sFlt-1 Levels as a Predicting Tool in Placental Dysfunction Complications in Multiple Pregnancies
Abstract
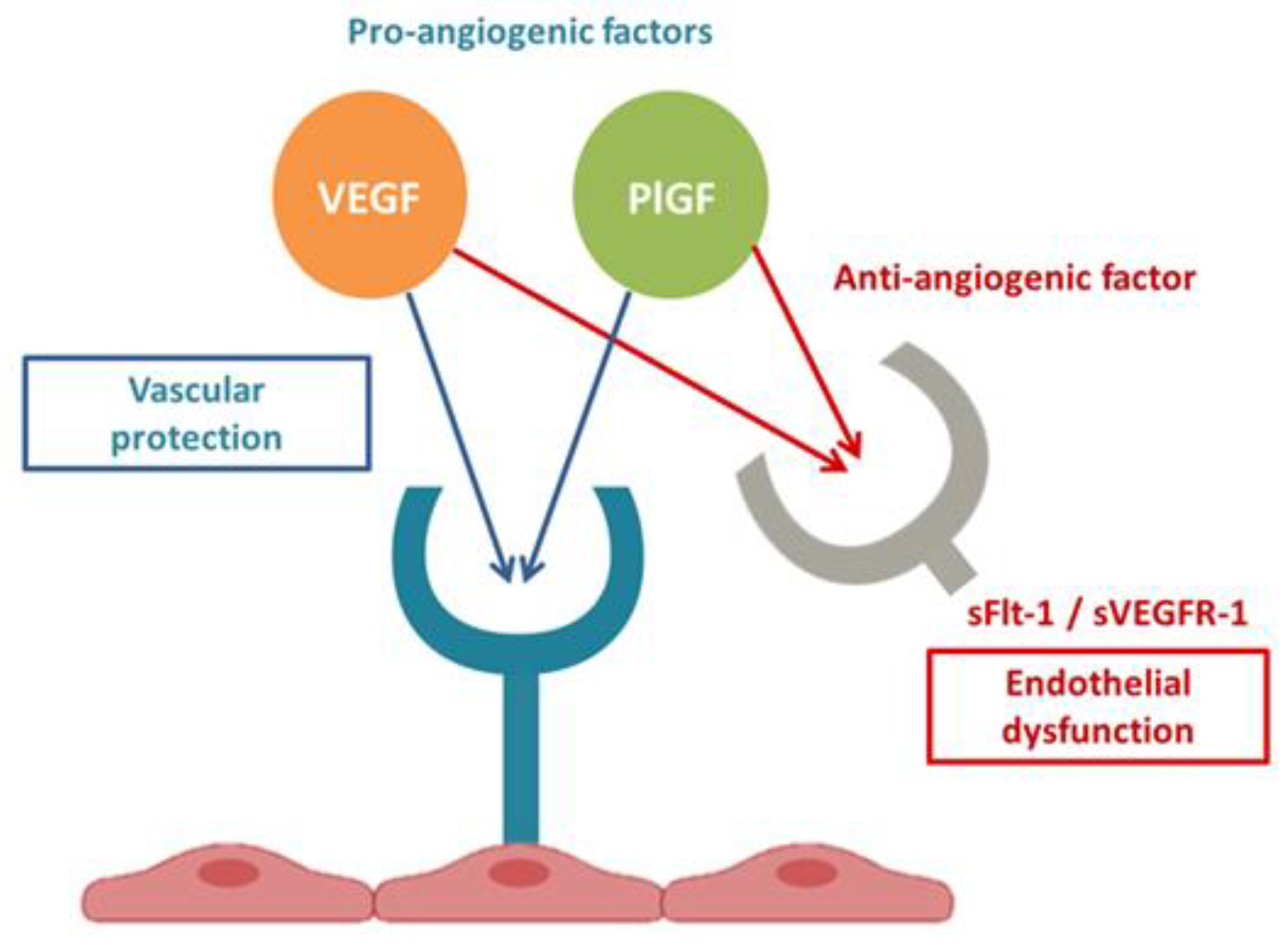
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gestations, M. Twin, Triplet, and Higher-Order Multifetal Pregnancies: ACOG Practice Bulletin, Number 231. Obstet. Gynecol. 2021, 137, e145–e162. [Google Scholar] [CrossRef]
- Santana, D.S.; Silveira, C.; Costa, M.L.; Souza, R.T.; Surita, F.G.; Souza, J.P.; Mazhar, S.B.; Jayaratne, K.; Qureshi, Z.; Sousa, M.H.; et al. WHO Multi-Country Survey on Maternal and Newborn Health Research Network. Perinatal outcomes in twin pregnancies complicated by maternal morbidity: Evidence from the WHO Multicountry Survey on Maternal and Newborn Health. BMC Pregnancy Childbirth 2018, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Kalikkot Thekkeveedu, R.; Dankhara, N.; Desai, J.; Klar, A.L.; Patel, J. Outcomes of multiple gestation births compared to singleton: Analysis of multicenter KID database. Matern. Health Neonatol. Perinatol. 2021, 7, 15. [Google Scholar] [CrossRef]
- Narang, K.; Szymanski, L.M. Multiple Gestations and Hypertensive Disorders of Pregnancy: What Do We Know? Curr. Hypertens. Rep. 2020, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Laine, K.; Murzakanova, G.; Sole, K.B.; Pay, A.D.; Heradstveit, S.; Räisänen, S. Prevalence and risk of pre-eclampsia and gestational hypertension in twin pregnancies: A population-based register study. BMJ Open 2019, 9, e029908. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.S.; Rebarber, A.; Klauser, C.K.; Roman, A.S.; Saltzman, D.H. Intrauterine growth restriction in twin pregnancies: Incidence and associated risk factors. Am. J. Perinatol. 2011, 28, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.; Khalil, A. Fetal growth restriction in twins. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 79–88. [Google Scholar] [CrossRef]
- Odibo, A.O.; McDonald, R.E.; Stamilio, D.M.; Ural, S.H.; Macones, G.A. Perinatal outcomes in growth-restricted twins compared with age-matched growth-restricted singletons. Am. J. Perinatol. 2005, 22, 269–273. [Google Scholar] [CrossRef]
- Filipecka-Tyczka, D.; Jakiel, G.; Kajdy, A.; Rabijewski, M. Is growth restriction in twin pregnancies a double challenge? A narrative review. J. Mother. Child. 2021, 24, 24–30. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Bardin, N.; Murthi, P.; Alfaidy, N. Normal and pathological placental angiogenesis. Biomed. Res. Int. 2015, 2015, 354359. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Venkatesha, S.; Thadhani, R.; Karumanchi, S.A. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr. Res. 2005, 57 Pt 2, 1R–7R. [Google Scholar] [CrossRef]
