The Negative Impact of Insulin Resistance/Hyperinsulinemia on Chronic Heart Failure and the Potential Benefits of Its Screening and Treatment
Abstract
1. Introduction
- I.
- Heart failure with reduced ejection fraction (HFrEF) is defined by an LVEF ≤ 40%.
- II.
- Heart failure with mildly reduced EF (HFmrEF) is defined by an EF between 41% and 49%.
- III.
- Heart failure with preserved EF (HFpEF) is defined by an LVEF ≥ 50% in the presence of symptoms and signs of HF associated with structural and/or functional cardiac abnormalities and/or increased natriuretic peptides [1].
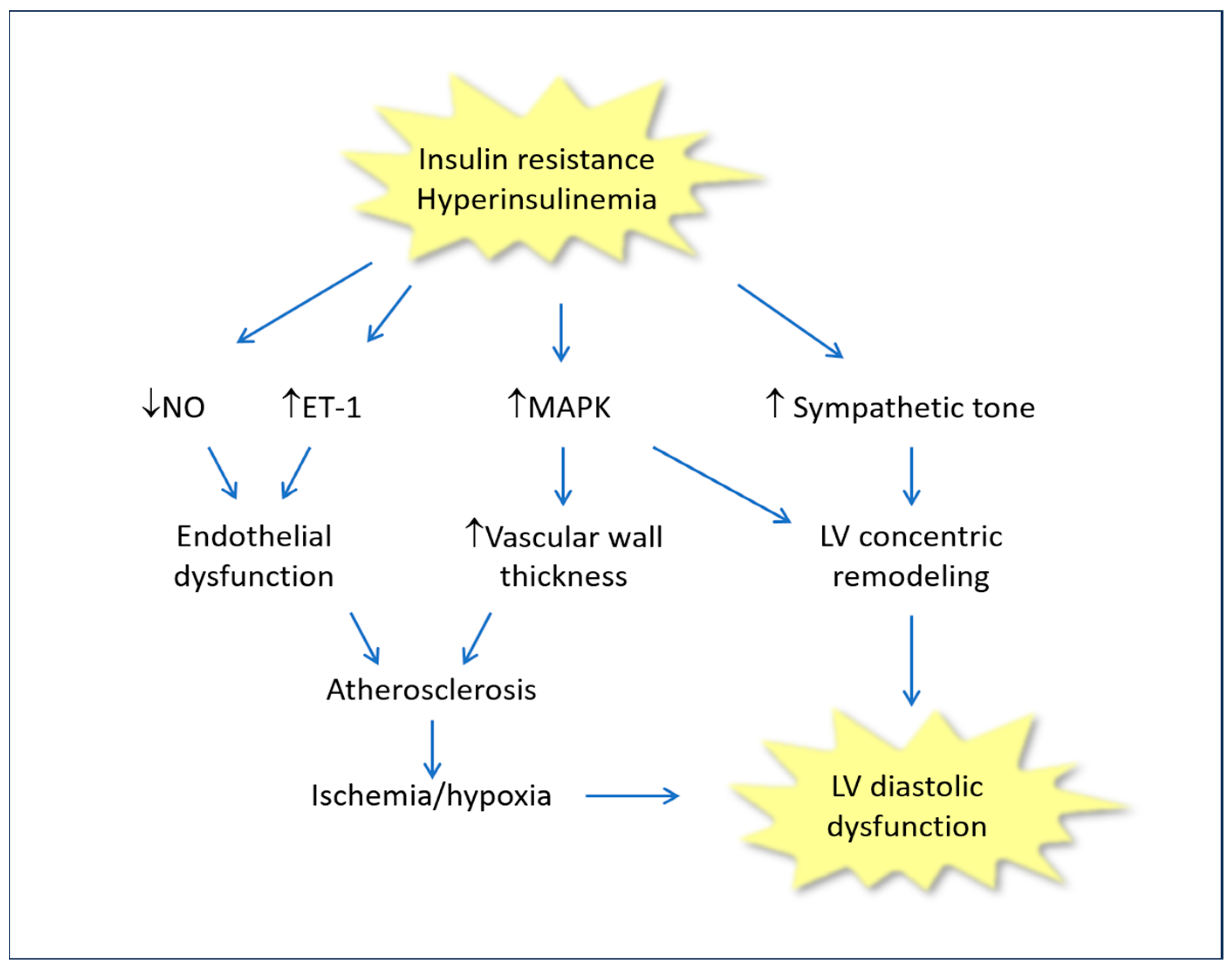
2. Insulin Resistance/Hyperinsulinemia and Chronic Heart Failure
3. Treatment of Insulin Resistance/Hyperinsulinemia: Possible Beneficial Effects in Patients with Chronic HF
3.1. Role of Diet
3.2. Sodium-Glucose Cotransporter-2 Inhibitors
3.3. Metformin
3.4. Berberine
3.5. Glucagon-like Peptide-1 Receptor Agonists
3.6. Interactions of Some Drugs, Used in HF, with IR/Hyperins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Meigs, J.B.; Bonadonna, R.C.; Muggeo, M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: The Bruneck study. Diabetes Care 2007, 30, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Luo, H.; Fan, L.; Liu, X.; Gao, C. Heart failure with preserved ejection fraction: An update on pathophysiology, diagnosis, treatment, and prognosis. Braz. J. Med. Biol. Res. 2020, 53, e9646. [Google Scholar] [CrossRef]
- Naing, P.; Forrester, D.; Kangaharan, N.; Muthumala, A.; Myint, S.M.; Playford, D. Heart failure with preserved ejection fraction: A growing global epidemic. Aust. J. Gen. Pract. 2019, 48, 465–471. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Kronmal, R.; Eng, J.; Folsom, A.; Burke, G.; Carr, J.J.; Shea, S.; Lima, J.A.C.; Bluemke, D.A.; O’brien, C.; et al. Left Ventricular Mass at MRI and Long-term Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2019, 293, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Rame, J.; Marino, E.K.; Gottdiener, J.S.; Kitzman, D.W.; Gardin, J.M.; Manolio, T.A.; Dries, D.L.; Siscovick, D.S. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2004, 43, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.; DeVore, A.D.; Wu, J.; Matsouaka, R.A.; Fonarow, G.C.; Heidenreich, P.A.; Yancy, C.W.; Green, J.B.; Altman, N.; Hernandez, A.F. Heart Failure with Preserved Ejection Fraction and Diabetes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Riehle, C.; Abel, E.D. Insulin Signaling and Heart Failure. Circ. Res. 2016, 118, 1151–1169. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Esmailzadehha, N.; Oveisi, S.; Ghorbani, A.; Ghanei, L. The threshold value of homeostasis model assessment for insulin resistance in Qazvin Metabolic Diseases Study (QMDS): Assessment of metabolic syndrome. J. Res. Health Sci. 2015, 15, 94–100. [Google Scholar] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison with the Euglycemic-Hyperinsulinemic Clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D. Insulin signaling in the heart. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E130–E145. [Google Scholar] [CrossRef]
