The Role and Therapeutic Implications of Inflammation in the Pathogenesis of Brain Arteriovenous Malformations
Abstract
1. Overview
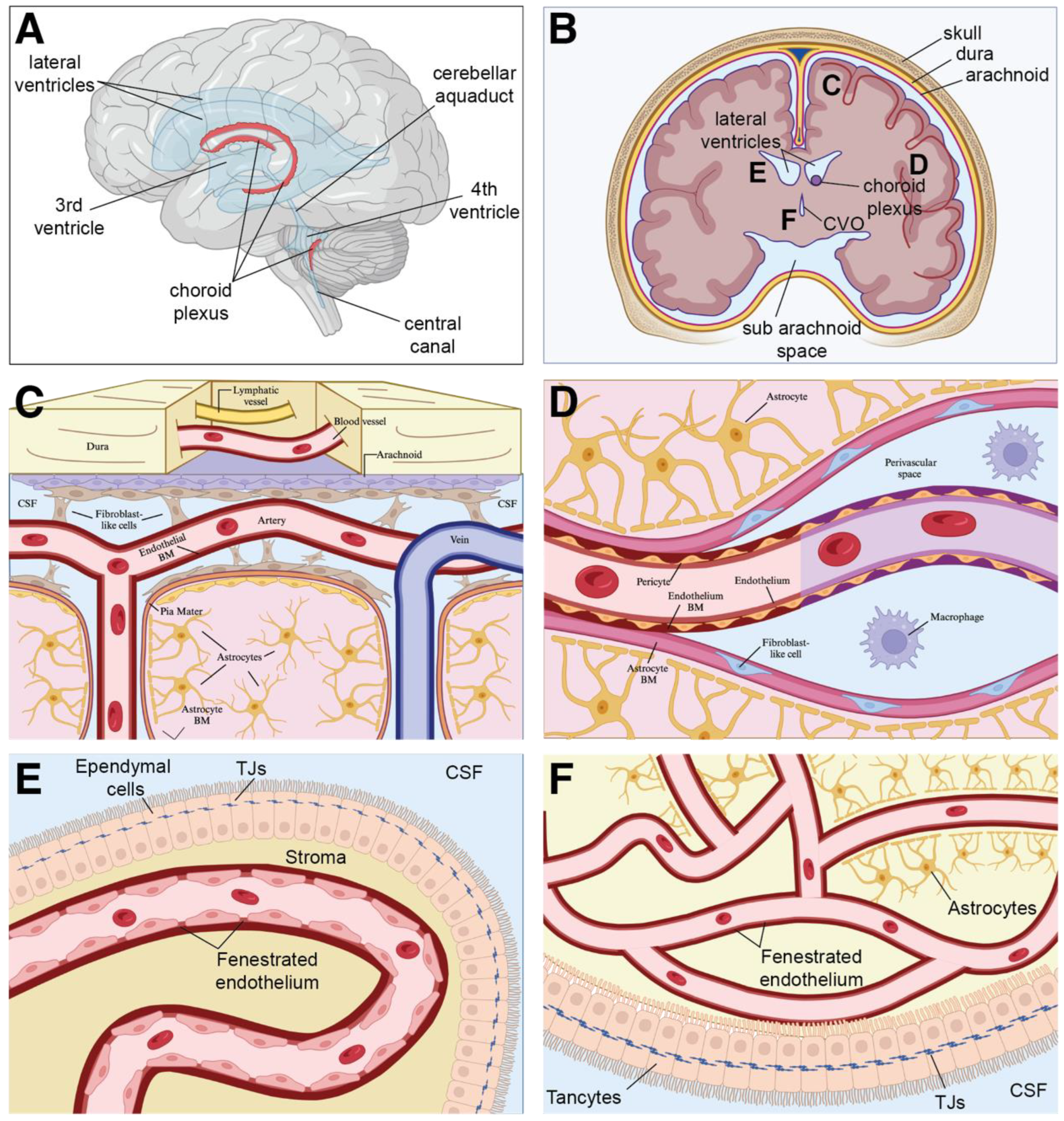
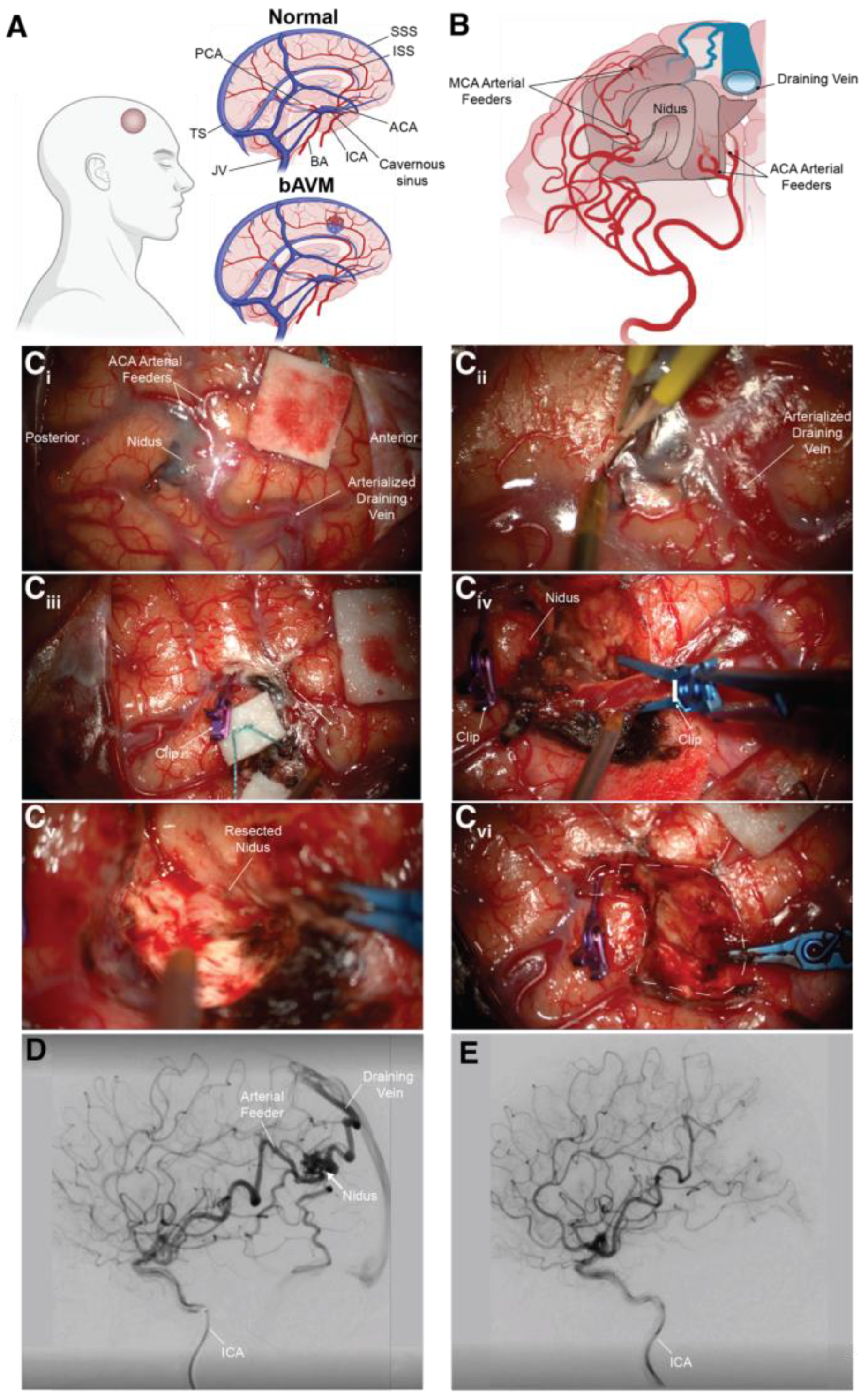
2. The Etiology and Sequalae of Brain Arteriovenous Malformations
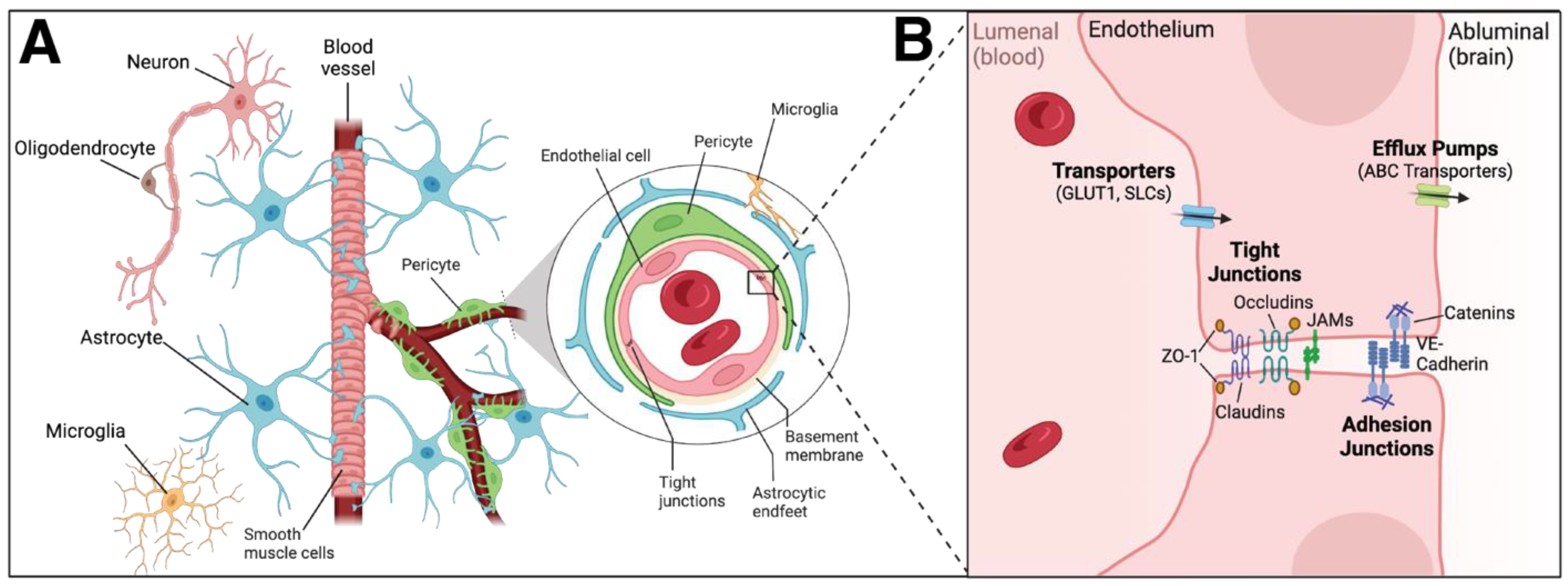
3. Inflammation and the Neurovascular Unit
4. Immune Cell Populations within the Brain
4.1. Resident Immune Cells in the Brain—Microglia
4.2. Resident Cells in the Brain—Astrocytes
4.3. Resident Cells in the Brain—Pericytes
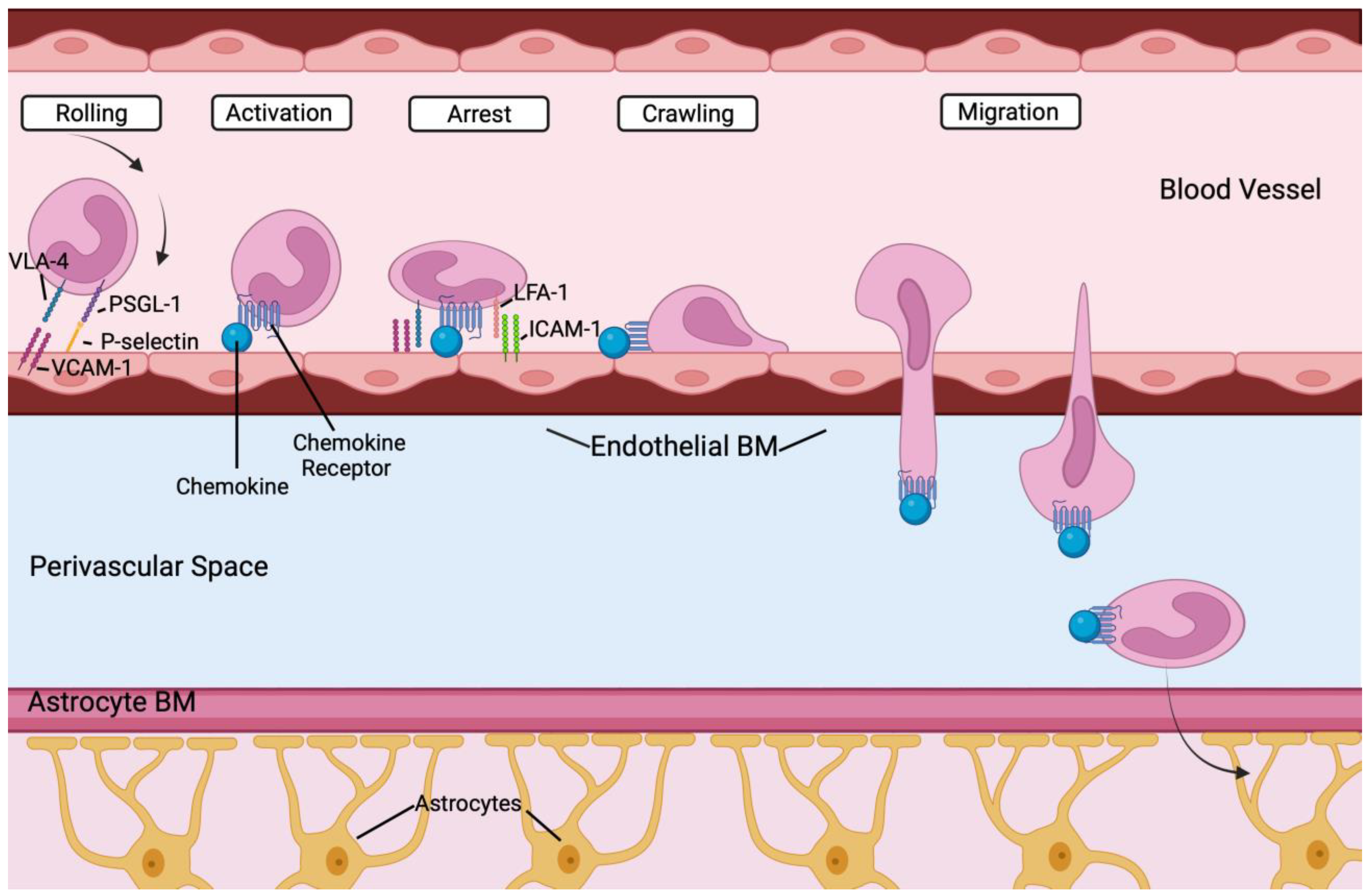
4.4. Peripheral Immune Cells in the Brain
4.5. Monocytes
4.6. Dendritic Cells
4.7. Neutrophils
4.8. T and B Cells
5. Inflammation, bAVM Rupture, and Intracranial Hemorrhage
6. Genetic Variants Associated with Increased Risk of bAVM Rupture
Inflammatory Genes and Biomarkers in Unruptured bAVM
7. Anti-Inflammatory Therapies for Treatment of bAVMs
8. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalton, A.; Dobson, G.; Prasad, M.; Mukerji, N. De novo intracerebral arteriovenous malformations and a review of the theories of their formation. Br. J. Neurosurg. 2018, 32, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.T.; Suen, J.Y. Clinical course of arteriovenous malformations of the head and neck: A case series. Otolaryngol. Head. Neck Surg. 2010, 142, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Uller, W.; Alomari, A.I.; Richter, G.T. Arteriovenous malformations. Semin. Pediatr. Surg. 2014, 23, 203–207. [Google Scholar] [CrossRef]
- Mulligan, P.R.; Prajapati, H.J.; Martin, L.G.; Patel, T.H. Vascular anomalies: Classification, imaging characteristics and implications for interventional radiology treatment approaches. Br. J. Radiol. 2014, 87, 20130392. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Rutledge, W.C.; Kim, H.; Stapf, C.; Whitehead, K.J.; Li, D.Y.; Krings, T.; terBrugge, K.; Kondziolka, D.; Morgan, M.K.; et al. Brain arteriovenous malformations. Nat. Rev. Dis. Primers 2015, 1, 15008. [Google Scholar] [CrossRef]
- Meyer-Heim, A.D.; Boltshauser, E. Spontaneous intracranial haemorrhage in children: Aetiology, presentation and outcome. Brain Dev. 2003, 25, 416–421. [Google Scholar] [CrossRef]
- Celli, P.; Ferrante, L.; Palma, L.; Cavedon, G. Cerebral arteriovenous malformations in children. Clinical features and outcome of treatment in children and in adults. Surg. Neurol. 1984, 22, 43–49. [Google Scholar] [CrossRef]
- Hernesniemi, J.A.; Dashti, R.; Juvela, S.; Vaart, K.; Niemela, M.; Laakso, A. Natural history of brain arteriovenous malformations: A long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 2008, 63, 823–829; discussion 829–831. [Google Scholar] [CrossRef]
- Kim, H.; Sidney, S.; McCulloch, C.E.; Poon, K.Y.; Singh, V.; Johnston, S.C.; Ko, N.U.; Achrol, A.S.; Lawton, M.T.; Higashida, R.T.; et al. Racial/Ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke 2007, 38, 2430–2437. [Google Scholar] [CrossRef]
- Rutledge, W.C.; Ko, N.U.; Lawton, M.T.; Kim, H. Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Transl. Stroke Res. 2014, 5, 538–542. [Google Scholar] [CrossRef]
- Stapf, C.; Mast, H.; Sciacca, R.R.; Choi, J.H.; Khaw, A.V.; Connolly, E.S.; Pile-Spellman, J.; Mohr, J.P. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006, 66, 1350–1355. [Google Scholar] [CrossRef]
- Yamada, S.; Takagi, Y.; Nozaki, K.; Kikuta, K.; Hashimoto, N. Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J. Neurosurg. 2007, 107, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Al-Shahi Salman, R.; McCulloch, C.E.; Stapf, C.; Young, W.L.; Coinvestigators, M. Untreated brain arteriovenous malformation: Patient-level meta-analysis of hemorrhage predictors. Neurology 2014, 83, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Buscarini, E.; Botella, L.M.; Geisthoff, U.; Kjeldsen, A.D.; Mager, H.J.; Pagella, F.; Suppressa, P.; Zarrabeitia, R.; Dupuis-Girod, S.; Shovlin, C.L.; et al. Safety of thalidomide and bevacizumab in patients with hereditary hemorrhagic telangiectasia. Orphanet J. Rare Dis. 2019, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Fults, D.; Kelly, D.L., Jr. Natural history of arteriovenous malformations of the brain: A clinical study. Neurosurgery 1984, 15, 658–662. [Google Scholar] [CrossRef]
- van Beijnum, J.; Lovelock, C.E.; Cordonnier, C.; Rothwell, P.M.; Klijn, C.J.; Al-Shahi Salman, R.; Committee, S.S.; SIVMS Steering Committee and the Oxford Vascular Study. Outcome after spontaneous and arteriovenous malformation-related intracerebral haemorrhage: Population-based studies. Brain 2009, 132, 537–543. [Google Scholar] [CrossRef]
- Brown, R.D., Jr.; Wiebers, D.O.; Forbes, G.; O’Fallon, W.M.; Piepgras, D.G.; Marsh, W.R.; Maciunas, R.J. The natural history of unruptured intracranial arteriovenous malformations. J. Neurosurg. 1988, 68, 352–357. [Google Scholar] [CrossRef]
- Morris, Z.; Whiteley, W.N.; Longstreth, W.T., Jr.; Weber, F.; Lee, Y.C.; Tsushima, Y.; Alphs, H.; Ladd, S.C.; Warlow, C.; Wardlaw, J.M.; et al. Incidental findings on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2009, 339, b3016. [Google Scholar] [CrossRef]
- Gabriel, R.A.; Kim, H.; Sidney, S.; McCulloch, C.E.; Singh, V.; Johnston, S.C.; Ko, N.U.; Achrol, A.S.; Zaroff, J.G.; Young, W.L. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke 2010, 41, 21–26. [Google Scholar] [CrossRef]
- ApSimon, H.T.; Reef, H.; Phadke, R.V.; Popovic, E.A. A population-based study of brain arteriovenous malformation: Long-term treatment outcomes. Stroke 2002, 33, 2794–2800. [Google Scholar] [CrossRef]
- McDonald, J.; Stevenson, D.A. Hereditary Hemorrhagic Telangiectasia. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Buscarini, E.; Danesino, C.; Olivieri, C.; Lupinacci, G.; De Grazia, F.; Reduzzi, L.; Blotta, P.; Gazzaniga, P.; Pagella, F.; Grosso, M.; et al. Doppler ultrasonographic grading of hepatic vascular malformations in hereditary hemorrhagic telangiectasia -- results of extensive screening. Ultraschall Med. 2004, 25, 348–355. [Google Scholar] [CrossRef]