- Karumanchi, S.A.; Epstein, F.H. Placental ischemia and soluble FMS-like tyrosine kinase 1: Cause or consequence of preeclampsia? Kidney Int. 2007, 71, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.R.; LaMarca, B.B.; Parrish, M.; Cockrell, K.; Granger, J.P. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: Role of tumor necrosis factor-α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R130–R135. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Schuetz, P.; Yano, K.; Sorasaki, M.; Parikh, S.M.; Jones, A.E.; Trzeciak, S.; Ngo, L.; Aird, W.C. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit. Care 2010, 14, R182. [Google Scholar] [CrossRef]
- Page, A.V.; Liles, W.C. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 2013, 4, 507–516. [Google Scholar] [CrossRef]
- Draker, N.; Torry, D.S.; Torry, R.J. Placenta growth factor and sFlt-1 as biomarkers in ischemic heart disease and heart failure: A review. Biomark. Med. 2019, 13, 785–799. [Google Scholar] [CrossRef]
- Dumnicka, P.; Sporek, M.; Mazur-Laskowska, M.; Ceranowicz, P.; Kuźniewski, M.; Drożdż, R.; Ambroży, T.; Olszanecki, R.; Kuśnierz-Cabala, B. Serum Soluble FMS-Like Tyrosine Kinase 1 (sFlt-1) Predicts the Severity of Acute Pancreatitis. Int. J. Mol. Sci. 2016, 17, 2038. [Google Scholar] [CrossRef]
- Giardini, V.; Carrer, A.; Casati, M.; Contro, E.; Vergani, P.; Gambacorti-Passerini, C. Increased sFLT-1/PlGF ratio in COVID-19: A novel link to angiotensin II-mediated endothelial dysfunction. Am. J. Hematol. 2020, 95, E188–E191. [Google Scholar] [CrossRef] [PubMed]
- Negro, A.; Fama, A.; Penna, D.; Belloni, L.; Zerbini, A.; Giuri, P.G. SFLT-1 levels in COVID-19 patients: Association with outcome and thrombosis. Am. J. Hematol. 2021, 96, E41–E43. [Google Scholar] [CrossRef]
- McElwain, C.J.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows into Future Cardiometabolic Health? Front. Endocrinol. 2020, 11, 655. [Google Scholar]
- Giardini, V.; Gambacorti-Passerini, C.; Casati, M.; Carrer, A.; Vergani, P. Can Similarities between the Pathogenesis of preeclampsia and COVID-19 increase the understanding of COVID-19? Int. J. Transl. Med. 2022, 2, 186–197. [Google Scholar] [CrossRef]
- Stepan, H.; Hund, M.; Andraczek, T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension 2020, 75, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wei, Q.; Liang, Q.; Song, S.; Li, J. Diagnostic capacity of sFlt-1/PlGF ratio in fetal growth restriction: A systematic review and meta-analysis. Placenta 2022, 127, 37–42. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef]