- Cai, W.; Sakaguchi, M.; Kleinridders, A.; Pino, G.G.-D.; Dreyfuss, J.M.; O’neill, B.T.; Ramirez, A.K.; Pan, H.; Winnay, J.N.; Boucher, J.; et al. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat. Commun. 2017, 8, 14892. [Google Scholar] [CrossRef]
- Chopra, I.; Li, H.F.; Wang, H.; Webster, K.A. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia 2012, 55, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Mercurio, V.; Carlomagno, G.; Fazio, V.; Fazio, S. Insulin resistance: Is it time for primary prevention? World J. Cardiol. 2012, 4, 1–7. [Google Scholar] [CrossRef]
- Bachmann, K.N.; Deger, S.M.; Alsouqi, A.; Huang, S.; Xu, M.; Ferguson, J.F.; Su, Y.R.; Niswender, K.D.; Ikizler, T.A.; Wang, T.J. Acute effects of insulin on circulating natriuretic peptide levels in humans. PLoS ONE 2018, 13, e0196869. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.W. Insulin as a mitogenic factor: Role in the pathogenesis of cardiovascular disease. Am. J. Med. 1991, 90, S62–S65. [Google Scholar] [CrossRef]
- Buse, J.B.; Ginsberg, H.N.; Bakris, G.L.; Clark, N.G.; Costa, F.; Fonseca, V.; Grundy, S.; Nesto, R.W.; Porte, D.; Eckel, R.; et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007, 115, 114–126. [Google Scholar] [CrossRef]
- Alzadjali, M.A.; Godfrey, V.; Khan, F.; Choy, A.; Doney, A.S.; Wong, A.K.; Petrie, J.R.; Struthers, A.D.; Lang, C.C. Insulin Resistance Is Highly Prevalent and Is Associated with Reduced Exercise Tolerance in Nondiabetic Patients with Heart Failure. J. Am. Coll. Cardiol. 2009, 53, 747–753. [Google Scholar] [CrossRef]
- Mohan, M.; Dihoum, A.; Mordi, I.R.; Choy, A.-M.; Rena, G.; Lang, C.C. Left Ventricular Hypertrophy in Diabetic Cardiomyopathy: A Target for Intervention. Front. Cardiovasc. Med. 2021, 8, 746382. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Russo, C.; Bella, J.N. Treatment of isolated left ventricular diastolic dysfunction in hypertension: Reaching blood pressure target matters. Hypertension 2010, 55, 224–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tai, C.; Gan, T.; Zou, L.; Sun, Y.; Zhang, Y.; Chen, W.; Li, J.; Zhang, J.; Xu, Y.; Lu, H.; et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on cardiovascular events in patients with heart failure: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2017, 17, 257. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Levelt, E.; Mahmod, M.; Piechnik, S.K.; Ariga, R.; Francis, J.M.; Rodgers, C.T.; Clarke, W.T.; Sabharwal, N.; Schneider, J.E.; Karamitsos, T.D.; et al. Relationship Between Left Ventricular Structural and Metabolic Remodeling in Type 2 Diabetes. Diabetes 2016, 65, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, D.; Winhofer, Y.; Promintzer-Schifferl, M.; Wohlschläger-Krenn, E.; Anderwald, C.H.; Wolf, P.; Scherer, T.; Reiter, G.; Trattnig, S.; Luger, A.; et al. Effects of Insulin Therapy on Myocardial Lipid Content and Cardiac Geometry in Patients with Type-2 Diabetes Mellitus. PLoS ONE 2012, 7, e50077. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.V.; Abbasi, S.A.; Heydari, B.; Rickers, C.; Jacobs, D.R., Jr.; Wang, L.; Kwong, R.W.; Bluemke, D.A.; Lima, J.A.C.; Jerosch-Herold, M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2013, 61, 1698–1706. [Google Scholar] [CrossRef]