- Ianora, A.A.; Memeo, M.; Sabba, C.; Cirulli, A.; Rotondo, A.; Angelelli, G. Hereditary hemorrhagic telangiectasia: Multi-detector row helical CT assessment of hepatic involvement. Radiology 2004, 230, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Iyer, V.N.; Wood, C.P.; Lanzino, G. Prevalence and characteristics of brain arteriovenous malformations in hereditary hemorrhagic telangiectasia: A systematic review and meta-analysis. J. Neurosurg. 2017, 127, 302–310. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Lang-Robertson, K.; Buscarini, E.; Deslandres, E.; Kasthuri, R.S.; Lausman, A.; Poetker, D.; et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2020, 173, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.A.; Sanz-Rodriguez, F.; Blanco, F.J.; Bernabeu, C.; Botella, L.M. Hereditary hemorrhagic telangiectasia, a vascular dysplasia affecting the TGF-beta signaling pathway. Clin. Med. Res. 2006, 4, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Hetts, S.W.; Shieh, J.T.; Ohliger, M.A.; Conrad, M.B. Hereditary Hemorrhagic Telangiectasia: The Convergence of Genotype, Phenotype, and Imaging in Modern Diagnosis and Management of a Multisystem Disease. Radiology 2021, 300, 17–30. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-beta Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- McAllister, K.A.; Lennon, F.; Bowles-Biesecker, B.; McKinnon, W.C.; Helmbold, E.A.; Markel, D.S.; Jackson, C.E.; Guttmacher, A.E.; Pericak-Vance, M.A.; Marchuk, D.A. Genetic heterogeneity in hereditary haemorrhagic telangiectasia: Possible correlation with clinical phenotype. J. Med. Genet. 1994, 31, 927–932. [Google Scholar] [CrossRef][Green Version]
- Shovlin, C.L.; Hughes, J.M.; Scott, J.; Seidman, C.E.; Seidman, J.G. Characterization of endoglin and identification of novel mutations in hereditary hemorrhagic telangiectasia. Am. J. Hum. Genet. 1997, 61, 68–79. [Google Scholar] [CrossRef]
- Johnson, D.W.; Berg, J.N.; Baldwin, M.A.; Gallione, C.J.; Marondel, I.; Yoon, S.J.; Stenzel, T.T.; Speer, M.; Pericak-Vance, M.A.; Diamond, A.; et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet. 1996, 13, 189–195. [Google Scholar] [CrossRef]
- Gallione, C.J.; Repetto, G.M.; Legius, E.; Rustgi, A.K.; Schelley, S.L.; Tejpar, S.; Mitchell, G.; Drouin, E.; Westermann, C.J.; Marchuk, D.A. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004, 363, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, S.; Graves, T.J.; Shimonty, A.; Kerr, K.; Kilner, J.; Xiao, S.; Slade, R.; Sroya, M.; Alikian, M.; Curetean, E.; et al. Identification and validation of a novel pathogenic variant in GDF2 (BMP9) responsible for hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. Am. J. Med. Genet. A 2022, 188, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Wooderchak-Donahue, W.L.; McDonald, J.; O’Fallon, B.; Upton, P.D.; Li, W.; Roman, B.L.; Young, S.; Plant, P.; Fulop, G.T.; Langa, C.; et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am. J. Hum. Genet. 2013, 93, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.M.; Roman, B.L. An update on preclinical models of hereditary haemorrhagic telangiectasia: Insights into disease mechanisms. Front. Med. 2022, 9, 973964. [Google Scholar] [CrossRef]
- Drapé, E.; Anquetil, T.; Larrivée, B.; Dubrac, A. Brain arteriovenous malformation in hereditary hemorrhagic telangiectasia: Recent advances in cellular and molecular mechanisms. Front. Hum. Neurosci. 2022, 16, 1006115. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Bernabeu, C. The physiological role of endoglin in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H959–H974. [Google Scholar] [CrossRef]
- Ruiz-Llorente, L.; Gallardo-Vara, E.; Rossi, E.; Smadja, D.M.; Botella, L.M.; Bernabeu, C. Endoglin and alk1 as therapeutic targets for hereditary hemorrhagic telangiectasia. Expert. Opin. Ther. Targets 2017, 21, 933–947. [Google Scholar] [CrossRef]
- Snellings, D.A.; Gallione, C.J.; Clark, D.S.; Vozoris, N.T.; Faughnan, M.E.; Marchuk, D.A. Somatic Mutations in Vascular Malformations of Hereditary Hemorrhagic Telangiectasia Result in Bi-allelic Loss of ENG or ACVRL1. Am. J. Hum. Genet. 2019, 105, 894–906. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Rezai Jahromi, B.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.R.; et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef]
- Bameri, O.; Salarzaei, M.; Parooie, F. KRAS/BRAF mutations in brain arteriovenous malformations: A systematic review and meta-analysis. Interv. Neuroradiol. 2021, 27, 539–546. [Google Scholar] [CrossRef]
- Gao, S.; Nelson, J.; Weinsheimer, S.; Winkler, E.A.; Rutledge, C.; Abla, A.A.; Gupta, N.; Shieh, J.T.; Cooke, D.L.; Hetts, S.W.; et al. Somatic mosaicism in the MAPK pathway in sporadic brain arteriovenous malformation and association with phenotype. J. Neurosurg. 2022, 136, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.A.; Huang, A.Y.; Smith, E.; Konczyk, D.J.; Smits, P.J.; Sudduth, C.L.; Stapleton, C.; Patel, A.; Alexandrescu, S.; Warman, M.L.; et al. Somatic mutations in intracranial arteriovenous malformations. PLoS ONE 2019, 14, e0226852. [Google Scholar] [CrossRef]
- Hong, T.; Yan, Y.; Li, J.; Radovanovic, I.; Ma, X.; Shao, Y.W.; Yu, J.; Ma, Y.; Zhang, P.; Ling, F.; et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 2019, 142, 23–34. [Google Scholar] [CrossRef]
- Oka, M.; Kushamae, M.; Aoki, T.; Yamaguchi, T.; Kitazato, K.; Abekura, Y.; Kawamata, T.; Mizutani, T.; Miyamoto, S.; Takagi, Y. KRAS G12D or G12V Mutation in Human Brain Arteriovenous Malformations. World Neurosurg. 2019, 126, e1365–e1373. [Google Scholar] [CrossRef] [PubMed]