- Sapantzoglou, I.; Rouvali, A.; Koutras, A.; Chatziioannou, M.I.; Prokopakis, I.; Fasoulakis, Z.; Zachariou, E.; Douligeris, A.; Mortaki, A.; Perros, P.; et al. sFLT1, PlGF, the sFLT1/PlGF Ratio and Their Association with Pre-Eclampsia in Twin Pregnancies-A Review of the Literature. Medicina 2023, 59, 1232. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble FMS-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Stepan, H.; Galindo, A.; Hund, M.; Schlembach, D.; Sillman, J.; Surbek, D.; Vatish, M. Clinical utility of sFlt-1 and PlGF in screening, prediction, diagnosis and monitoring of pre-eclampsia and fetal growth restriction. Ultrasound Obstet. Gynecol. 2023, 61, 168–180. [Google Scholar] [CrossRef]
- Redman, C.W.; Staff, A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am. J. Obstet. Gynecol. 2015, 213, S9.e1–S9.e11. [Google Scholar] [CrossRef]
- De La Calle, M.; Delgado, J.L.; Verlohren, S.; Escudero, A.I.; Bartha, J.L.; Campillos, J.M.; Aguarón De La Cruz, A.; Chantraine, F.; García Hernández, J.Á.; Herraiz, I.; et al. Gestational Age-Specific Reference Ranges for the sFlt-1/PlGF Immunoassay Ratio in Twin Pregnancies. Fetal Diagn. Ther. 2021, 48, 288–296. [Google Scholar] [CrossRef]
- Binder, J.; Palmrich, P.; Pateisky, P.; Kalafat, E.; Kuessel, L.; Zeisler, H.; Munkhbaatar, M.; Windsperger, K.; Thilaganathan, B.; Khalil, A. The Prognostic Value of Angiogenic Markers in Twin Pregnancies to Predict Delivery Due to Maternal Complications of Preeclampsia. Hypertension 2020, 76, 176–183. [Google Scholar] [CrossRef]
- Saleh, L.; Tahitu, S.I.M.; Danser, A.H.J.; van den Meiracker, A.H.; Visser, W. The predictive value of the sFlt-1/PlGF ratio on short-term absence of preeclampsia and maternal and fetal or neonatal complications in twin pregnancies. Pregnancy Hypertens. 2018, 14, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Dröge, L.; Herraìz, I.; Zeisler, H.; Schlembach, D.; Stepan, H.; Küssel, L.; Henrich, W.; Galindo, A.; Verlohren, S. Maternal serum sFlt-1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obstet. Gynecol. 2015, 45, 286–293. [Google Scholar] [CrossRef]
- Shinohara, S.; Sunami, R.; Kasai, M.; Yasuda, G.; Uchida, Y. Predictive value of the sFlt-1/PlGF ratio for preeclampsia in twin pregnancies: A retrospective study. Hypertens. Pregnancy 2021, 40, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Varea, A.; Martínez-Sáez, C.; Domenech, J.; Desco-Blay, J.; Monfort-Pitarch, S.; Hueso, M.; Diago-Almela, V. sFlt-1/PlGF Ratio at 24 Weeks Gestation in Twin Pregnancies as a Predictor of Preeclampsia or Fetal Growth Restriction. Fetal Diagn. Ther. 2022, 49, 206–214. [Google Scholar] [CrossRef]