- Packer, M. Differential Pathophysiological Mechanisms in Heart Failure with a Reduced or Preserved Ejection Fraction in Diabetes. JACC Heart Fail. 2021, 9, 535–549. [Google Scholar] [CrossRef]
- Hasegawa, H.; Komuro, I. CHARM study-new strategy for the treatment of heart failure. Nihon Rinsho. Jpn. J. Clin. Med. 2004, 62, 995–1002. [Google Scholar]
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997, 336, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Andresen, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- Lindman, B.R.; Davila-Roman, V.G.; Mann, D.L.; McNulty, S.; Semigran, M.J.; Lewis, G.D.; de la Fuentes, L.; Vader, J.; Hernanderz, A.H.; Redfield, M.M.; et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: A RELAX trial ancillary study. J. Am. Coll. Cardiol. 2014, 64, 541–549. [Google Scholar] [CrossRef]
- Bellavite, P.; Fazio, S.; Affuso, F. A Descriptive Review of the Action Mechanisms of Berberine, Quercetin and Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Prevention. Molecules 2023, 28, 4491. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.; Braunwald, E.; Anstrom, K.J.; Hernanderz, A.H.; O’Connor, C.M.; et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2013, 309, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.D.; Pan, W.Q.; Feng, S.; Quan, J.W.; Chen, J.W.; Shu, X.Y.; Aihemaiti, M.; Ding, F.H.; Shen, W.F.; Lu, L.; et al. Insulin Resistance Is Associated with Heart Failure with Recovered Ejection Fraction in Patients Without Diabetes. J. Am. Heart Assoc. 2022, 11, e026184. [Google Scholar] [CrossRef] [PubMed]
- Jurczewska, J.; Ostrowska, J.; Che’chowska, M.; Panczyk, M.; Rudnicka, E.; Kucharski, M.; Smolarczyk, R.; Szostak-Węgierek, D. Physical activity, rather than diet, is linked to lower insulin resistance in PCOS women—A case control study. Nutrients 2023, 15, 2011. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Pherwani, S.; Ketema, E.B. Ketone metabolism in the failing heart. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158813. [Google Scholar] [CrossRef]
- Hshemlu, L.; Esmaeili, R.; Bahramnezhad, F.; Rohani, C. A systematic review on clinical guidelines of nome health care in heart failure patients. BMC Nurs. 2023, 22, 127. [Google Scholar]
- Butler, J.; Usman, M.S.; Khan, M.S.; Greene, S.J.; Friede, T.; Vaduganathan, M.; Filippatos, G.; Coats, A.J.S.; Anker, S.D. Efficacy and safety of SGLT2 inhibitors in heart failure: Systematic review and meta-analysis. ESC Heart Fail. 2020, 7, 3298–3309. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; De Mets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Shah, S.J.