.; Al-Saedi, D.A.; Saddeek, S.; Almowallad, S.; AlMassabi, R.F.; Huwait, E. Atheroprotective Effect of Fucoidan in THP-1 Macrophages by Potential Upregulation of ABCA1. Biomedicines 2023, 11, 2929. https://doi.org/10.3390/biomedicines11112929
Mirza Z, Al-Saedi DA, Saddeek S, Almowallad S, AlMassabi RF, Huwait E. Atheroprotective Effect of Fucoidan in THP-1 Macrophages by Potential Upregulation of ABCA1. Biomedicines. 2023; 11(11):2929. https://doi.org/10.3390/biomedicines11112929
Chicago/Turabian StyleMirza, Zeenat, Dalal A. Al-Saedi, Salma Saddeek, Sanaa Almowallad, Rehab F. AlMassabi, and Etimad Huwait. 2023. "Atheroprotective Effect of Fucoidan in THP-1 Macrophages by Potential Upregulation of ABCA1" Biomedicines 11, no. 11: 2929. https://doi.org/10.3390/biomedicines11112929
APA StyleMirza, Z., Al-Saedi, D. A., Saddeek, S., Almowallad, S., AlMassabi, R. F., & Huwait, E. (2023). Atheroprotective Effect of Fucoidan in THP-1 Macrophages by Potential Upregulation of ABCA1. Biomedicines, 11(11), 2929. https://doi.org/10.3390/biomedicines11112929