Identification of Mispairing Omic Signatures in Chinese Hamster Ovary (CHO) Cells Producing a Tri-Specific Antibody
Abstract
:1. Introduction
2. Materials and Methods
3. Results
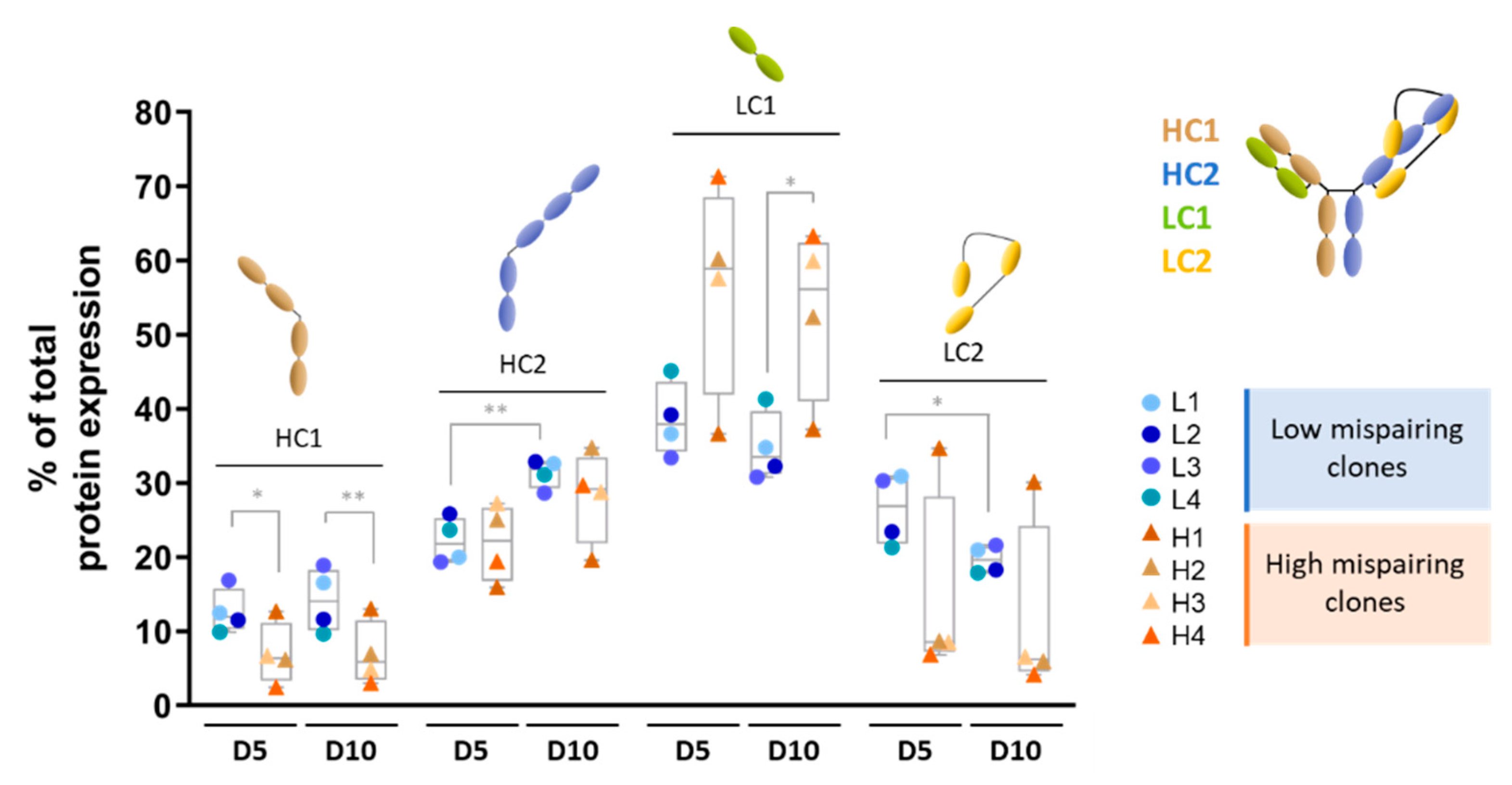
3.1. Mispairing Profile of Clones
3.2. Transcriptomic and Proteomic Analysis of tsAb-Producing Clones Reveals Enrichment in Key Pathways in Low vs. High Mispairing Clones
3.2.1. Exponential Growth Phase (Day 5)
3.2.2. tsAb Production Phase (Day 10)
3.2.3. RNA-Seq Validation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Lu, Y.-W.; Liu, Z.; Stevens, J.; Murawsky, C.M.; Wilson, V.; Hu, Z.; Richards, W.G.; Michaels, M.L.; Zhang, J.; et al. A biparatopic agonistic antibody that mimics fibroblast growth factor 21 ligand activity. J. Biol. Chem. 2018, 293, 5909–5919. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, T.; Igawa, T.; Sampei, Z.; Muto, A.; Kojima, T.; Soeda, T.; Yoshihashi, K.; Okuyama-Nishida, Y.; Saito, H.; Tsunoda, H.; et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat. Med. 2012, 18, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.; Callaghan, M.U. The role of emicizumab, a bispecific factor IXa- and factor X-directed antibody, for the prevention of bleeding episodes in patients with hemophilia A. Ther. Adv. Hematol. 2018, 9, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Correnti, C.E.; Laszlo, G.S.; de van der Schueren, W.J.; Godwin, C.D.; Bandaranayake, A.; Busch, M.A.; Gudgeon, C.J.; Bates, O.M.; Olson, J.M.; Mehlin, C.; et al. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia 2018, 32, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.-E.; Bargou, R. Blinatumomab: A CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk. Lymphoma 2016, 57, 1021–1032. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Jimeno, A. Bispecific antibodies for cancer therapy: A review. Pharmacol. Ther. 2018, 185, 122–134. [Google Scholar] [CrossRef]
- Wu, L.; Seung, E.; Xu, L.; Rao, E.; Lord, D.M.; Wei, R.R.; Cortez-Retamozo, V.; Ospina, B.; Posternak, V.; Ulinski, G.; et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 2019, 1, 86–98. [Google Scholar] [CrossRef]
- Seung, E.; Xing, Z.; Wu, L.; Rao, E.; Cortez-Retamozo, V.; Ospina, B.; Chen, L.; Beil, C.; Song, Z.; Zhang, B.; et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature 2022, 603, 328–334. [Google Scholar] [CrossRef]
- Xu, L.; Pegu, A.; Rao, E.; Doria-Rose, N.; Beninga, J.; McKee, K.; Lord, D.M.; Wei, R.R.; Deng, G.; Louder, M.; et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 2017, 358, 85–90. [Google Scholar] [CrossRef]
- Linke, R.; Klein, A.; Seimetz, D. Catumaxomab: Clinical Development and Future Directions. mAbs 2010, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jen, E.Y.; Xu, Q.; Schetter, A.; Przepiorka, D.; Shen, Y.L.; Roscoe, D.; Sridhara, R.; Deisseroth, A.; Philip, R.; Farrell, A.T.; et al. FDA Approval: Blinatumomab for Patients with B-cell Precursor Acute Lymphoblastic Leukemia in Morphologic Remission with Minimal Residual Disease. Clin. Cancer Res. 2019, 25, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Simi, A.; Sabari, J.; Vijayaraghavan, S.; Moores, S.; Spira, A. Amivantamab, an