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Pabel, S.; Hamdani, N.; Luedde, M.; Sossalla, S. SGLT2 Inhibitors and Their Mode of Action in Heart Failure-Has the Mystery Been Unravelled? Curr. Heart Fail Rep. 2021, 18, 315–328. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Ogawa, W. SGLT2 inhibitors for genetic and acquired insulin resistance: Considerations for clinical use. J. Diabetes Investig. 2020, 11, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Verma, S.; Santos-Gallego, C.G.; Bhatt, A.S.; Vaduganathan, M.; Khan, M.S.; Lopes, R.D.; Al’aref, S.J.; McGuire, D.K.; Fudim, M. Sodium-Glucose Cotransporter 2 Inhibitors and Cardiac Remodeling. J. Cardiovasc. Transl. Res. 2022, 15, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.K.; Mistry, N.; Puar, P.; Verma, R.; Anker, S.; Mazer, C.D.; Verma, S. SGLT2 inhibitors and cardiac remodelling: A systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021, 8, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-T.; Wei, Y.-L.; Rui, Y.-F.; Fan, L. Echocardiographic evaluation of the effect of dapagliflozin on epicardial adipose tissue and left ventricular systolic function in type 2 diabetes mellitus. J. Diabetes Complicat. 2023, 37, 108509. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Zhong, W.; Shu, J.; Abu Much, A.; Lotan, D.; Grupper, A.; Younis, A.; Dai, H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur. J. Prev. Cardiol. 2021, 28, 1682–1690. [Google Scholar] [CrossRef]
- Halabi, A.; Sen, J.; Huynh, Q.; Marwick, T.H. Metformin treatment in heart failure with preserved ejection fraction: A systematic review and meta-regression analysis. Cardiovasc. Diabetol. 2020, 19, 124. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of Metformin in Heart Failure: From Pathophysiological Rationale to Clinical Evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- García, N.; Zazueta, C.; Aguilera-Aguirre, L. Oxidative Stress and Inflammation in Cardiovascular Disease. Oxidative Med. Cell. Longev. 2017, 2017, 5853238. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.M.; Sabry, N.; Farid, S. Effect of metformin on left ventricular mass and functional parameters in non-diabetic patients: A meta-analysis of randomized clinical trials. BMC Cardiovasc. Disord. 2022, 22, 405. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.K.; Symon, R.; AlZadjali, M.A.; Ang, D.S.; Ogston, S.; Choy, A.; Petrie, J.R.; Struthers, A.D.; Lang, C.C. The effect of metformin on insulin resistance and exercise parameters in patients with heart failure. Eur. J. Heart Fail. 2012, 14, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, X.; Ma, W.; Song, H.; Gong, Z.; Wang, Q.; Che, L.; Xu, W.; Jiang, J.; Xu, J.; et al. VE/VCO2 slope and its prognostic value in patients with chronic heart failure. Exp. Ther. Med. 2015, 9, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic Potential of Quercetin to Alleviate Endothelial Dysfunction in Age-Related Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 658400. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Milajerdi, A.; Dadgostar, E.; Kolahdooz, F.; Chamani, M.; Amirani, E.; Mirzaei, H.; Asemi, Z. The Effects of Quercetin Supplementation on Blood Pressures and Endothelial Function Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2019, 25, 1372–1384. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytotherapy Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, R.; Zhou, N.; Wang, X.; Liu, Q.; Bai, Y.; Bai, Y.; Liu, Z.; Yang, H.; Zou, J.; et al. Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model. Biomed. Rep. 2013, 1, 71–76. [Google Scholar] [CrossRef]
- Kang, J.S.; Jeon, Y.J.; Kim, H.M.; Han, S.H.; Yang, K.-H. Inhibition of Inducible Nitric-Oxide Synthase Expression by Silymarin in Lipopolysaccharide-Stimulated Macrophages. J. Pharmacol. Exp. Ther. 2002, 302, 138–144. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Wang, Y.; Zhu, T. Silymarin improved diet-induced liver damage and insulin resistance by decreasing inflammation in mice. Pharm. Biol. 2016, 54, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Huang, B.; Jing, Y.; Shen, S.; Feng, W.; Wang, W.; Meng, R.; Zhu, D. Silymarin ameliorates the disordered glucose metabolism of mice with diet-induced obesity by activating the hepatic SIRT1 pathway. Cell. Signal. 2021, 84, 110023. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yang, Y.R.; Mo, Z.J.; Ding, Y.; Jiang, W.J. Silibinin improves palmitate-induced insulin resistance in C2C12 myotubes by attenuating IRS-1/PI3K/Akt pathway inhibition. Braz. J. Med. Biol. Res. 2015, 48, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bouderba, S.; Sanchez-Martin, C.; Villanueva, G.R.; Detaille, D.; Koceïr, E.A. Beneficial effects of silibinin against the progression of metabolic syndrome, increased oxidative stress, and liver steatosis in Psammomys obesus, a relevant animal model of human obesity and diabetes. J. Diabetes 2014, 6, 184–192. [Google Scholar] [CrossRef]
- Zeng, X.-H.; Li, Y.-Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2003, 92, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zeng, X. Relationship between the clinical effects of berberine on severe congestive heart failure and its concentration in plasma studied by HPLC. Biomed. Chromatogr. 1999, 13, 442–444. [Google Scholar] [CrossRef]
- Carlomagno, G.; Affuso, F.; Napoli, R.; Mercurio, V.; Fazio, V.; Micillo, F.; Pirozzi, C.; Ruvolo, A.; Saccá, L.; Fazio, S. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J. Cardiol. 2012, 4, 77–83. [Google Scholar] [CrossRef][Green Version]
- Mercurio, V.; Pucci, G.; Bosso, G.; Fazio, V.; Battista, F.; Iannuzzi, A.; Brambilla, N.; Vitalini, C.; D’Amato, M.; Giacovelli, G.; et al. A nutraceutical combination reduces left ventricular mass in subjects with metabolic syndrome and left ventricular hypertrophy: A multicenter, randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2020, 39, 1379–1384. [Google Scholar] [CrossRef]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-lime peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Saraiva, F.; Sharma, A.; Vasques-Nóvoa, F.; Angélico-Gonçalves, A.; Leite, A.R.; Borges-Canha, M.; Carvalho, D.; Packer, M.; Zannad, F.; et al. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: A meta-analysis of randomized placebo-controlled outcome trials. Diabetes Obes. Metab. 2023, 25, 1495–1502. [Google Scholar] [CrossRef]