- Priemer, D.S.; Vortmeyer, A.O.; Zhang, S.; Chang, H.Y.; Curless, K.L.; Cheng, L. Activating KRAS mutations in arteriovenous malformations of the brain: Frequency and clinicopathologic correlation. Hum. Pathol. 2019, 89, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.A.; Huang, A.Y.; Konczyk, D.J.; Goss, J.A.; Fishman, S.J.; Mulliken, J.B.; Warman, M.L.; Greene, A.K. Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation. Am. J. Hum. Genet. 2017, 100, 546–554. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, S.; Liu, B.; Zhang, Q.; Li, Y.; Liu, J.; Shen, Y.; Ding, X.; Lin, J.; Wu, Y.; et al. Perturbations of BMP/TGF-beta and VEGF/VEGFR signalling pathways in non-syndromic sporadic brain arteriovenous malformations (BAVM). J. Med. Genet. 2018, 55, 675–684. [Google Scholar] [CrossRef]
- Fukui, H.; Hanaoka, R.; Kawahara, A. Noncanonical activity of seryl-tRNA synthetase is involved in vascular development. Circ. Res. 2009, 104, 1253–1259. [Google Scholar] [CrossRef]
- Herzog, W.; Müller, K.; Huisken, J.; Stainier, D.Y.R. Genetic Evidence for a Noncanonical Function of Seryl-tRNA Synthetase in Vascular Development. Circ. Res. 2009, 104, 1260–1266. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, C.; Li, H.; Shi, L.; Liu, N.; Sun, Y.; Lu, S.; Liu, Y.; Sun, L.; Li, X.; et al. Nir1 promotes invasion of breast cancer cells by binding to chemokine (C-C motif) ligand 18 through the PI3K/Akt/GSK3beta/Snail signalling pathway. Eur. J. Cancer 2013, 49, 3900–3913. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, X.; Zhang, Q.; Liu, J.; Zhang, Y.; Zhang, Y.; Tian, Z.; Li, W.; Zhu, W.; Kang, H.; et al. Exome sequencing of 112 trios identifies recessive genetic variants in brain arteriovenous malformations. J. Neurointerv Surg. 2021, 13, 568–573. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, B.; Sun, Z.; Wu, Y.; Shi, W.; Wang, J.; Meng, X.; Ge, W.; Wang, G. Quantitative protein profiling and pathway analysis of spinal arteriovenous malformations. Microvasc. Res. 2018, 120, 47–54. [Google Scholar] [CrossRef]
- Wallace, S.E.; Regalado, E.S.; Gong, L.; Janda, A.L.; Guo, D.C.; Russo, C.F.; Kulmacz, R.J.; Hanna, N.; Jondeau, G.; Boileau, C.; et al. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genet. Med. 2019, 21, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lapek, J.; Fujimura, K.; Strnadel, J.; Liu, B.; Gonzalez, D.J.; Zhang, W.; Watson, F.; Yu, V.; Liu, C.; et al. Pseudopodium-enriched atypical kinase 1 mediates angiogenesis by modulating GATA2-dependent VEGFR2 transcription. Cell Discov. 2018, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Patel, S.; Shah, J. Emerging role of Piezo ion channels in cardiovascular development. Dev. Dyn. 2022, 251, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.R.; Dhawan, A.; Farach-Carson, M.C. Modular Proteoglycan Perlecan/HSPG2: Mutations, Phenotypes, and Functions. Genes 2018, 9, 556. [Google Scholar] [CrossRef]
- Pera, J.; Korostynski, M.; Krzyszkowski, T.; Czopek, J.; Slowik, A.; Dziedzic, T.; Piechota, M.; Stachura, K.; Moskala, M.; Przewlocki, R.; et al. Gene expression profiles in human ruptured and unruptured intracranial aneurysms: What is the role of inflammation? Stroke 2010, 41, 224–231. [Google Scholar] [CrossRef]
- Catapano, J.S.; Frisoli, F.A.; Nguyen, C.L.; Labib, M.A.; Cole, T.S.; Baranoski, J.F.; Kim, H.; Spetzler, R.F.; Lawton, M.T. Intermediate-grade brain arteriovenous malformations and the boundary of operability using the supplemented Spetzler-Martin grading system. J. Neurosurg. 2022, 136, 125–133. [Google Scholar] [CrossRef]
- Hafez, A.; Koroknay-Pal, P.; Oulasvirta, E.; Elseoud, A.A.; Lawton, M.T.; Niemela, M.; Laakso, A. The Application of the Novel Grading Scale (Lawton-Young Grading System) to Predict the Outcome of Brain Arteriovenous Malformation. Neurosurgery 2019, 84, 529–536. [Google Scholar] [CrossRef]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 1986, 65, 476–483. [Google Scholar] [CrossRef]
- Zaki Ghali, M.G.; Kan, P.; Britz, G.W. Curative Embolization of Arteriovenous Malformations. World Neurosurg. 2019, 129, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T. The role of AVM microsurgery in the aftermath of a randomized trial of unruptured brain arteriovenous malformations. AJNR Am. J. Neuroradiol. 2015, 36, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Derdeyn, C.P.; Zipfel, G.J.; Albuquerque, F.C.; Cooke, D.L.; Feldmann, E.; Sheehan, J.P.; Torner, J.C.; American Heart Association Stroke, C. Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e200–e224. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Casasco, A.; Kusak, M.E.; Martinez, N.; Rey, G.; Martinez, R. Results for a series of 697 arteriovenous malformations treated by gamma knife: Influence of angiographic features on the obliteration rate. Neurosurgery 2014, 75, 568–583, dicussion 582–563, quiz 583. [Google Scholar] [CrossRef]
- Ilyas, A.; Chen, C.J.; Ding, D.; Taylor, D.G.; Moosa, S.; Lee, C.C.; Cohen-Inbar, O.; Sheehan, J.P. Volume-staged versus dose-staged stereotactic radiosurgery outcomes for large brain arteriovenous malformations: A systematic review. J. Neurosurg. 2018, 128, 154–164. [Google Scholar] [CrossRef]
- Karlsson, B.; Lindqvist, M.; Blomgren, H.; Wan-Yeo, G.; Soderman, M.; Lax, I.; Yamamoto, M.; Bailes, J. Long-term results after fractionated radiation therapy for large brain arteriovenous malformations. Neurosurgery 2005, 57, 42–49, dicussion 42–49. [Google Scholar] [CrossRef]
- Moosa, S.; Chen, C.J.; Ding, D.; Lee, C.C.; Chivukula, S.; Starke, R.M.; Yen, C.P.; Xu, Z.; Sheehan, J.P. Volume-staged versus dose-staged radiosurgery outcomes for large intracranial arteriovenous malformations. Neurosurg. Focus. 2014, 37, E18. [Google Scholar] [CrossRef]