- Herraiz, I.; Dröge, L.A.; Gómez-Montes, E.; Henrich, W.; Galindo, A.; Verlohren, S. Characterization of the soluble FMS-like tyrosine kinase-1 to placental growth factor ratio in pregnancies complicated by fetal growth restriction. Obstet. Gynecol. 2014, 124 Pt 1, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Hacker, M.R.; Modest, A.M.; Salahuddin, S.; Lim, K.H.; Verlohren, S.; Perschel, F.H.; Karumanchi, S.A. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 2012, 60, 451–458. [Google Scholar] [CrossRef]
- Karge, A.; Seiler, A.; Flechsenhar, S.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Axt-Fliedner, R.; Enzensberger, C.; Abel, K.; Kuschel, B.; et al. Prediction of adverse perinatal outcome and the mean time until delivery in twin pregnancies with suspected pre-eclampsia using sFlt-1/PIGF ratio. Pregnancy Hypertens. 2021, 24, 37–43. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts up-regulate soluble FMS-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef]
- Makris, A.; Thornton, C.; Thompson, J.; Thomson, S.; Martin, R.; Ogle, R.; Waugh, R.; McKenzie, P.; Kirwan, P.; Hennessy, A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007, 71, 977–984. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble FMS-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, L.B.; de Lucca, L.; Dorneles, B.N.; Konopka, C.K.; Gonçalves, T.L. Evaluation of oxidative stress and δ-aminolevulinate dehydratase activity in twin pregnancies. J. Matern. Fetal Neonatal Med. 2020, 33, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Moore Simas, T.A.; Solitro, M.J.; Rajan, A.; Crawford, S.; Soderland, P.; Meyer, B.A. Circulating angiogenic factors in singleton vs multiple-gestation pregnancies. Am. J. Obstet. Gynecol. 2008, 198, 200.e1–200.e7. [Google Scholar] [CrossRef]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161.e1–161.e11. [Google Scholar] [CrossRef]
- Kozłowski, S.; Stelmaszczyk-Emmel, A.; Szymusik, I.; Saletra-Bielińska, A.; Brawura-Biskupski-Samaha, R.; Pietruski, P.; Osińska, A.; Kosińska-Kaczyńska, K. sFlt-1, Not PlGF, Is Related to Twin Gestation Choronicity in the First and Third Trimesters of Pregnancy. Diagnostics 2021, 11, 1181. [Google Scholar] [CrossRef]
| Multiple Pregnancies—n = 39 | High Levels of sFlt-1 | Low Levels of sFlt-1 | p Value |
|---|---|---|---|
| sFlt-1 ≥ 15,802 pg/mL | sFlt-1 < 15,802 pg/mL | ||
| n = 15 (38) | n = 24 (62) | ||
| Types | |||
| DD twins | 12 (80) | 22 (92) | 0.354 |