- Merza, N.; Akram, M.; Mengal, A.; Rashid, A.M.; Mahboob, A.; Faryad, M.; Fatima, Z.; Ahmed, M.; Ansari, S.A. The Safety and Efficacy of GLP-1 Receptor Agonists in Heart Failure Patients: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101602. [Google Scholar] [CrossRef] [PubMed]
- Wicklmayr, M.; Rett, K.; Dietze, G.; Mehnert, H. Effects of beta-blocking agents on insulin secretion and glucose disposal. Horm. Metab. Res. Suppl. 1990, 22, 29–33. [Google Scholar] [PubMed]
- Jacob, S. Beta-blocking agents in patients with insulin resistance: Effects of vasodilating beta-blockers. Blood Press 1999, 8, 261–268. [Google Scholar] [CrossRef]
- Buscemi, S.; Nicolucci, A.; Lucisano, G.; Galvano, F.; Grosso, G.; Massenti, F.M.; Amodio, E.; Bonura, A.; Sprini, D.; Rini, G.B. Impact of chronic diuretic treatment on glucose homeostasis. Diabetol. Metab. Syndr. 2013, 5, 80. [Google Scholar] [CrossRef]
- Ramsay, L.E.; Yeo, W.W.; Jackson, P.R. Diabetes, impaired glucose tolerance and insulin resistance with diuretics. Eur. Heart J. 1992, 13, 68–71. [Google Scholar] [CrossRef]
- Dronavalli, S.; Bakris, G.L.; Kaplan, N.M.; Raheja, P.; Price, A.; Wang, Z.; Arbique, D.; Adams-Huet, B.; Auchus, R.J.; Vongpatanasin, W.; et al. Mechanistic Insights into Diuretic-Induced Insulin Resistance. Hypertension 2008, 52, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Lamendola, C.; Harris, C.S.; Harris, V.; Tsai, M.-S.; Tripathi, P.; Abbas, F.; Reaven, G.M.; Reaven, P.D.; Snyder, M.P.; et al. Statins Are Associated with Increased Insulin Resistance and Secretion. Arter. Thromb. Vasc. Biol. 2021, 41, 2786–2797. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, S.; Mercurio, V.; Affuso, F.; Bellavite, P. The Negative Impact of Insulin Resistance/Hyperinsulinemia on Chronic Heart Failure and the Potential Benefits of Its Screening and Treatment. Biomedicines 2023, 11, 2928. https://doi.org/10.3390/biomedicines11112928
Fazio S, Mercurio V, Affuso F, Bellavite P. The Negative Impact of Insulin Resistance/Hyperinsulinemia on Chronic Heart Failure and the Potential Benefits of Its Screening and Treatment. Biomedicines. 2023; 11(11):2928. https://doi.org/10.3390/biomedicines11112928
Chicago/Turabian StyleFazio, Serafino, Valentina Mercurio, Flora Affuso, and Paolo Bellavite. 2023. "The Negative Impact of Insulin Resistance/Hyperinsulinemia on Chronic Heart Failure and the Potential Benefits of Its Screening and Treatment" Biomedicines 11, no. 11: 2928. https://doi.org/10.3390/biomedicines11112928
APA StyleFazio, S., Mercurio, V., Affuso, F., & Bellavite, P. (2023). The Negative Impact of Insulin Resistance/Hyperinsulinemia on Chronic Heart Failure and the Potential Benefits of Its Screening and Treatment. Biomedicines, 11(11), 2928. https://doi.org/10.3390/biomedicines11112928