- Pollock, B.E.; Kline, R.W.; Stafford, S.L.; Foote, R.L.; Schomberg, P.J. The rationale and technique of staged-volume arteriovenous malformation radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 817–824. [Google Scholar] [CrossRef]
- Edwards, E.A.; Phelps, A.S.; Cooke, D.; Frieden, I.J.; Zapala, M.A.; Fullerton, H.J.; Shimano, K.A. Monitoring Arteriovenous Malformation Response to Genotype-Targeted Therapy. Pediatrics 2020, 146, e20193206. [Google Scholar] [CrossRef]
- Fish, J.E.; Flores Suarez, C.P.; Boudreau, E.; Herman, A.M.; Gutierrez, M.C.; Gustafson, D.; DiStefano, P.V.; Cui, M.; Chen, Z.; De Ruiz, K.B.; et al. Somatic Gain of KRAS Function in the Endothelium Is Sufficient to Cause Vascular Malformations That Require MEK but Not PI3K Signaling. Circ. Res. 2020, 127, 727–743. [Google Scholar] [CrossRef]
- Lekwuttikarn, R.; Lim, Y.H.; Admani, S.; Choate, K.A.; Teng, J.M.C. Genotype-Guided Medical Treatment of an Arteriovenous Malformation in a Child. JAMA Dermatol. 2019, 155, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Soon, K.; Li, M.; Wu, R.; Zhou, A.; Khosraviani, N.; Turner, W.D.; Wythe, J.D.; Fish, J.E.; Nunes, S.S. A human model of arteriovenous malformation (AVM)-on-a-chip reproduces key disease hallmarks and enables drug testing in perfused human vessel networks. Biomaterials 2022, 288, 121729. [Google Scholar] [CrossRef]
- Winkler, E.; Wu, D.; Gil, E.; McCoy, D.; Narsinh, K.; Sun, Z.; Mueller, K.; Ross, J.; Kim, H.; Weinsheimer, S.; et al. Endoluminal Biopsy for Molecular Profiling of Human Brain Vascular Malformations. Neurology 2022, 98, e1637–e1647. [Google Scholar] [CrossRef] [PubMed]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Allen, S.; Blake, J.F.; Bowcut, V.; Briere, D.M.; Calinisan, A.; Dahlke, J.R.; Fell, J.B.; Fischer, J.P.; Gunn, R.J.; et al. Identification of MRTX1133, a Noncovalent, Potent, and Selective KRAS(G12D) Inhibitor. J. Med. Chem. 2022, 65, 3123–3133. [Google Scholar] [CrossRef] [PubMed]
- Kun, E.; Tsang, Y.T.M.; Ng, C.W.; Gershenson, D.M.; Wong, K.K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 2021, 92, 102137. [Google Scholar] [CrossRef]
- Proietti, I.; Skroza, N.; Bernardini, N.; Tolino, E.; Balduzzi, V.; Marchesiello, A.; Michelini, S.; Volpe, S.; Mambrin, A.; Mangino, G.; et al. Mechanisms of Acquired BRAF Inhibitor Resistance in Melanoma: A Systematic Review. Cancers 2020, 12, 2801. [Google Scholar] [CrossRef]
- Blaquier, J.B.; Cardona, A.F.; Recondo, G. Resistance to KRAS(G12C) Inhibitors in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 787585. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. The KRAS-G12C inhibitor: Activity and resistance. Cancer Gene Ther. 2022, 29, 875–878. [Google Scholar] [CrossRef]
- Boon, L.M.; Dekeuleneer, V.; Coulie, J.; Marot, L.; Bataille, A.-C.; Hammer, F.; Clapuyt, P.; Jeanjean, A.; Dompmartin, A.; Vikkula, M. Case report study of thalidomide therapy in 18 patients with severe arteriovenous malformations. Nat. Cardiovasc. Res. 2022, 1, 562–567. [Google Scholar] [CrossRef]
- Lebrin, F.; Srun, S.; Raymond, K.; Martin, S.; van den Brink, S.; Freitas, C.; Breant, C.; Mathivet, T.; Larrivee, B.; Thomas, J.L.; et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat. Med. 2010, 16, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, W.; Zou, D.; Wang, L.; Bao, C.; Zhan, L.; Saw, D.; Wang, S.; Winkler, E.; Li, Z.; et al. Thalidomide Reduces Hemorrhage of Brain Arteriovenous Malformations in a Mouse Model. Stroke 2018, 49, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Tu, S.; Shao, A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1079. [Google Scholar] [CrossRef]
- Herz, J.; Filiano, A.J.; Wiltbank, A.T.; Yogev, N.; Kipnis, J. Myeloid Cells in the Central Nervous System. Immunity 2017, 46, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Wieghofer, P.; Jordao, M.J.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M.; et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Prinz, M.; Geissmann, F.; Gomez Perdiguero, E. Development and function of tissue resident macrophages in mice. Semin. Immunol. 2015, 27, 369–378. [Google Scholar] [CrossRef]
- Prinz, M.; Erny, D.; Hagemeyer, N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 2017, 18, 385–392. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.H.; Hong, Y.K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.L.; Xia, Z.L.; Wang, J.R.; Yuan, H.; Li, W.X.; Chen, Y.S.; Yang, M.F.; Zhang, S.M. Effects of blockade of cerebral lymphatic drainage on regional cerebral blood flow and brain edema after subarachnoid hemorrhage. Clin. Hemorheol. Microcirc. 2006, 34, 227–232. [Google Scholar] [PubMed]
- Hsu, M.; Rayasam, A.; Kijak, J.A.; Choi, Y.H.; Harding, J.S.; Marcus, S.A.; Karpus, W.J.; Sandor, M.; Fabry, Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 2019, 10, 229. [Google Scholar] [CrossRef]
- Kovacs, M.A.; Cowan, M.N.; Babcock, I.W.; Sibley, L.A.; Still, K.; Batista, S.J.; Labuzan, S.A.; Sethi, I.; Harris, T.H. Meningeal lymphatic drainage promotes T cell responses against Toxoplasma gondii but is dispensable for parasite control in the brain. eLife 2022, 11, e80775. [Google Scholar] [CrossRef]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Kipnis, J. Brain borders at the central stage of neuroimmunology. Nature 2022, 612, 417–429. [Google Scholar] [CrossRef]
- Krithika, S.; Sumi, S. Neurovascular inflammation in the pathogenesis of brain arteriovenous malformations. J. Cell Physiol. 2021, 236, 4841–4856. [Google Scholar] [CrossRef] [PubMed]
- Mundt, S.; Mrdjen, D.; Utz, S.G.; Greter, M.; Schreiner, B.; Becher, B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 2019, 4, eaau8380. [Google Scholar] [CrossRef]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, K.; Li, P.; Zhu, L.; Xu, J.; Yang, B.; Hu, X.; Lu, Z.; Chen, J. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res. Rev. 2017, 34, 77–87. [Google Scholar] [CrossRef]
- Forro, T.; Bajko, Z.; Balasa, A.; Balasa, R. Dysfunction of the Neurovascular Unit in Ischemic Stroke: Highlights on microRNAs and Exosomes as Potential Biomarkers and Therapy. Int. J. Mol. Sci. 2021, 22, 5621. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A critical role in ischemic stroke. CNS Neurosci. Ther. 2021, 27, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zarekiani, P.; Nogueira Pinto, H.; Hol, E.M.; Bugiani, M.; de Vries, H.E. The neurovascular unit in leukodystrophies: Towards solving the puzzle. Fluids Barriers CNS 2022, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Pansieri, J.; Hadley, G.; Lockhart, A.; Pisa, M.; DeLuca, G.C. Regional contribution of vascular dysfunction in white matter dementia: Clinical and neuropathological insights. Front. Neurol. 2023, 14, 1199491. [Google Scholar] [CrossRef]
- Cramer, S.P.; Simonsen, H.; Frederiksen, J.L.; Rostrup, E.; Larsson, H.B. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin. 2014, 4, 182–189. [Google Scholar] [CrossRef]
- Geraldes, R.; Esiri, M.M.; DeLuca, G.C.; Palace, J. Age-related small vessel disease: A potential contributor to neurodegeneration in multiple sclerosis. Brain Pathol. 2017, 27, 707–722. [Google Scholar] [CrossRef]
- Wuerfel, J.; Bellmann-Strobl, J.; Brunecker, P.; Aktas, O.; McFarland, H.; Villringer, A.; Zipp, F. Changes in cerebral perfusion precede plaque formation in multiple sclerosis: A longitudinal perfusion MRI study. Brain 2004, 127, 111–119. [Google Scholar] [CrossRef]
- Binnewijzend, M.A.; Benedictus, M.R.; Kuijer, J.P.; van der Flier, W.M.; Teunissen, C.E.; Prins, N.D.; Wattjes, M.P.; van Berckel, B.N.; Scheltens, P.; Barkhof, F. Cerebral perfusion in the predementia stages of Alzheimer’s disease. Eur. Radiol. 2016, 26, 506–514. [Google Scholar] [CrossRef]
- de la Torre, J. The Vascular Hypothesis of Alzheimer’s Disease: A Key to Preclinical Prediction of Dementia Using Neuroimaging. J. Alzheimers Dis. 2018, 63, 35–52. [Google Scholar] [CrossRef]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of Alzheimer’s disease--A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Hays, C.C.; Zlatar, Z.Z.; Wierenga, C.E. The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cell Mol. Neurobiol. 2016, 36, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T. Vascular pathology in Alzheimer’s disease. Psychogeriatrics 2010, 10, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Wälchli, T.; Ghobrial, M.; Schwab, M.; Takada, S.; Zhong, H.; Suntharalingham, S.; Vetiska, S.; Gonzalez, D.R.; Rehrauer, H.; Wu, R.; et al. Molecular atlas of the human brain vasculature at the single-cell level. bioRxiv 2021. [Google Scholar] [CrossRef]
- Winkler, E.A.; Kim, C.N.; Ross, J.M.; Garcia, J.H.; Gil, E.; Oh, I.; Chen, L.Q.; Wu, D.; Catapano, J.S.; Raygor, K.; et al. A single-cell atlas of the normal and malformed human brain vasculature. Science 2022, 375, eabi7377. [Google Scholar] [CrossRef]
- Mastorakos, P.; McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4, eaav0492. [Google Scholar] [CrossRef]
- Chen, W.; Choi, E.J.; McDougall, C.M.; Su, H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl. Stroke Res. 2014, 5, 316–329. [Google Scholar] [CrossRef]
- Hanania, R.; Sun, H.S.; Xu, K.; Pustylnik, S.; Jeganathan, S.; Harrison, R.E. Classically activated macrophages use stable microtubules for matrix metalloproteinase-9 (MMP-9) secretion. J. Biol. Chem. 2012, 287, 8468–8483. [Google Scholar] [CrossRef]
- Billack, B. Macrophage activation: Role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharm. Educ. 2006, 70, 102. [Google Scholar] [CrossRef]
- Buxade, M.; Huerga Encabo, H.; Riera-Borrull, M.; Quintana-Gallardo, L.; Lopez-Cotarelo, P.; Tellechea, M.; Martinez-Martinez, S.; Redondo, J.M.; Martin-Caballero, J.; Flores, J.M.; et al. Macrophage-specific MHCII expression is regulated by a remote Ciita enhancer controlled by NFAT5. J. Exp. Med. 2018, 215, 2901–2918. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, N.; Isibasi, A.; Ortiz-Navarrete, V. Macrophages present exogenous antigens by class I major histocompatibility complex molecules via a secretory pathway as a consequence of interferon-gamma activation. Immunology 2001, 103, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.H.; Taylor, D.K.; Turka, L.A. The contribution of direct TLR signaling to T cell responses. Immunol. Res. 2009, 45, 25–36. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Holscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Savchenko, V.L.; Nikonenko, I.R.; Skibo, G.G.; McKanna, J.A. Distribution of microglia and astrocytes in different regions of the normal adult rat brain. Neurophysiology 1997, 29, 343–351. [Google Scholar] [CrossRef]
- Stowell, R.D.; Wong, E.L.; Batchelor, H.N.; Mendes, M.S.; Lamantia, C.E.; Whitelaw, B.S.; Majewska, A.K. Cerebellar microglia are dynamically unique and survey Purkinje neurons in vivo. Dev. Neurobiol. 2018, 78, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef]
- Verdonk, F.; Roux, P.; Flamant, P.; Fiette, L.; Bozza, F.A.; Simard, S.; Lemaire, M.; Plaud, B.; Shorte, S.L.; Sharshar, T.; et al. Phenotypic clustering: A novel method for microglial morphology analysis. J. Neuroinflammation 2016, 13, 153. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Bennett, F.C.; Molofsky, A.V. The immune system and psychiatric disease: A basic science perspective. Clin. Exp. Immunol. 2019, 197, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Cheadle, L.; Rivera, S.A.; Phelps, J.S.; Ennis, K.A.; Stevens, B.; Burkly, L.C.; Lee, W.-C.A.; Greenberg, M.E. Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism. Neuron 2020, 108, 451–468.e9. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Brennan, F.H.; Popovich, P.G.; Zhou, M. Microglia maintain the normal structure and function of the hippocampal astrocyte network. Glia 2022, 70, 1359–1379. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.-D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflam. 2021, 18, 258. [Google Scholar] [CrossRef]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Zengeler, K.E.; Lukens, J.R. Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat. Rev. Immunol. 2021, 21, 454–468. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Wang, Y.; Szretter, K.J.; Vermi, W.; Gilfillan, S.; Rossini, C.; Cella, M.; Barrow, A.D.; Diamond, M.S.; Colonna, M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012, 13, 753–760. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Mordelt, A.; de Witte, L.D. Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: Hype or hope? Curr. Opin. Neurobiol. 2023, 79, 102674. [Google Scholar] [CrossRef]
- Kang, R.; Gamdzyk, M.; Lenahan, C.; Tang, J.; Tan, S.; Zhang, J.H. The Dual Role of Microglia in Blood-Brain Barrier Dysfunction after Stroke. Curr. Neuropharmacol. 2020, 18, 1237–1249. [Google Scholar] [CrossRef]
- Hallenbeck, J.M. The many faces of tumor necrosis factor in stroke. Nat. Med. 2002, 8, 1363–1368. [Google Scholar] [CrossRef]
- da Fonseca, A.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Xing, C.; Li, W.; Deng, W.; Ning, M.; Lo, E.H. A potential gliovascular mechanism for microglial activation: Differential phenotypic switching of microglia by endothelium versus astrocytes. J. Neuroinflammation 2018, 15, 143. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Collins, L.E.; Murphy, R.P.; Cummins, P.M. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: Consequences for interendothelial adherens and tight junctions. PLoS ONE 2014, 9, e101815. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Wang, Z.; Liu, Y.; Wang, P.; Xue, Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 2011, 44, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Tagami, M.; Takenaga, F.; Yamori, Y.; Itoh, S. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol. Dis. 2004, 17, 491–499. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.A.; Srinivasan, K.; Ayalon, G.; Meilandt, W.J.; Lin, H.; Huntley, M.A.; Cao, Y.; Lee, S.H.; Haddick, P.C.G.; Ngu, H.; et al. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Rep. 2018, 22, 832–847. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.; Liao, Y.; Sun, Q.; Luo, C.; Wang, G.; Zhao, F.; Jin, Y. Association of NF-kappaB and AP-1 with MMP-9 Overexpression in 2-Chloroethanol Exposed Rat Astrocytes. Cells 2018, 7, 96. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- van Splunder, H.; Villacampa, P.; Martinez-Romero, A.; Graupera, M. Pericytes in the disease spotlight. Trends Cell Biol. 2023. [Google Scholar] [CrossRef]
- Medina-Flores, F.; Hurtado-Alvarado, G.; Deli, M.A.; Gómez-González, B. The Active Role of Pericytes During Neuroinflammation in the Adult Brain. Cell. Mol. Neurobiol. 2023, 43, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Mae, M.A.; He, L.; Nordling, S.; Vazquez-Liebanas, E.; Nahar, K.; Jung, B.; Li, X.; Tan, B.C.; Chin Foo, J.; Cazenave-Gassiot, A.; et al. Single-Cell Analysis of Blood-Brain Barrier Response to Pericyte Loss. Circ. Res. 2021, 128, e46–e62. [Google Scholar] [CrossRef]
- Shepro, D.; Morel, N.M. Pericyte physiology. FASEB J. 1993, 7, 1031–1038. [Google Scholar] [CrossRef]
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Arimura, K.; Ago, T.; Kamouchi, M.; Nakamura, K.; Ishitsuka, K.; Kuroda, J.; Sugimori, H.; Ooboshi, H.; Sasaki, T.; Kitazono, T. PDGF receptor beta signaling in pericytes following ischemic brain injury. Curr. Neurovasc Res. 2012, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Pleiotrophin: Activity and mechanism. Adv. Clin. Chem. 2020, 98, 51–89. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ. Transplant. 2016, 21, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Roediger, B.; Weninger, W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. 2014, 291, 22–31. [Google Scholar] [CrossRef]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical patrolling monocyte function in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef]
- van Furth, R.; Cohn, Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968, 128, 415–435. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Landsman, L.; Varol, C.; Jung, S. Distinct differentiation potential of blood monocyte subsets in the lung. J. Immunol. 2007, 178, 2000–2007. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, A.; McGovern, N.; Teo, P.; Zelante, T.; Atarashi, K.; Low, D.; Ho, A.W.; See, P.; Shin, A.; Wasan, P.S.; et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013, 38, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Marzaioli, V.; Canavan, M.; Floudas, A.; Wade, S.C.; Low, C.; Veale, D.J.; Fearon, U. Monocyte-Derived Dendritic Cell Differentiation in Inflammatory Arthritis Is Regulated by the JAK/STAT Axis via NADPH Oxidase Regulation. Front. Immunol. 2020, 11, 1406. [Google Scholar] [CrossRef]
- Randolph, G.J.; Inaba, K.; Robbiani, D.F.; Steinman, R.M.; Muller, W.A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 1999, 11, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Carlin, L.M.; Stamatiades, E.G.; Auffray, C.; Hanna, R.N.; Glover, L.; Vizcay-Barrena, G.; Hedrick, C.C.; Cook, H.T.; Diebold, S.; Geissmann, F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013, 153, 362–375. [Google Scholar] [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ruiz, S.R.; Torres-Aguilar, H.; Gonzalez-Dominguez, E.; Narvaez, J.; Gonzalez-Perez, G.; Vargas-Ayala, G.; Meraz-Rios, M.A.; Garcia-Zepeda, E.A.; Sanchez-Torres, C. Human CD16+ and CD16- monocyte subsets display unique effector properties in inflammatory conditions in vivo. J. Leukoc. Biol. 2011, 90, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Thaler, B.; Hohensinner, P.J.; Krychtiuk, K.A.; Matzneller, P.; Koller, L.; Brekalo, M.; Maurer, G.; Huber, K.; Zeitlinger, M.; Jilma, B.; et al. Differential in vivo activation of monocyte subsets during low-grade inflammation through experimental endotoxemia in humans. Sci. Rep. 2016, 6, 30162. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Grip, O.; Bredberg, A.; Lindgren, S.; Henriksson, G. Increased subpopulations of CD16(+) and CD56(+) blood monocytes in patients with active Crohn’s disease. Inflamm. Bowel Dis. 2007, 13, 566–572. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Seiler, S.; Zawada, A.M.; Reichart, B.; Herath, E.; Roth, D.; Ulrich, C.; Fliser, D.; Heine, G.H. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur. Heart J. 2011, 32, 84–92. [Google Scholar] [CrossRef]
- Rossol, M.; Kraus, S.; Pierer, M.; Baerwald, C.; Wagner, U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012, 64, 671–677. [Google Scholar] [CrossRef]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef]
- Anandasabapathy, N.; Victora, G.D.; Meredith, M.; Feder, R.; Dong, B.; Kluger, C.; Yao, K.; Dustin, M.L.; Nussenzweig, M.C.; Steinman, R.M.; et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 2011, 208, 1695–1705. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Ruitenberg, M.J.; McMenamin, P.G. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J. Neuropathol. Exp. Neurol. 2010, 69, 896–909. [Google Scholar] [CrossRef]
- McMenamin, P.G. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J. Comp. Neurol. 1999, 405, 553–562. [Google Scholar] [CrossRef]
- Serot, J.M.; Foliguet, B.; Bene, M.C.; Faure, G.C. Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. Neuroreport 1997, 8, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Murphy, T.L.; Grajales-Reyes, G.E.; Wu, X.; Tussiwand, R.; Briseno, C.G.; Iwata, A.; Kretzer, N.M.; Durai, V.; Murphy, K.M. Transcriptional Control of Dendritic Cell Development. Annu. Rev. Immunol. 2016, 34, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.H.; Wang, L.; Ho, P.C. Metabolic programming in dendritic cells tailors immune responses and homeostasis. Cell Mol. Immunol. 2022, 19, 370–383. [Google Scholar] [CrossRef]
- Merad, M.; Manz, M.G. Dendritic cell homeostasis. Blood 2009, 113, 3418–3427. [Google Scholar] [CrossRef]
- Fine, N.; Tasevski, N.; McCulloch, C.A.; Tenenbaum, H.C.; Glogauer, M. The Neutrophil: Constant Defender and First Responder. Front. Immunol. 2020, 11, 571085. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Cassatella, M.A. Social networking of human neutrophils within the immune system. Blood 2014, 124, 710–719. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, W.; Bollen, A.W.; Lawton, M.T.; Barbaro, N.M.; Dowd, C.F.; Hashimoto, T.; Yang, G.Y.; Young, W.L. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery 2008, 62, 1340–1349; discussion 1349–1350. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Borregaard, N.; Kjeldsen, L.; Moses, M.A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001, 276, 37258–37265. [Google Scholar] [CrossRef]
- Yang, Q.; Jeremiah Bell, J.; Bhandoola, A. T-cell lineage determination. Immunol. Rev. 2010, 238, 12–22. [Google Scholar] [CrossRef]
- Kurosaki, T. B-lymphocyte biology. Immunol. Rev. 2010, 237, 5–9. [Google Scholar] [CrossRef]
- Nagasawa, T. Microenvironmental niches in the bone marrow required for B-cell development. Nat. Rev. Immunol. 2006, 6, 107–116. [Google Scholar] [CrossRef]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef]
- Stone, J.D.; Cochran, J.R.; Stern, L.J. T-cell activation by soluble MHC oligomers can be described by a two-parameter binding model. Biophys. J. 2001, 81, 2547–2557. [Google Scholar] [CrossRef]
- Ahn, J.J.; Abu-Rub, M.; Miller, R.H. B Cells in Neuroinflammation: New Perspectives and Mechanistic Insights. Cells 2021, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.W.; Yong, V.W. B cells in central nervous system disease: Diversity, locations and pathophysiology. Nat. Rev. Immunol. 2022, 22, 513–524. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Tang, A.T.; Choi, J.P.; Kotzin, J.J.; Yang, Y.; Hong, C.C.; Hobson, N.; Girard, R.; Zeineddine, H.A.; Lightle, R.; Moore, T.; et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 2017, 545, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Nelsen, B.; Frias-Anaya, E.; Gallego-Gutierrez, H.; Orecchioni, M.; Herrera, V.; Ortiz, E.; Sun, H.; Mesarwi, O.A.; Ley, K.; et al. Neuroinflammation Plays a Critical Role in Cerebral Cavernous Malformation Disease. Circ. Res. 2022, 131, 909–925. [Google Scholar] [CrossRef]
- Chalouhi, N.; Hoh, B.L.; Hasan, D. Review of cerebral aneurysm formation, growth, and rupture. Stroke 2013, 44, 3613–3622. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Banda, M.J.; Leppert, D. Matrix metalloproteinases in immunity. J. Immunol. 1996, 156, 1–4. [Google Scholar] [CrossRef]
- Zajac, E.; Schweighofer, B.; Kupriyanova, T.A.; Juncker-Jensen, A.; Minder, P.; Quigley, J.P.; Deryugina, E.I. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 2013, 122, 4054–4067. [Google Scholar] [CrossRef]
- Montaner, J.; Ramiro, L.; Simats, A.; Hernandez-Guillamon, M.; Delgado, P.; Bustamante, A.; Rosell, A. Matrix metalloproteinases and ADAMs in stroke. Cell Mol. Life Sci. 2019, 76, 3117–3140. [Google Scholar] [CrossRef]
- Shan, Y.; Tan, S.; Lin, Y.; Liao, S.; Zhang, B.; Chen, X.; Wang, J.; Deng, Z.; Zeng, Q.; Zhang, L.; et al. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J. Neuroinflammation 2019, 16, 242. [Google Scholar] [CrossRef]