| MD twins | 1 (7) | 1 (4) | 1.000 |
| MM twins | 1 (7) | 0 | 0.384 |
| Triplets | 1 (7) | 1 (4) | 1.000 |
| Maternal anamnestic characteristics | |||
| Age (years) | 37 ± 5 | 35 ± 6 | 0.136 |
| Nulliparous | 11 (73) | 19 (79) | 0.711 |
| Obesity (BMI > 30 kg/m2) | 3 (20) | 5 (21) | 1.000 |
| Diabetes/GDM | 4 (27) | 4 (17) | 0.685 |
| Chronic hypertension | 2 (13) | 0 | 0.141 |
| sFlt-1 | |||
| GA at blood test (weeks.days) | 33.6 ± 3.5 | 35.4 ± 1.3 | 0.136 |
| Latency time between blood test and delivery (days) | 3 ± 3 | 7 ± 4 | 0.007 |
| Pregnancy complications at blood test | |||
| HD | 9 (60) | 9 (38) | 0.202 |
| HD + FGR | 3 (20) | 6 (25) | 1.000 |
| FGR | 3 (20) | 9 (38) | 0.305 |
| GA at 1st hospitalization for HD/FGR (weeks.days) | 32.5 ± 4.2 | 34.6 ± 1.3 | 0.126 |
| GA at delivery (weeks.days) | 34.2 ± 3.5 | 36.4 ± 1.0 | 0.060 |
| Adverse outcomes | |||
| Birth < 34 weeks | 4 (27) | 0 | 0.016 |
| Urgent cesarean section for HD/FGR | 4 (27) | 0 | 0.016 |
| HELLP syndrome | 2 (13) | 0 | 0.141 |
| ICU admission for HD complications | 2 (13) | 0 | 0.141 |
| Antihypertensive therapy before delivery | 7 (47) | 1 (4) | 0.002 |
| Antihypertensive therapy at postpartum discharge | 7 (47) | 4 (17) | 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giardini, V.; Grilli, L.; Terzaghi, A.; Todyrenchuk, L.; Zavettieri, C.; Mazzoni, G.; Cozzolino, S.; Casati, M.; Vergani, P.; Locatelli, A. sFlt-1 Levels as a Predicting Tool in Placental Dysfunction Complications in Multiple Pregnancies. Biomedicines 2023, 11, 2917. https://doi.org/10.3390/biomedicines11112917
Giardini V, Grilli L, Terzaghi A, Todyrenchuk L, Zavettieri C, Mazzoni G, Cozzolino S, Casati M, Vergani P, Locatelli A. sFlt-1 Levels as a Predicting Tool in Placental Dysfunction Complications in Multiple Pregnancies. Biomedicines. 2023; 11(11):2917. https://doi.org/10.3390/biomedicines11112917
Chicago/Turabian StyleGiardini, Valentina, Leonora Grilli, Alessandra Terzaghi, Lyudmyla Todyrenchuk, Caterina Zavettieri, Giulia Mazzoni, Sabrina Cozzolino, Marco Casati, Patrizia Vergani, and Anna Locatelli. 2023. "sFlt-1 Levels as a Predicting Tool in Placental Dysfunction Complications in Multiple Pregnancies" Biomedicines 11, no. 11: 2917. https://doi.org/10.3390/biomedicines11112917
APA StyleGiardini, V., Grilli, L., Terzaghi, A., Todyrenchuk, L., Zavettieri, C., Mazzoni, G., Cozzolino, S., Casati, M., Vergani, P., & Locatelli, A. (2023). sFlt-1 Levels as a Predicting Tool in Placental Dysfunction Complications in Multiple Pregnancies. Biomedicines, 11(11), 2917. https://doi.org/10.3390/biomedicines11112917