Epidermal Growth Factor Receptor (EGFR) and Mesenchymal-epithelial Transition Factor (MET) Bispecific Antibody, Designed to Enable Multiple Mechanisms of Action and Broad Clinical Applications. Clin. Lung Cancer 2022, 24, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sahni, J.; Patel, S.S.; Dugel, P.U.; Khanani, A.M.; Jhaveri, C.D.; Wykoff, C.C.; Hershberger, V.S.; Pauly-Evers, M.; Sadikhov, S.; Szczesny, P.; et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema BOULEVARD Phase 2 Randomized Trial. Ophthalmology 2019, 126, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Byrne, H.; Conroy, P.J.; Whisstock, J.C.; O’kennedy, R.J. A tale of two specificities: Bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol. 2013, 31, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Kammila, S.; Das, D.; Bhatnagar, P.K.; Sunwoo, H.H.; Zayas-Zamora, G.; King, M.; Suresh, M.R. A rapid point of care immunoswab assay for SARS-CoV detection. J. Virol. Methods 2008, 152, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wagstaffe, S.J.; Hill, K.E.; Williams, D.W.; Randle, B.J.; Thomas, D.W.; Stephens, P.; Riley, D.J. Bispecific Antibody-Mediated Detection of the Staphylococcus aureus Thermonuclease. Anal. Chem. 2012, 84, 5876–5884. [Google Scholar] [CrossRef]
- Amaral, M.; Hölper, S.; Lange, C.; Jung, J.; Sjuts, H.; Weil, S.; Fischer, M.; Radoevic, K.; Rao, E. Engineered Technologies and Bioanalysis of multispecific Antibody Formats. J. Appl. Bioanal. 2020, 6, 26–51. [Google Scholar] [CrossRef]
- Tousi, F.; Jiang, Y.; Sivendran, S.; Song, Y.; Elliott, S.; Paiva, A.; Lund, A.; Albee, K.; Lee, K. Intact Protein Mass Spectrometry of Cell Culture Harvest Guides Cell Line Development for Trispecific Antibodies. Anal. Chem. 2020, 92, 2764–2769. [Google Scholar] [CrossRef]
- Li, H.; Saw, P.E.; Song, E. Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics. Cells Mol. Immunol. 2020, 17, 451–461. [Google Scholar] [CrossRef]
- Ridgway, J.B.B.; Presta, L.G.; Carter, P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. Des. Sel. 1996, 9, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Tsunoda, H.; Kikuchi, Y.; Yoshida, M.; Tanaka, M.; Koga, A.; Sekimori, Y.; Orita, T.; Aso, Y.; Hattori, K.; et al. VH/VL interface engineering to promote selective expression and inhibit conformational isomerization of thrombopoietin receptor agonist single-chain diabody. Protein Eng. Des. Sel. 2010, 23, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Furtmann, N.; Schneider, M.; Spindler, N.; Steinmann, B.; Li, Z.; Focken, I.; Meyer, J.; Dimova, D.; Kroll, K.; Leuschner, W.D.; et al. An end-to-end automated platform process for high-throughput engineering of next-generation multi-specific antibody therapeutics. mAbs 2021, 13, 1955433. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. A brief introduction of IgG-like bispecific antibody purification: Methods for removing product-related impurities. Protein Expr. Purif. 2019, 155, 112–119. [Google Scholar] [CrossRef]
- Wang, C.; Vemulapalli, B.; Cao, M.; Gadre, D.; Wang, J.; Hunter, A.; Wang, X.; Liu, D. A systematic approach for analysis and characterization of mispairing in bispecific antibodies with asymmetric architecture. mAbs 2018, 10, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Abecasis, B.; Aguiar, T.; Arnault, É.; Costa, R.; Gomes-Alves, P.; Aspegren, A.; Serra, M.; Alves, P.M. Expansion of 3D human induced pluripotent stem cell aggregates in bioreactors: Bioprocess intensification and scaling-up approaches. J. Biotechnol. 2017, 246, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, M.J.; Gomes-Alves, P.; Reis, I.; Sanchez, B.; Palacios, I.; Serra, M.; Alves, P.M. Bioreactor-based 3D human myocardial ischemia/reperfusion in vitro model: A novel tool to unveil key paracrine factors upon acute myocardial infarction. Transl. Res. 2020, 215, 57–74. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Alberti, S.; Böhse, K.; Arndt, V.; Schmitz, A.; Höhfeld, J. The Cochaperone HspBP1 Inhibits the CHIP Ubiquitin Ligase and Stimulates the Maturation of the Cystic Fibrosis Transmembrane Conductance Regulator. Mol. Biol. Cell 2004, 15, 4003–4010. [Google Scholar] [CrossRef]
- Blanco, N.; Williams, A.J.; Tang, D.; Zhan, D.; Misaghi, S.; Kelley, R.F.; Simmons, L.C. Tailoring translational strength using Kozak sequence variants improves bispecific antibody assembly and reduces product-related impurities in CHO cells. Biotechnol. Bioeng. 2020, 117, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; D’antona, A.M. Recent Advances in the Molecular Design and Applications of Multispecific Biotherapeutics. Antibodies 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; Wieczorek, A.; Lu, F.; Bruno, R.; Diaz, L.; Agrawal, N.J.; Daris, K. Culture temperature modulates half antibody and aggregate formation in a Chinese hamster ovary cell line expressing a bispecific antibody. Biotechnol. Bioeng. 2018, 115, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, P.; Aziz, A.H.; Majewska, N.I.; Ahuja, S.; Handlogten, M.W. Perfusion reduces bispecific antibody aggregation via mitigating mitochondrial dysfunction-induced glutathione oxidation and ER stress in CHO cells. Sci. Rep. 2020, 10, 16620. [Google Scholar] [CrossRef]
- Lee, A.P.; Kok, Y.J.; Lakshmanan, M.; Leong, D.; Zheng, L.; Lim, H.L.; Chen, S.; Mak, S.Y.; Ang, K.S.; Templeton, N.; et al. Multi-omics profiling of a CHO cell culture system unravels the effect of culture pH on cell growth, antibody titer, and product quality. Biotechnol. Bioeng. 2021, 118, 4305–4316. [Google Scholar] [CrossRef] [PubMed]
- Budge, J.D.; Knight, T.J.; Povey, J.; Roobol, J.; Brown, I.R.; Singh, G.; Dean, A.; Turner, S.; Jaques, C.M.; Young, R.J.; et al. Engineering of Chinese hamster ovary cell lipid metabolism results in an expanded ER and enhanced recombinant biotherapeutic protein production. Metab. Eng. 2020, 57, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Pybus, L.P.; Dean, G.; West, N.R.; Smith, A.; Daramola, O.; Field, R.; Wilkinson, S.J.; James, D.C. Model-directed engineering of “difficult-to-express” monoclonal antibody production by Chinese hamster ovary cells. Biotechnol. Bioeng. 2014, 111, 372–385. [Google Scholar] [CrossRef]