- Takata, F.; Sumi, N.; Nishioku, T.; Harada, E.; Wakigawa, T.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Oncostatin M induces functional and structural impairment of blood-brain barriers comprised of rat brain capillary endothelial cells. Neurosci. Lett. 2008, 441, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Takata, F.; Dohgu, S.; Sakaguchi, S.; Sakai, K.; Yamanaka, G.; Iwao, T.; Matsumoto, J.; Kimura, I.; Sezaki, Y.; Tanaka, Y.; et al. Oncostatin-M-Reactive Pericytes Aggravate Blood-Brain Barrier Dysfunction by Activating JAK/STAT3 Signaling In Vitro. Neuroscience 2019, 422, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.A.; Xu, L.; Tang, X.N.; Qiao, Y.; Giffard, R.G. Microglia potentiate damage to blood-brain barrier constituents: Improvement by minocycline in vivo and in vitro. Stroke 2006, 37, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, Y.; Deng, X.; Jiang, W.; Li, B.; Fu, Z.; Du, M.; Ding, R. Hemoglobin-induced nitric oxide synthase overexpression and nitric oxide production contribute to blood-brain barrier disruption in the rat. J. Mol. Neurosci. 2013, 51, 352–363. [Google Scholar] [CrossRef]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell Longev. 2016, 2016, 1203285. [Google Scholar] [CrossRef]
- Hu, X.; Tao, C.; Gan, Q.; Zheng, J.; Li, H.; You, C. Oxidative Stress in Intracerebral Hemorrhage: Sources, Mechanisms, and Therapeutic Targets. Oxid. Med. Cell Longev. 2016, 2016, 3215391. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef]
- Kurki, M.I.; Hakkinen, S.K.; Frosen, J.; Tulamo, R.; von und zu Fraunberg, M.; Wong, G.; Tromp, G.; Niemela, M.; Hernesniemi, J.; Jaaskelainen, J.E.; et al. Upregulated signaling pathways in ruptured human saccular intracranial aneurysm wall: An emerging regulative role of Toll-like receptor signaling and nuclear factor-kappaB, hypoxia-inducible factor-1A, and ETS transcription factors. Neurosurgery 2011, 68, 1667–1675; discussion 1675–1666. [Google Scholar] [CrossRef]
- Nakaoka, H.; Tajima, A.; Yoneyama, T.; Hosomichi, K.; Kasuya, H.; Mizutani, T.; Inoue, I. Gene expression profiling reveals distinct molecular signatures associated with the rupture of intracranial aneurysm. Stroke 2014, 45, 2239–2245. [Google Scholar] [CrossRef]
- Chaudhury, A.; Howe, P.H. The tale of transforming growth factor-beta (TGFbeta) signaling: A soigne enigma. IUBMB Life 2009, 61, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.M.; Chen, C.M.; Lee, Y.S.; Chang, K.H.; Chen, H.W.; Chen, S.T.; Chen, Y.C. Association of MMP-9 Haplotypes and TIMP-1 Polymorphism with Spontaneous Deep Intracerebral Hemorrhage in the Taiwan Population. PLoS ONE 2015, 10, e0125397. [Google Scholar] [CrossRef]
- Marchese, E.; Vignati, A.; Albanese, A.; Nucci, C.G.; Sabatino, G.; Tirpakova, B.; Lofrese, G.; Zelano, G.; Maira, G. Comparative evaluation of genome-wide gene expression profiles in ruptured and unruptured human intracranial aneurysms. J. Biol. Regul. Homeost. Agents 2010, 24, 185–195. [Google Scholar] [PubMed]
- Fan, Y.; Lu, H.; Liang, W.; Hu, W.; Zhang, J.; Chen, Y.E. Kruppel-like factors and vascular wall homeostasis. J. Mol. Cell Biol. 2017, 9, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Laakso, A.; Hernesniemi, J. Arteriovenous malformations: Epidemiology and clinical presentation. Neurosurg. Clin. N. Am. 2012, 23, 1–6. [Google Scholar] [CrossRef]
- Li, H.; Yan, Z.; Huo, R.; Ya, X.; Xu, H.; Liu, Z.; Jiao, Y.; Weng, J.; Wang, J.; Wang, S.; et al. RNA sequencing analysis between ruptured and un-ruptured brain AVM. Chin. Neurosurg. J. 2022, 8, 13. [Google Scholar] [CrossRef]
- Weinsheimer, S.M.; Xu, H.; Achrol, A.S.; Stamova, B.; McCulloch, C.E.; Pawlikowska, L.; Tian, Y.; Ko, N.U.; Lawton, M.T.; Steinberg, G.K.; et al. Gene expression profiling of blood in brain arteriovenous malformation patients. Transl. Stroke Res. 2011, 2, 575–587. [Google Scholar] [CrossRef]
- Mouchtouris, N.; Jabbour, P.M.; Starke, R.M.; Hasan, D.M.; Zanaty, M.; Theofanis, T.; Ding, D.; Tjoumakaris, S.I.; Dumont, A.S.; Ghobrial, G.M.; et al. Biology of cerebral arteriovenous malformations with a focus on inflammation. J. Cereb. Blood Flow. Metab. 2015, 35, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.P.; Secord, E. Innate Immunity. Immunol. Allergy Clin. North. Am. 2021, 41, 535–541. [Google Scholar] [CrossRef]
- Hasan, D.M.; Amans, M.; Tihan, T.; Hess, C.; Guo, Y.; Cha, S.; Su, H.; Martin, A.J.; Lawton, M.T.; Neuwelt, E.A.; et al. Ferumoxytol-enhanced MRI to Image Inflammation within Human Brain Arteriovenous Malformations: A Pilot Investigation. Transl. Stroke Res. 2012, 3, 166–173. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Poon, K.Y.; Achrol, A.S.; Lawton, M.T.; Zhu, Y.; McCulloch, C.E.; Hashimoto, T.; Lee, C.; Barbaro, N.M.; et al. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front. Biosci. 2006, 11, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pawlikowska, L.; Yao, J.S.; Shen, F.; Zhai, W.; Achrol, A.S.; Lawton, M.T.; Kwok, P.Y.; Yang, G.Y.; Young, W.L. Interleukin-6 involvement in brain arteriovenous malformations. Ann. Neurol. 2006, 59, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Sugita, Y.; Nakashima, S.; Okada, Y.; Yoshitomi, M.; Kimura, Y.; Miyoshi, H.; Morioka, M.; Ohshima, K. Alternatively Activated Macrophages Play an Important Role in Vascular Remodeling and Hemorrhaging in Patients with Brain Arteriovenous Malformation. J. Stroke Cerebrovasc. Dis. 2016, 25, 600–609. [Google Scholar] [CrossRef]
- Wright, R.; Jarvelin, P.; Pekonen, H.; Keranen, S.; Rauramaa, T.; Frosen, J. Histopathology of brain AVMs part II: Inflammation in arteriovenous malformation of the brain. Acta Neurochir. 2020, 162, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, Y.; Walker, E.J.; Shen, F.; Jun, K.; Oh, S.P.; Degos, V.; Lawton, M.T.; Tihan, T.; Davalos, D.; et al. Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 305–310. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Z.; Han, Z.; Jun, K.; Camus, M.; Wankhede, M.; Mao, L.; Arnold, T.; Young, W.L.; Su, H. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 2014, 45, 900–902. [Google Scholar] [CrossRef]
- Choi, E.J.; Chen, W.; Jun, K.; Arthur, H.M.; Young, W.L.; