Targeting Cytochrome P450 Enzymes in Ovarian Cancers: New Approaches to Tumor-Selective Intervention
Abstract
1. Introduction
2. CYPS in Ovarian Cancers
2.1. CYP1A1
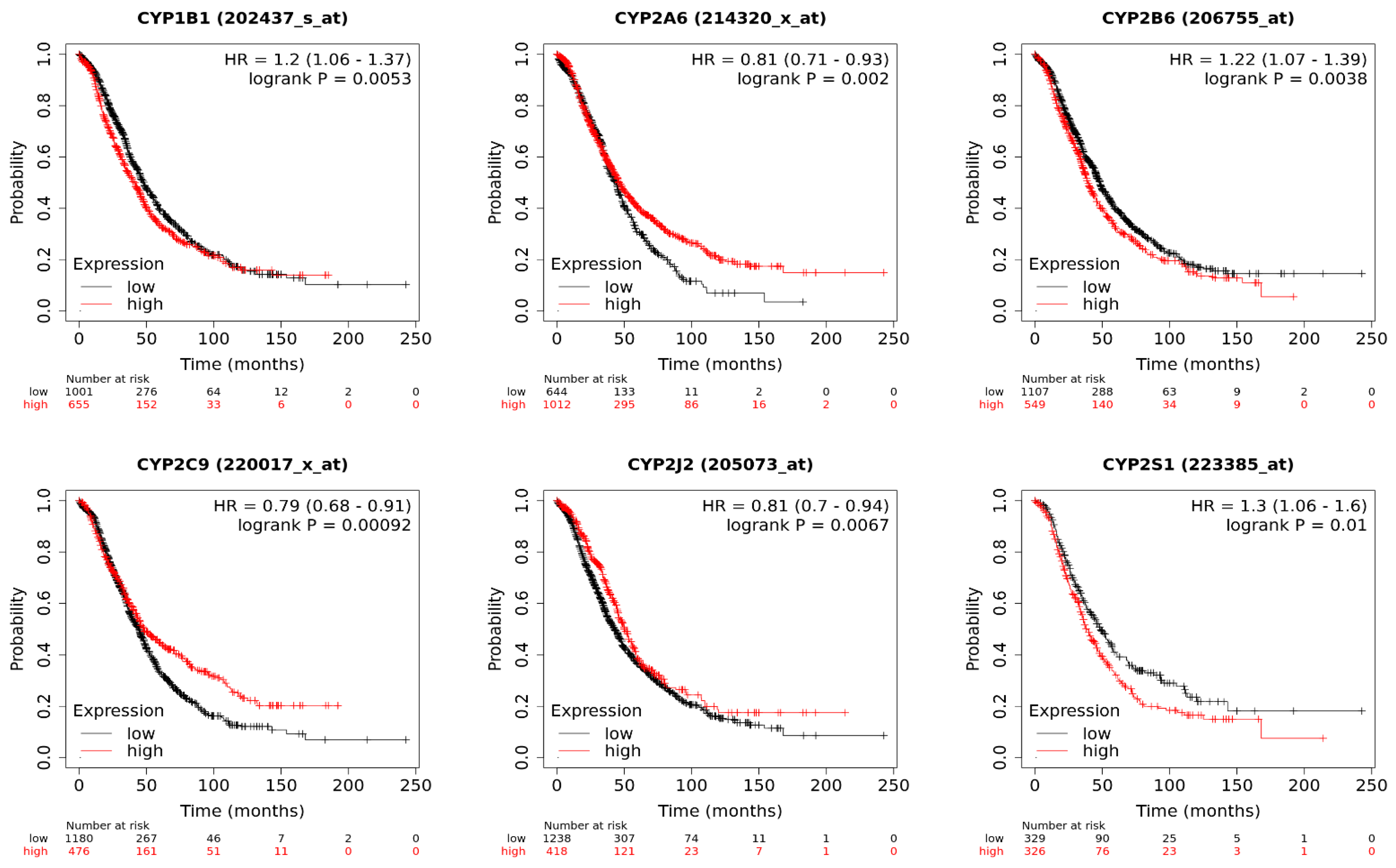
2.2. CYP1B1
2.3. CYP2A6 and CYP2B6
2.4. CYP2C8
2.5. CYP2C9
2.6. CYP2E1
2.7. CYP2J2
2.8. CYP2S1
2.9. CYP2U1
2.10. CYP3A4
2.11. CYP3A5
2.12. CYP3A7
2.13. CYP3A43
2.14. CYP4B1
2.15. CYP4Z1
2.16. CYP26A1
2.17. CYP51A1
3. Impact of CYP Polymorphisms on Risk and Prognosis of Ovarian Cancer
4. Impact of CYP Polymorphisms on Chemotherapeutic Agent Metabolism in Ovarian Cancer
5. Implications of CYP Isoform Expression for Patient Survival
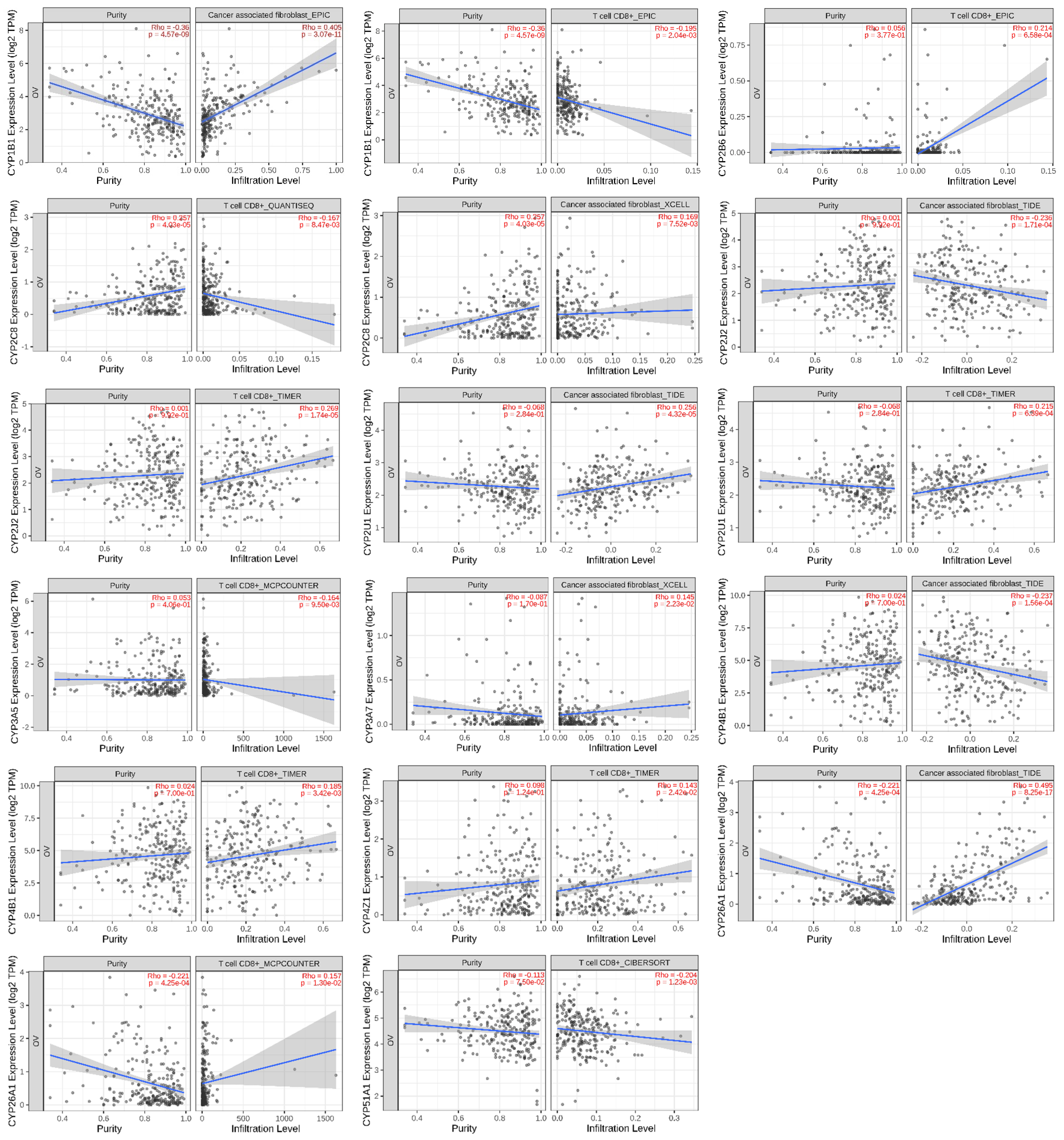
6. Association of CYP Isoform Expression with Immune Infiltration
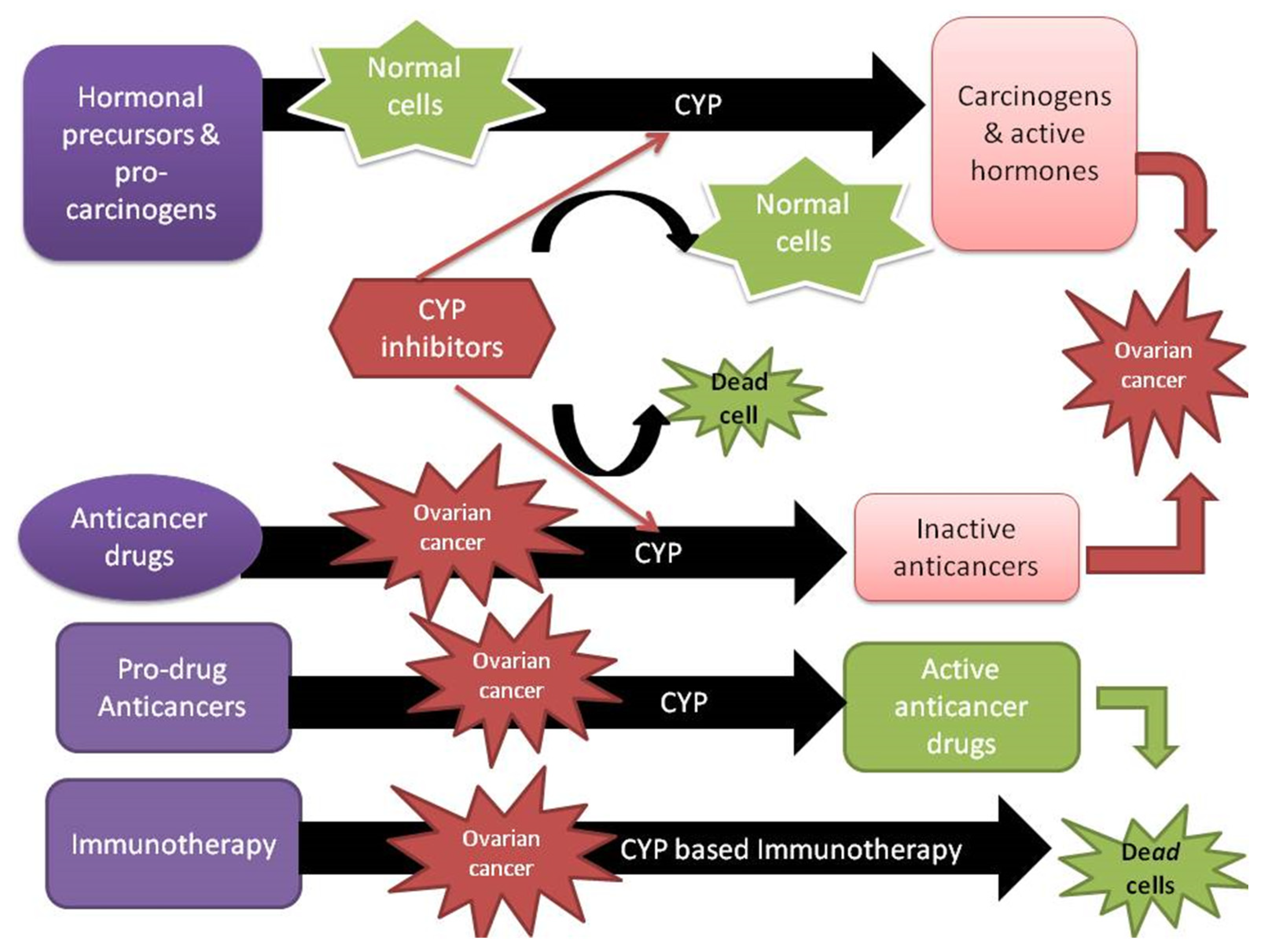
7. Future Perspectives
8. Methods
8.1. Survival Prognosis Analysis of CYP Isoforms
8.2. Immune Infiltration Analysis
8.3. Search Criteria
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AhR | Aryl hydrocarbon receptor |
| AP1 | Protein 1 |
| CAR | Constitutive androstane receptor |
| CDC20 | Cell division cycle 20 homolog |
| CREB | CAMP-response element binding |
| CYP | Cytochrome P450 |
| DAPK1 | Death-associated protein kinase-1 |
| EGA | European Genome-Phenome Archive |
| GEO | Gene Expression Omnibus |
| GR | Glucocorticoid receptor |
| 20-HETE | 20-hydroxyeicosatetraenoic acid |
| HNF4a | Hepatic nuclear factor-4a |
| PKC | Protein kinase C |
| PXR | Pregnane X receptor |
| RA | Retinoic acid |
| RXR | Retinoid X receptor |
| TCGA | The Cancer Genome Atlas |
| TIMP-2 | Tissue inhibitor of metalloproteinases 2 |
| VEGF A | Vascular endothelial growth factor A |
| VDR | Vitamin D receptor |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef]
- Carter, J.S.; Downs, L.S., Jr. Ovarian Cancer Tests and Treatment. Female Patient 2011, 36, 30–35. [Google Scholar] [PubMed]
- Sneha, S.; Baker, S.C.; Green, A.; Storr, S.; Aiyappa, R.; Martin, S.; Pors, K. Intratumoural Cytochrome P450 Expression in Breast Cancer: Impact on Standard of Care Treatment and New Efforts to Develop Tumour-Selective Therapies. Biomedicines 2021, 9, 290. [Google Scholar] [CrossRef]
- Lim, S.; Alshagga, M.; Ong, C.E.; Chieng, J.Y.; Pan, Y. Cytochrome P450 4B1 (CYP4B1) as a target in cancer treatment. Hum. Exp. Toxicol. 2020, 39, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Bruno, R.D.; Njar, V.C.O. Targeting cytochrome P450 enzymes: A new approach in anti-cancer drug development. Bioorg. Med. Chem. 2007, 15, 5047–5060. [Google Scholar] [CrossRef]
- Luo, B.; Yan, D.; Yan, H.; Yuan, J. Cytochrome P450: Implications for human breast cancer. Oncol. Lett. 2021, 22, 548. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.; Youssef, A.M.M.; Al-Sarayreh, S.; Almuhaisen, G.H.; Alnawaiseh, N.; Al Shuneigat, J.M.; Alrawashdeh, H.M. Profiling of CYP4Z1 and CYP1B1 expression in bladder cancers. Sci. Rep. 2021, 11, 5581. [Google Scholar] [CrossRef]
- Alshammari, F.O.F.O.; Al-Saraireh, Y.M.; Youssef, A.M.M.; Al-Sarayra, Y.M.; Alrawashdeh, H.M. Cytochrome P450 1B1 Overexpression in Cervical Cancers: Cross-sectional Study. Interact J. Med. Res. 2021, 10, e31150. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Alboaisa, N.S.; Alrawashdeh, H.M.; Hamdan, O.; Al-Sarayreh, S.; Al-Shuneigat, J.M.; Nofal, M.N. Screening of cytochrome 4Z1 expression in human non-neoplastic, pre-neoplastic and neoplastic tissues. Ecancermedicalscience 2020, 14, 1114. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.; Youssef, A.M.M.; Al-Sarayra, Y.M.; Al-Saraireh, R.A.; Al-Muhaisen, G.H.; Al-Mahdy, Y.S.; Al-Kharabsheh, A.M.; Abufraijeh, S.M.; Alrawashdeh, H.M. Cytochrome 4Z1 Expression Is Correlated with Poor Prognosis in Patients with Cervical Cancer. Curr. Oncol. 2021, 28, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.; Youssef, A.M.M.; Al-Sarayreh, S.; Almuhaisen, G.H.; Alnawaiseh, N.; Al-Shuneigat, J.M.; Alrawashdeh, H.M. Cytochrome 4Z1 Expression is Associated with Poor Prognosis in Colon Cancer Patients. OncoTargets Ther. 2021, 14, 5249–5260. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.; Youssef, A.M.M.; Al-Tarawneh, F.; Al-Sarayreh, S.; Almuhaisen, G.H.; Satari, A.O.; Al-Shuneigat, J.; Alrawashdeh, H.M. Cytochrome 4Z1 Expression is Associated with Unfavorable Survival in Triple-Negative Breast Cancers. Breast Cancer 2021, 13, 565–574. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Alshammari, F.; Satari, A.O.; Al-Mahdy, Y.S.; Almuhaisen, G.H.; Abu-Azzam, O.H.; Uwais, A.N.; Abufraijeh, S.M.; Al-Kharabsheh, A.M.; Al-Dalain, S.M.; et al. Cytochrome 4Z1 Expression Connotes Unfavorable Prognosis in Ovarian Cancers. Medicina 2022, 58, 1263. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Expression of CYP1A1, CYP1B1 and MnSOD in a panel of human cancer cell lines. Mol. Cell. Biochem. 2013, 383, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.K.; Lau, K.M.; Mobley, J.; Jiang, Z.; Ho, S.M. Overexpression of cytochrome P450 1A1 and its novel spliced variant in ovarian cancer cells: Alternative subcellular enzyme compartmentation may contribute to carcinogenesis. Cancer Res. 2005, 65, 3726–3734. [Google Scholar] [CrossRef]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; DiSaia, P.; Gabra, H.; Glenn, P.; et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Downie, D.; McFadyen, M.C.; Rooney, P.H.; Cruickshank, M.E.; Parkin, D.E.; Miller, I.D.; Telfer, C.; Melvin, W.T.; Murray, G.I. Profiling cytochrome P450 expression in ovarian cancer: Identification of prognostic markers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 7369–7375. [Google Scholar] [CrossRef]
- Rodriguez, M.; Potter, D.A. CYP1A1 regulates breast cancer proliferation and survival. Mol. Cancer Res. 2013, 11, 780–792. [Google Scholar] [CrossRef]
- Al-Dhfyan, A.; Alhoshani, A.; Korashy, H.M. Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-Catenin and Akt activation. Mol. Cancer 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Shnyder, S.D.; Loadman, P.M.; Sutherland, M.; Sheldrake, H.M.; Searcey, M.; Patterson, L.H.; Pors, K. Abstract 4541: Tumor-selective bioactivation of duocarmycin bioprecursors by cytochrome P450 enzymes provides an opportunity to treat drug-resistant breast cancer cells. Cancer Res. 2015, 75, 4541. [Google Scholar] [CrossRef]
- Sutherland, M.; Gill, J.H.; Loadman, P.M.; Laye, J.P.; Sheldrake, H.M.; Illingworth, N.A.; Alandas, M.N.; Cooper, P.A.; Searcey, M.; Pors, K.; et al. Antitumor Activity of a Duocarmycin Analogue Rationalized to Be Metabolically Activated by Cytochrome P450 1A1 in Human Transitional Cell Carcinoma of the Bladder. Mol. Cancer Ther. 2013, 12, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Travica, S.; Pors, K.; Loadman, P.M.; Shnyder, S.D.; Johansson, I.; Alandas, M.N.; Sheldrake, H.M.; Mkrtchian, S.; Patterson, L.H.; Ingelman-Sundberg, M. Colon cancer-specific cytochrome P450 2W1 converts duocarmycin analogues into potent tumor cytotoxins. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 2952–2961. [Google Scholar] [CrossRef]
- Fortin, S.; Charest-Morin, X.; Turcotte, V.; Lauvaux, C.; Lacroix, J.; Côté, M.F.; Gobeil, S.; C.-Gaudreault, R. Activation of Phenyl 4-(2-Oxo-3-alkylimidazolidin-1-yl)benzenesulfonates Prodrugs by CYP1A1 as New Antimitotics Targeting Breast Cancer Cells. J. Med. Chem. 2017, 60, 4963–4982. [Google Scholar] [CrossRef]
- McFadyen, M.C.; Cruickshank, M.E.; Miller, I.D.; McLeod, H.L.; Melvin, W.T.; Haites, N.E.; Parkin, D.; Murray, G.I. Cytochrome P450 CYP1B1 over-expression in primary and metastatic ovarian cancer. Br. J. Cancer 2001, 85, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Buters, J.; Quintanilla-Martinez, L.; Schober, W.; Soballa, V.J.; Hintermair, J.; Wolff, T.; Gonzalez, F.J.; Greim, H. CYP1B1 determines susceptibility to low doses of 7,12-dimethylbenz[a]anthracene-induced ovarian cancers in mice: Correlation of CYP1B1-mediated DNA adducts with carcinogenicity. Carcinogenesis 2003, 24, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, Y.; Chang, I.; Fukuhara, S.; Hiraki, M.; Arichi, N.; Yasumoto, H.; Hirata, H.; Yamamura, S.; Shahryari, V.; Deng, G.; et al. CYP1B1 promotes tumorigenesis via altered expression of CDC20 and DAPK1 genes in renal cell carcinoma. BMC Cancer 2015, 15, 942. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Baek, H.S.; Ye, D.J.; Shin, S.; Kim, D.; Chun, Y.J. CYP1B1 Enhances Cell Proliferation and Metastasis through Induction of EMT and Activation of Wnt/β-Catenin Signaling via Sp1 Upregulation. PLoS ONE 2016, 11, e0151598. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Guo, Y.; He, J.; Chen, Z.; Qiu, S.; Zhang, Y.; Ding, H.; Pan, J.; Pan, Y. CYP1B1 inhibits ferroptosis and induces anti-PD-1 resistance by degrading ACSL4 in colorectal cancer. Cell Death Dis. 2023, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Mu, Y.; Qi, C.; Wang, J.; Xi, G.; Guo, J.; Mi, R.; Zhao, F. CYP1B1 enhances the resistance of epithelial ovarian cancer cells to paclitaxel in vivo and in vitro. Int. J. Mol. Med. 2015, 35, 340–348. [Google Scholar] [CrossRef] [PubMed]
- DeLoia, J.A.; Zamboni, W.C.; Jones, J.M.; Strychor, S.; Kelley, J.L.; Gallion, H.H. Expression and activity of taxane-metabolizing enzymes in ovarian tumors. Gynecol. Oncol. 2008, 108, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; O’Connor, R.; Liang, Y.; Clynes, M. CYP1B1 expression is induced by docetaxel: Effect on cell viability and drug resistance. Br. J. Cancer 2008, 98, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Backman, J.T.; Filppula, A.M.; Niemi, M.; Neuvonen, P.J. Role of Cytochrome P450 2C8 in Drug Metabolism and Interactions. Pharm. Rev. 2016, 68, 168–241. [Google Scholar] [CrossRef]
- Aquilante, C.L.; Niemi, M.; Gong, L.; Altman, R.B.; Klein, T.E. PharmGKB summary: Very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 8. Pharm. Genom. 2013, 23, 721–728. [Google Scholar] [CrossRef]
- Klose, T.S.; Blaisdell, J.A.; Goldstein, J.A. Gene structure of CYP2C8 and extrahepatic distribution of the human CYP2Cs. J. Biochem. Mol. Toxicol. 1999, 13, 289–295. [Google Scholar] [CrossRef]
- Evangelista, E.A.; Cho, C.W.; Aliwarga, T.; Totah, R.A. Expression and Function of Eicosanoid-Producing Cytochrome P450 Enzymes in Solid Tumors. Front. Pharmacol. 2020, 11, 828. [Google Scholar] [CrossRef]
- Freedman, R.S.; Wang, E.; Voiculescu, S.; Patenia, R.; Bassett, R.L., Jr.; Deavers, M.; Marincola, F.M.; Yang, P.; Newman, R.A. Comparative analysis of peritoneum and tumor eicosanoids and pathways in advanced ovarian cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 5736–5744. [Google Scholar] [CrossRef]
- Leung, T.; Rajendran, R.; Singh, S.; Garva, R.; Krstic-Demonacos, M.; Demonacos, C. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res. 2013, 15, R107. [Google Scholar] [CrossRef]
- Trousil, S.; Lee, P.; Edwards, R.J.; Maslen, L.; Lozan-Kuehne, J.P.; Ramaswami, R.; Aboagye, E.O.; Clarke, S.; Liddle, C.; Sharma, R. Altered cytochrome 2E1 and 3A P450-dependent drug metabolism in advanced ovarian cancer correlates to tumour-associated inflammation. Br. J. Pharmacol. 2019, 176, 3712–3722. [Google Scholar] [CrossRef]
- Xu, M.; Ju, W.; Hao, H.; Wang, G.; Li, P. Cytochrome P450 2J2: Distribution, function, regulation, genetic polymorphisms and clinical significance. Drug Metab. Rev. 2013, 45, 311–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.G.; Chen, C.L.; Card, J.W.; Yang, S.; Chen, J.X.; Fu, X.N.; Ning, Y.G.; Xiao, X.; Zeldin, D.C.; Wang, D.W. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005, 65, 4707–4715. [Google Scholar] [CrossRef]
- Chen, C.; Wei, X.; Rao, X.; Wu, J.; Yang, S.; Chen, F.; Ma, D.; Zhou, J.; Dackor, R.T.; Zeldin, D.C.; et al. Cytochrome P450 2J2 Is Highly Expressed in Hematologic Malignant Diseases and Promotes Tumor Cell Growth. J. Pharmacol. Exp. Ther. 2011, 336, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, G.; Liao, W.; Wu, J.; Liu, L.; Ma, D.; Zhou, J.; Elbekai, R.H.; Edin, M.L.; Zeldin, D.C.; et al. Selective Inhibitors of CYP2J2 Related to Terfenadine Exhibit Strong Activity against Human Cancers in Vitro and in Vivo. J. Pharmacol. Exp. Ther. 2009, 329, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Saarikoski, S.T.; Wikman, H.A.; Smith, G.; Wolff, C.H.; Husgafvel-Pursiainen, K. Localization of cytochrome P450 CYP2S1 expression in human tissues by in situ hybridization and immunohistochemistry. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2005, 53, 549–556. [Google Scholar] [CrossRef]
- Guo, H.; Zeng, B.; Wang, L.; Ge, C.; Zuo, X.; Li, Y.; Ding, W.; Deng, L.; Zhang, J.; Qian, X.; et al. Knockdown CYP2S1 inhibits lung cancer cells proliferation and migration. Cancer Biomark. 2021, 32, 531–539. [Google Scholar] [CrossRef]
- Kuban, W.; Daniel, W.A. Cytochrome P450 expression and regulation in the brain. Drug Metab. Rev. 2021, 53, 1–29. [Google Scholar] [CrossRef]
- Chuang, S.S.; Helvig, C.; Taimi, M.; Ramshaw, H.A.; Collop, A.H.; Amad, M.; White, J.A.; Petkovich, M.; Jones, G.; Korczak, B. CYP2U1, a novel human thymus- and brain-specific cytochrome P450, catalyzes omega- and (omega-1)-hydroxylation of fatty acids. J. Biol. Chem. 2004, 279, 6305–6314. [Google Scholar] [CrossRef]
- Bièche, I.; Narjoz, C.; Asselah, T.; Vacher, S.; Marcellin, P.; Lidereau, R.; Beaune, P.; de Waziers, I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharm. Genom. 2007, 17, 731–742. [Google Scholar] [CrossRef]
- Marsh, S.; Paul, J.; King, C.R.; Gifford, G.; McLeod, H.L.; Brown, R. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: The Scottish Randomised Trial in Ovarian Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4528–4535. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.E.; Han, L.W.; Mao, Q.; Lampe, J.N. Digging Deeper into CYP3A Testosterone Metabolism: Kinetic, Regioselectivity, and Stereoselectivity Differences between CYP3A4/5 and CYP3A7. Drug Metab. Dispos. 2017, 45, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Lolodi, O.; Wang, Y.M.; Wright, W.C.; Chen, T. Differential Regulation of CYP3A4 and CYP3A5 and its Implication in Drug Discovery. Curr. Drug Metab. 2017, 18, 1095–1105. [Google Scholar] [CrossRef]
- Sevrioukova, I.F. Structural Basis for the Diminished Ligand Binding and Catalytic Ability of Human Fetal-Specific CYP3A7. Int. J. Mol. Sci. 2021, 22, 5831. [Google Scholar] [CrossRef]
- Li, H.; Lampe, J.N. Neonatal cytochrome P450 CYP3A7: A comprehensive review of its role in development, disease, and xenobiotic metabolism. Arch. Biochem. Biophys. 2019, 673, 108078. [Google Scholar] [CrossRef] [PubMed]
- Westlind, A.; Malmebo, S.; Johansson, I.; Otter, C.; Andersson, T.B.; Ingelman-Sundberg, M.; Oscarson, M. Cloning and tissue distribution of a novel human cytochrome p450 of the CYP3A subfamily, CYP3A43. Biochem. Biophys. Res. Commun. 2001, 281, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Machalz, D.; Liu, S.; Wolf, C.A.; Wolber, G.; Parr, M.K.; Bureik, M. Metabolism of the antipsychotic drug olanzapine by CYP3A43. Xenobiotica Fate Foreign Compd. Biol. Syst. 2022, 52, 413–425. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Lau, A.T.Y.; Mo, H.Y.; Zhong, Q.H.; Zhao, X.Y.; Yu, F.Y.; Han, J.; Wu, Y.Y.; Xu, Y.M. Effects of CYP3A43 Expression on Cell Proliferation and Migration of Lung Adenocarcinoma and Its Clinical Significance. Int. J. Mol. Sci. 2022, 24, 113. [Google Scholar] [CrossRef]
- Imaoka, S.; Yoneda, Y.; Sugimoto, T.; Hiroi, T.; Yamamoto, K.; Nakatani, T.; Funae, Y. CYP4B1 is a possible risk factor for bladder cancer in humans. Biochem. Biophys. Res. Commun. 2000, 277, 776–780. [Google Scholar] [CrossRef]
- Satih, S.; Chalabi, N.; Rabiau, N.; Bosviel, R.; Fontana, L.; Bignon, Y.-J.; Bernard-Gallon, D.J. Gene expression profiling of breast cancer cell lines in response to soy isoflavones using a pangenomic microarray approach. OMICS 2010, 14, 231–238. [Google Scholar] [CrossRef]
- Liu, X.; Jia, Y.; Shi, C.; Kong, D.; Wu, Y.; Zhang, T.; Wei, A.; Wang, D. CYP4B1 is a prognostic biomarker and potential therapeutic target in lung adenocarcinoma. PLoS ONE 2021, 16, e0247020. [Google Scholar] [CrossRef] [PubMed]
- Barlin, J.N.; Jelinic, P.; Olvera, N.; Bogomolniy, F.; Bisogna, M.; Dao, F.; Barakat, R.R.; Chi, D.S.; Levine, D.A. Validated gene targets associated with curatively treated advanced serous ovarian carcinoma. Gynecol. Oncol. 2013, 128, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Savas, U.; Hsu, M.H.; Griffin, K.J.; Bell, D.R.; Johnson, E.F. Conditional regulation of the human CYP4X1 and CYP4Z1 genes. Arch. Biochem. Biophys. 2005, 436, 377–385. [Google Scholar] [CrossRef]
- Zollner, A.; Dragan, C.A.; Pistorius, D.; Muller, R.; Bode, H.B.; Peters, F.T.; Maurer, H.H.; Bureik, M. Human CYP4Z1 catalyzes the in-chain hydroxylation of lauric acid and myristic acid. Biol. Chem. 2009, 390, 313–317. [Google Scholar] [CrossRef]
- McDonald, M.G.; Ray, S.; Amorosi, C.J.; Sitko, K.A.; Kowalski, J.P.; Paco, L.; Nath, A.; Gallis, B.; Totah, R.A.; Dunham, M.J.; et al. Expression and Functional Characterization of Breast Cancer-Associated Cytochrome P450 4Z1 in Saccharomyces cerevisiae. Drug Metab. Dispos. 2017, 45, 1364–1371. [Google Scholar] [CrossRef]
- Rieger, M.A.; Ebner, R.; Bell, D.R.; Kiessling, A.; Rohayem, J.; Schmitz, M.; Temme, A.; Rieber, E.P.; Weigle, B. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Res. 2004, 64, 2357–2364. [Google Scholar] [CrossRef]
- Tradonsky, A.; Rubin, T.; Beck, R.; Ring, B.; Seitz, R.; Mair, S. A search for reliable molecular markers of prognosis in prostate cancer: A study of 240 cases. Am. J. Clin. Pathol. 2012, 137, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Khayeka-Wandabwa, C.; Ma, X.; Jia, Y.; Bureik, M. Monitoring of autoantibodies against CYP4Z1 in patients with colon, ovarian, or prostate cancer. Immunobiology 2022, 227, 152174. [Google Scholar] [CrossRef]
- Nunna, V.; Jalal, N.; Bureik, M. Anti-CYP4Z1 autoantibodies detected in breast cancer patients. Cell Mol. Immunol 2017, 14, 572–574. [Google Scholar] [CrossRef]
- Yu, W.; Chai, H.; Li, Y.; Zhao, H.; Xie, X.; Zheng, H.; Wang, C.; Wang, X.; Yang, G.; Cai, X. Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer. Toxicol. Appl. Pharmacol. 2012, 264, 73–83. [Google Scholar] [CrossRef]
- Zheng, L.; Li, X.; Gu, Y.; Lv, X.; Xi, T. The 3′ UTR of the pseudogene CYP4Z2P promotes tumor angiogenesis in breast cancer by acting as a ceRNA for CYP4Z1. Breast Cancer Res. Treat. 2015, 150, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Guo, Q.; Xiang, C.; Liu, S.; Jiang, Y.; Gao, L.; Ni, H.; Wang, T.; Zhao, Q.; Liu, H. Transcriptional factor six2 promotes the competitive endogenous RNA network between CYP4Z1 and pseudogene CYP4Z2P responsible for maintaining the stemness of breast cancer cells. J. Hematol. Oncol. 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Osanai, M.; Sawada, N.; Lee, G.H. Oncogenic and cell survival properties of the retinoic acid metabolizing enzyme, CYP26A1. Oncogene 2010, 29, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Stevison, F.; Jing, J.; Tripathy, S.; Isoherranen, N. Chapter Eleven—Role of Retinoic Acid-Metabolizing Cytochrome P450s, CYP26, in Inflammation and Cancer. In Advances in Pharmacology; Hardwick, J.P., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 74, pp. 373–412. [Google Scholar]
- Foti, R.S.; Isoherranen, N.; Zelter, A.; Dickmann, L.J.; Buttrick, B.R.; Diaz, P.; Douguet, D. Identification of Tazarotenic Acid as the First Xenobiotic Substrate of Human Retinoic Acid Hydroxylase CYP26A1 and CYP26B1. J. Pharmacol. Exp. Ther. 2016, 357, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhskiy, L.; Ershov, P.; Yablokov, E.; Shkel, T.; Grabovec, I.; Mezentsev, Y.; Gnedenko, O.; Usanov, S.; Shabunya, P.; Fatykhava, S.; et al. Human Lanosterol 14-Alpha Demethylase (CYP51A1) Is a Putative Target for Natural Flavonoid Luteolin 7,3’-Disulfate. Molecules 2021, 26, 2237. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Sivakumaran, S.; Yazlovitskaya, E.M.; Hiebert, S.W.; Guengerich, F.P.; Waterman, M.R.; Lepesheva, G.I. Human sterol 14α-demethylase as a target for anticancer chemotherapy: Towards structure-aided drug design1. J. Lipid Res. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- PDQ Cancer Genetics Editorial Board. Genetics of Breast and Gynecologic Cancers (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Aktas, D.; Guney, I.; Alikasifoglu, M.; Yüce, K.; Tuncbilek, E.; Ayhan, A. CYP1A1 gene polymorphism and risk of epithelial ovarian neoplasm. Gynecol. Oncol. 2002, 86, 124–128. [Google Scholar] [CrossRef]
- Heubner, M.; Wimberger, P.; Riemann, K.; Kasimir-Bauer, S.; Otterbach, F.; Kimmig, R.; Siffert, W. The CYP1A1 Ile462Val polymorphism and platinum resistance of epithelial ovarian neoplasms. Oncol. Res. 2010, 18, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; McDuffie, K.; Kolonel, L.N.; Terada, K.; Donlon, T.A.; Wilkens, L.R.; Guo, C.; Le Marchand, L. Case-control study of ovarian cancer and polymorphisms in genes involved in catecholestrogen formation and metabolism. Cancer Epidemiol. Biomark. Prev. 2001, 10, 209–216. [Google Scholar]
- Seremak-Mrozikiewicz, A.; Drews, K.; Semczuk, A.; Jakowicki, J.A.; Mrozikiewicz, P.M. CYP1A1 alleles in female genital cancers in the Polish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 246–250. [Google Scholar] [CrossRef]
- Sergentanis, T.N.; Economopoulos, K.P.; Choussein, S.; Vlahos, N.F. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and ovarian cancer risk: A meta-analysis. Mol. Biol. Rep. 2012, 39, 9921–9930. [Google Scholar] [CrossRef]
- Huang, M.; Chen, Q.; Xiao, J.; Zhao, X.; Liu, C. CYP1A1 Ile462Val is a risk factor for ovarian cancer development. Cytokine 2012, 58, 73–78. [Google Scholar] [CrossRef]
- Goodman, M.T.; Tung, K.-H.; McDuffie, K.; Wilkens, L.R.; Donlon, T.A. Association of caffeine intake and CYP1A2 genotype with ovarian cancer. Nutr. Cancer 2003, 46, 23–29. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.-L.; Zhao, L.; Zhang, C.-L. Role of CYP1A2* 1F polymorphism in cancer risk: Evidence from a meta-analysis of 46 case–control studies. Gene 2013, 524, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sellers, T.A.; Schildkraut, J.M.; Pankratz, V.S.; Vierkant, R.A.; Fredericksen, Z.S.; Olson, J.E.; Cunningham, J.; Taylor, W.; Liebow, M.; McPherson, C.; et al. Estrogen bioactivation, genetic polymorphisms, and ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2536–2543. [Google Scholar] [CrossRef]
- Delort, L.; Chalabi, N.; Satih, S.; Rabiau, N.; Kwiatkowski, F.; Bignon, Y.J.; Bernard-Gallon, D.J. Association between genetic polymorphisms and ovarian cancer risk. Anticancer Res. 2008, 28, 3079–3081. [Google Scholar]
- Gajjar, K.; Owens, G.; Sperrin, M.; Martin-Hirsch, P.L.; Martin, F.L. Cytochrome P1B1 (CYP1B1) polymorphisms and ovarian cancer risk: A meta-analysis. Toxicology 2012, 302, 157–162. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Lou, M.; Deng, X.; Liu, C.; Li, L. The ovarian carcinoma risk with the polymorphisms of CYP1B1 come from the positive selection. Am. J. Transl. Res. 2021, 13, 4322–4341. [Google Scholar]
- Pearce, C.L.; Near, A.M.; Van Den Berg, D.J.; Ramus, S.J.; Gentry-Maharaj, A.; Menon, U.; Gayther, S.A.; Anderson, A.R.; Edlund, C.K.; Wu, A.H.; et al. Validating genetic risk associations for ovarian cancer through the international Ovarian Cancer Association Consortium. Br. J. Cancer 2009, 100, 412–420. [Google Scholar] [CrossRef]
- Assis, J.; Pereira, D.; Gomes, M.; Marques, D.; Marques, I.; Nogueira, A.; Catarino, R.; Medeiros, R. Influence of CYP3A4 genotypes in the outcome of serous ovarian cancer patients treated with first-line chemotherapy: Implication of a CYP3A4 activity profile. Int. J. Clin. Exp. Med. 2013, 6, 552–561. [Google Scholar] [PubMed]
- Spurdle, A.B.; Goodwin, B.; Hodgson, E.; Hopper, J.L.; Chen, X.; Purdie, D.M.; McCredie, M.R.; Giles, G.G.; Chenevix-Trench, G.; Liddle, C. The CYP3A4*1B polymorphism has no functional significance and is not associated with risk of breast or ovarian cancer. Pharmacogenetics 2002, 12, 355–366. [Google Scholar] [CrossRef]
- Fiszer-Maliszewska, Ł.; Łaczmański, Ł.; Dolińska, A.; Jagas, M.; Kołodziejska, E.; Jankowska, M.; Kuśnierczyk, P. Polymorphisms of ABCB1, CYP3A4 and CYP3A5 Genes in Ovarian Cancer and Treatment Response in Poles. Anticancer Res. 2018, 38, 1455–1459. [Google Scholar]
- Spurdle, A.B.; Chen, X.; Abbazadegan, M.; Martin, N.; Khoo, S.K.; Hurst, T.; Ward, B.; Webb, P.M.; Chenevix-Trench, G. CYP17 promotor polymorphism and ovarian cancer risk. Int. J. Cancer 2000, 86, 436–439. [Google Scholar] [CrossRef]
- Gowtham Kumar, G.; Paul, S.F.D.; Molia, C.; Manickavasagam, M.; Ramya, R.; Usha Rani, G.; Ganesan, N.; Andrea Mary, F. The association between CYP17A1, CYP19A1, and HSD17B1 gene polymorphisms of estrogen synthesis pathway and ovarian cancer predisposition. Meta Gene 2022, 31, 100985. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Vitonis, A.F.; Terry, K.L.; De Vivo, I.; Cramer, D.W.; Hankinson, S.E.; Tworoger, S.S. Coffee intake, variants in genes involved in caffeine metabolism, and the risk of epithelial ovarian cancer. Cancer Causes Control 2009, 20, 335–344. [Google Scholar] [CrossRef]
- Ayyob, A.N.; Al-Badran, A.I.; Abood, R.A. Association of TTTA polymorphism in CYP19 gene with endometrial and ovarian cancers risk in Basrah. Gene Rep. 2019, 16, 100453. [Google Scholar] [CrossRef]
- Park, J.-W.; Ji, Y.I. Differences in efficacy and toxicity in relation to genetic polymorphisms of the cytochrome P450 2C8 gene after chemotherapy in epithelial ovarian cancer. Eur. J. Gynaecol. Oncol. 2019, 40, 209–216. [Google Scholar] [CrossRef]
- Bryan, E.J.; Thomas, N.A.; Palmer, K.; Dawson, E.; Englefield, P.; Campbell, I.G. Refinement of an ovarian cancer tumour suppressor gene locus on chromosome arm 22q and mutation analysis of CYP2D6, SREBP2 and NAGA. Int. J. Cancer 2000, 87, 798–802. [Google Scholar] [CrossRef]
- Sarhanis, P.; Redman, C.; Perrett, C.; Brannigan, K.; Clayton, R.N.; Hand, P.; Musgrove, C.; Suarez, V.; Jones, P.; Fryer, A.A.; et al. Epithelial ovarian cancer: Influence of polymorphism at the glutathione S-transferase GSTM1 and GSTT1 loci on p53 expression. Br. J. Cancer 1996, 74, 1757–1761. [Google Scholar] [CrossRef][Green Version]
- Peethambaram, P.; Fridley, B.L.; Vierkant, R.A.; Larson, M.C.; Kalli, K.R.; Elliott, E.A.; Oberg, A.L.; White, K.L.; Rider, D.N.; Keeney, G.L.; et al. Polymorphisms in ABCB1 and ERCC2 associated with ovarian cancer outcome. Int. J. Mol. Epidemiol. Genet. 2011, 2, 185–195. [Google Scholar]
- Khrunin, A.V.; Ivanova, F.G.; Moiseev, A.A.; Gorbunova, V.A.; Limborskaia, S.A. CYP2E1 gene polymorphism and ovarian cancer risk in the Yakut population. Genetika 2011, 47, 1686–1688. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, M.C.; McLeod, H.L.; Jackson, F.C.; Melvin, W.T.; Doehmer, J.; Murray, G.I. Cytochrome P450 CYP1B1 protein expression: A novel mechanism of anticancer drug resistance. Biochem. Pharmacol. 2001, 62, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gagno, S.; Bartoletti, M.; Romualdi, C.; Poletto, E.; Scalone, S.; Sorio, R.; Zanchetta, M.; De Mattia, E.; Roncato, R.; Cecchin, E.; et al. Pharmacogenetic score predicts overall survival, progression-free survival and platinum sensitivity in ovarian cancer. Pharmacogenomics 2020, 21, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Gréen, H.; Söderkvist, P.; Rosenberg, P.; Mirghani, R.A.; Rymark, P.; Lundqvist, E.A.; Peterson, C. Pharmacogenetic studies of Paclitaxel in the treatment of ovarian cancer. Basic Clin. Pharmacol. Toxicol. 2009, 104, 130–137. [Google Scholar] [CrossRef]
- Nakajima, M.; Fujiki, Y.; Kyo, S.; Kanaya, T.; Nakamura, M.; Maida, Y.; Tanaka, M.; Inoue, M.; Yokoi, T. Pharmacokinetics of paclitaxel in ovarian cancer patients and genetic polymorphisms of CYP2C8, CYP3A4, and MDR1. J. Clin. Pharmacol. 2005, 45, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, T.K.; Brasch-Andersen, C.; Gréen, H.; Mirza, M.; Pedersen, R.S.; Nielsen, F.; Skougaard, K.; Wihl, J.; Keldsen, N.; Damkier, P.; et al. Impact of CYP2C8*3 on paclitaxel clearance: A population pharmacokinetic and pharmacogenomic study in 93 patients with ovarian cancer. Pharm. J. 2011, 11, 113–120. [Google Scholar] [CrossRef]
- Gréen, H.; Khan, M.S.; Jakobsen-Falk, I.; Åvall-Lundqvist, E.; Peterson, C. Impact of CYP3A5*3 and CYP2C8-HapC on paclitaxel/carboplatin-induced myelosuppression in patients with ovarian cancer. J. Pharm. Sci. 2011, 100, 4205–4209. [Google Scholar] [CrossRef]
- Hu, L.; Lv, Q.L.; Guo, Y.; Cheng, L.; Wu, N.Y.; Qin, C.Z.; Zhou, H.H. Genetic variation of CYP3A5 influences paclitaxel/carboplatin-induced toxicity in Chinese epithelial ovarian cancer patients. J. Clin. Pharmacol. 2016, 56, 349–354. [Google Scholar] [CrossRef]
- Klyushova, L.S.; Perepechaeva, M.L.; Grishanova, A.Y. The Role of CYP3A in Health and Disease. Biomedicines 2022, 10, 2686. [Google Scholar] [CrossRef]
- Yu, T.; Wang, X.; Zhu, G.; Han, C.; Su, H.; Liao, X.; Yang, C.; Qin, W.; Huang, K.; Peng, T. The prognostic value of differentially expressed CYP3A subfamily members for hepatocellular carcinoma. Cancer Manag. Res. 2018, 10, 1713–1726. [Google Scholar] [CrossRef]
- McFadyen, M.C.E.; Melvin, W.T.; Murray, G.I. Cytochrome P450 enzymes: Novel options for cancer therapeutics. Mol. Cancer Ther. 2004, 3, 363–371. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front. Cell Dev. Biol. 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.H.; DeFazio, A.; Kasherman, L. Immune checkpoint inhibitors in ovarian cancer: Where do we go from here? Cancer Drug Resist. 2023, 6, 358–377. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Murdaca, G.; Mirabile, G.; Gangemi, S. Redox Signaling Modulates Activity of Immune Checkpoint Inhibitors in Cancer Patients. Biomedicines 2023, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Yanev, S.; Stoyanova, T. Manipulating cytochrome P450 enzymes: New perspectives for cancer treatment. Biomed. Rev. 2018, 28, 120–124. [Google Scholar] [CrossRef]
- Singh, R.D.; Avadhesh, A.; Sharma, G.; Dholariya, S.; Shah, R.B.; Goyal, B.; Gupta, S.C. Potential of Cytochrome P450, a Family of Xenobiotic Metabolizing Enzymes, in Cancer Therapy. Antioxid. Redox Signal. 2023, 38, 853–876. [Google Scholar] [CrossRef]
- Mikstacka, R.; Dutkiewicz, Z. New perspectives of CYP1B1 inhibitors in the light of molecular studies. Processes 2021, 9, 817. [Google Scholar] [CrossRef]
- Capper, C.P.; Rae, J.M.; Auchus, R.J. The metabolism, analysis, and targeting of steroid hormones in breast and prostate cancer. Horm. Cancer 2016, 7, 149–164. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Albertella, M.R.; Loadman, P.M.; Jones, P.H.; Phillips, R.M.; Rampling, R.; Burnet, N.; Alcock, C.; Anthoney, A.; Vjaters, E.; Dunk, C.R. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: Results of a phase I study. Clin. Cancer Res. 2008, 14, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef] [PubMed]


| CYP Isoform | Functions | Candidate Drug |
|---|---|---|
| CYP1A1 | Metabolism of xenobiotics, steroids, and drugs | AFP464, Phortress, ICT2700, and AQ4N |
| CYP1B1 | Metabolism of xenobiotics, steroids, and drugs | DMU135, DMAKO-20, Phortress, and ZYC300 |
| CYP2A6 | Drug and steroid metabolism | |
| CYP2B6 | Drug and steroid metabolism | |
| CYP2C8 | Drug and steroid metabolism | |
| CYP2C9 | Drug and steroid metabolism | |
| CYP2J2 | Drug and steroid metabolism | Tanshinone IIA, decursin, and C-26 |
| CYP2S1 | Drug and steroid metabolism | AQ4N |
| CYP2U1 | Drug and steroid metabolism | |
| CYP3A4 | Drug and steroid (including testosterone) metabolism | |
| CYP3A5 | Drug and steroid (including testosterone) metabolism | |
| CYP3A7 | Drug and steroid (including testosterone) metabolism | |
| CYP3A43 | Drug and steroid (including testosterone) metabolism | |
| CYP4B1 | Arachidonic acid or fatty acid metabolism | 4-IPO |
| CYP4Z1 | Arachidonic acid or fatty acid metabolism | 1-benzylimidazole |
| CYP26A1 | Retinoic acid hydroxylase | |
| CYP51A1 | Cholesterol biosynthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-saraireh, Y.M.; Alshammari, F.O.F.O.; Abu-azzam, O.H.; Al-dalain, S.M.; Al-sarayra, Y.M.; Haddad, M.; Makeen, H.; Al-Qtaitat, A.; Almermesh, M.; Al-sarayreh, S.A. Targeting Cytochrome P450 Enzymes in Ovarian Cancers: New Approaches to Tumor-Selective Intervention. Biomedicines 2023, 11, 2898. https://doi.org/10.3390/biomedicines11112898
Al-saraireh YM, Alshammari FOFO, Abu-azzam OH, Al-dalain SM, Al-sarayra YM, Haddad M, Makeen H, Al-Qtaitat A, Almermesh M, Al-sarayreh SA. Targeting Cytochrome P450 Enzymes in Ovarian Cancers: New Approaches to Tumor-Selective Intervention. Biomedicines. 2023; 11(11):2898. https://doi.org/10.3390/biomedicines11112898
Chicago/Turabian StyleAl-saraireh, Yousef M., Fatemah O. F. O. Alshammari, Omar H. Abu-azzam, Sa’ed M. Al-dalain, Yahya M. Al-sarayra, Mansour Haddad, Hafiz Makeen, Aiman Al-Qtaitat, Mohammad Almermesh, and Sameeh A. Al-sarayreh. 2023. "Targeting Cytochrome P450 Enzymes in Ovarian Cancers: New Approaches to Tumor-Selective Intervention" Biomedicines 11, no. 11: 2898. https://doi.org/10.3390/biomedicines11112898
APA StyleAl-saraireh, Y. M., Alshammari, F. O. F. O., Abu-azzam, O. H., Al-dalain, S. M., Al-sarayra, Y. M., Haddad, M., Makeen, H., Al-Qtaitat, A., Almermesh, M., & Al-sarayreh, S. A. (2023). Targeting Cytochrome P450 Enzymes in Ovarian Cancers: New Approaches to Tumor-Selective Intervention. Biomedicines, 11(11), 2898. https://doi.org/10.3390/biomedicines11112898