- Florin, L.; Pegel, A.; Becker, E.; Hausser, A.; Olayioye, M.A.; Kaufmann, H. Heterologous expression of the lipid transfer protein CERT increases therapeutic protein productivity of mammalian cells. J. Biotechnol. 2009, 141, 84–90. [Google Scholar] [CrossRef]
- Johari, Y.B.; Estes, S.D.; Alves, C.S.; Sinacore, M.S.; James, D.C. Integrated cell and process engineering for improved transient production of a “difficult-to-express” fusion protein by CHO cells. Biotechnol. Bioeng. 2015, 112, 2527–2542. [Google Scholar] [CrossRef]
- Orellana, C.A.; Marcellin, E.; Schulz, B.L.; Nouwens, A.S.; Gray, P.P.; Nielsen, L.K. High-Antibody-Producing Chinese Hamster Ovary Cells Up-Regulate Intracellular Protein Transport and Glutathione Synthesis. J. Proteome Res. 2015, 14, 609–618. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Halim, A.; Schulz, M.A.; Frodin, M.; Rahman, S.H.; Vester-Christensen, M.B.; Behrens, C.; Kristensen, C.; Vakhrushev, S.Y.; et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat. Biotechnol. 2015, 33, 842–844. [Google Scholar] [CrossRef]
- del Val, I.J.; Fan, Y.; Weilguny, D. Dynamics of immature mAb glycoform secretion during CHO cell culture: An integrated modelling framework. Biotechnol. J. 2016, 11, 610–623. [Google Scholar] [CrossRef]
- Henry, M.; Gallagher, C.; Kelly, R.M.; Frye, C.C.; Osborne, M.D.; Brady, C.P.; Barron, N.; Clynes, M.; Meleady, P. Clonal variation in productivity and proteolytic clipping of an Fc-fusion protein in CHO cells: Proteomic analysis suggests a role for defective protein folding and the UPR. J. Biotechnol. 2018, 281, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, F.; Xiao, Q.; Catterall, H.B.; Robinson, J.H.; Wang, Z.; Mock, M.; Hubert, R. Expression liabilities in a four-chain bispecific molecule. Biotechnol. Bioeng. 2021, 118, 3744–3759. [Google Scholar] [CrossRef] [PubMed]
- Bhoskar, P.; Belongia, B.; Smith, R.; Yoon, S.; Carter, T.; Xu, J. Free light chain content in culture media reflects recombinant monoclonal antibody productivity and quality. Biotechnol. Prog. 2013, 29, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Magistrelli, G.; Pontini, G.; Poitevin, Y.; Malinge, P.; Bourguignon, J.; Gauye, F.; Fleury, E.; Plèche, N.; Galissaires, L.; Fischer, N. Tuning Relative Polypeptide Expression to Optimize Assembly, Yield and Downstream Processing of Bispecific Antibodies. Antibodies 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.L.; Koh, E.Y.C.; van Beers, M.; Mueller, M.; Wan, C.; Teo, G.; Song, Z.; Tong, Y.W.; Bardor, M.; Yang, Y. Control of IgG LC:HC ratio in stably transfected CHO cells and study of the impact on expression, aggregation, glycosylation and conformational stability. J. Biotechnol. 2013, 165, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yugami, M.; Kabe, Y.; Yamaguchi, Y.; Wada, T.; Handa, H. hnRNP-U enhances the expression of specific genes by stabilizing mRNA. FEBS Lett. 2006, 581, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Briata, P.; Bordo, D.; Puppo, M.; Gorlero, F.; Rossi, M.; Perrone-Bizzozero, N.; Gherzi, R. Diverse roles of the nucleic acid-binding protein KHSRP in cell differentiation and disease. Wiley Interdiscip. Rev. RNA 2015, 7, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M. A History of Poly A Sequences: From Formation to Factors to Function. Prog. Nucleic Acid Res. Mol. Biol. 2002, 71, 285–389. [Google Scholar] [CrossRef]
- Stewart, J.D.; Cowan, J.L.; Perry, L.S.; Coldwell, M.J.; Proud, C.G. ABC50 mutants modify translation start codon selection. Biochem. J. 2015, 467, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Garnon, J.; Lachance, C.; Di Marco, S.; Hel, Z.; Marion, D.; Ruiz, M.C.; Newkirk, M.M.; Khandjian, E.W.; Radzioch, D. Fragile X-related Protein FXR1P Regulates Proinflammatory Cytokine Tumor Necrosis Factor Expression at the Post-transcriptional Level. J. Biol. Chem. 2005, 280, 5750–5763. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Park, J.T.; Oh, S. The translational landscape as regulated by the RNA helicase DDX3. BMB Rep. 2022, 55, 125–135. [Google Scholar] [CrossRef]
- Shih, J.-W.; Tsai, T.-Y.; Chao, C.-H.; Lee, Y.-H.W. Candidate tumor suppressor DDX3 RNA helicase specifically represses cap-dependent translation by acting as an eIF4E inhibitory protein. Oncogene 2007, 27, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Calviello, L.; Venkataramanan, S.; Rogowski, K.J.; Wyler, E.; Wilkins, K.; Tejura, M.; Thai, B.; Krol, J.; Filipowicz, W.; Landthaler, M.; et al. DDX3 depletion represses translation of mRNAs with complex 5′ UTRs. Nucleic Acids Res. 2021, 49, 5336–5350. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.; Kaneyoshi, K.; Omasa, T. Improvement of Intracellular Traffic System by Overexpression of KDEL Receptor 1 in Antibody-Producing CHO Cells. Biotechnol. J. 2020, 15, 1900352. [Google Scholar] [CrossRef]
- Moncan, M.; Mnich, K.; Blomme, A.; Almanza, A.; Samali, A.; Gorman, A.M. Regulation of lipid metabolism by the unfolded protein response. J. Cells Mol. Med. 2021, 25, 1359–1370. [Google Scholar] [CrossRef]
- Vergnes, L.; Chin, R.G.; de Aguiar Vallim, T.; Fong, L.G.; Osborne, T.F.; Young, S.G.; Reue, K. SREBP-2-deficient and hypomorphic mice reveal roles for SREBP-2 in embryonic development and SREBP-1c expression. J. Lipid Res. 2016, 57, 410–421. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Soccio, R.E.; Duncan, E.M.; Sehayek, E.; Breslow, J.L. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid Res. 2003, 44, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Shah, N.A.; Warrington, J.A.; Anderson, N.N.; Park, S.W.; Brown, M.S.; Goldstein, J.L. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA 2003, 100, 12027–12032. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Sasaki-Osugi, K.; Oku, M.; Sawaguchi, S.; Tanakura, S.; Kawai, Y.; Wakabayashi, S.; Yoshida, H. MLX Is a Transcriptional Repressor of the Mammalian Golgi Stress Response. Cell Struct. Funct. 2016, 41, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.L.; Kyritsis, C.; Tampé, R.; Cresswell, P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat. Immunol. 2005, 6, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Popoff, V. Tracing the Retrograde Route in Protein Trafficking. Cell 2008, 135, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jönsson, F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front. Immunol. 2019, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of Fucose on Human IgG1 N-Linked Oligosaccharide Improves Binding to Human FcγRIII and Antibody-dependent Cellular Toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Cambay, F.; Forest-Nault, C.; Dumoulin, L.; Seguin, A.; Henry, O.; Durocher, Y.; De Crescenzo, G. Glycosylation of Fcγ receptors influences their interaction with various IgG1 glycoforms. Mol. Immunol. 2020, 121, 144–158. [Google Scholar] [CrossRef]
- Yogo, R.; Yamaguchi, Y.; Watanabe, H.; Yagi, H.; Satoh, T.; Nakanishi, M.; Onitsuka, M.; Omasa, T.; Shimada, M.; Maruno, T.; et al. The Fab portion of immunoglobulin G contributes to its binding to Fcγ receptor III. Sci. Rep. 2019, 9, 11957. [Google Scholar] [CrossRef]






| Transcript | Forward | Reverse |
|---|---|---|
| β-actin | 5′-ATGACGATATCGCTGCGCTC-3′ | 5′-ATGGCTACGTACATGGCTGG-3′ |
| Ntn1 | 5′-ACTGTGACTCCTATTGCAAGGC-3′ | 5′-TTGTC CGCTTTCAGGATGTGGA-3′ |
| Fcho1 | 5′-CTGCTGTCCAAGAACCTCTTCG-3′ | 5′-AAAGGGGATGGGCTGGATGTGA-3′ |
| Eps | 5′-GGCTCAATGACCACGGCAAGAA-3′ | 5′-ACTGGAAGTCCTTCAGCGTCTG-3′ |
| Rpl28 | 5′-CCACCATCAACAAGAATGCACGG-3′ | 5′-GTGCGCTTTCTCTTCACCACCA-3′ |
| Fbxl20 | 5′-CAGTAACTGGCAACGGATAGACC-3′ | 5′-CCTACTCCAAGACACCCACGAA-3′ |
| Bicd1 | 5′-CATCAAGGAAAGGAGAATCC-3′ | 5′-GTTTGTGACTCCTGGAGGTTGG-3′ |
| Ccl2 | 5′-GCTACAAGAGGATCACCAGCAG-3′ | 5′-GTCTGGACCCATTCCTTCTTGG-3′ |
| Grhl2 | 5′-GGACGTGAATGAAGAGGCAAAG-3′ | 5′-TTGACAGTACGCTCTGTGGATG-3′ |
| S100a16 | 5′-TGTTTCCAAGCACAGCCTGGTC-3′ | 5′-TGGTTGGCATCCAGGTTCTGGA-3′ |
| Vasn | 5′-CCAGCG TCCACCTGCCTGAATG-3′ | 5′-CTTGCCTCACAGGACTCTCACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastião, M.J.; Hoffman, M.; Escandell, J.; Tousi, F.; Zhang, J.; Figueroa, B.; DeMaria, C.; Gomes-Alves, P. Identification of Mispairing Omic Signatures in Chinese Hamster Ovary (CHO) Cells Producing a Tri-Specific Antibody. Biomedicines 2023, 11, 2890. https://doi.org/10.3390/biomedicines11112890
Sebastião MJ, Hoffman M, Escandell J, Tousi F, Zhang J, Figueroa B, DeMaria C, Gomes-Alves P. Identification of Mispairing Omic Signatures in Chinese Hamster Ovary (CHO) Cells Producing a Tri-Specific Antibody. Biomedicines. 2023; 11(11):2890. https://doi.org/10.3390/biomedicines11112890
Chicago/Turabian StyleSebastião, Maria João, Michael Hoffman, José Escandell, Fatemeh Tousi, Jin Zhang, Bruno Figueroa, Christine DeMaria, and Patrícia Gomes-Alves. 2023. "Identification of Mispairing Omic Signatures in Chinese Hamster Ovary (CHO) Cells Producing a Tri-Specific Antibody" Biomedicines 11, no. 11: 2890. https://doi.org/10.3390/biomedicines11112890
APA StyleSebastião, M. J., Hoffman, M., Escandell, J., Tousi, F., Zhang, J., Figueroa, B., DeMaria, C., & Gomes-Alves, P. (2023). Identification of Mispairing Omic Signatures in Chinese Hamster Ovary (CHO) Cells Producing a Tri-Specific Antibody. Biomedicines, 11(11), 2890. https://doi.org/10.3390/biomedicines11112890







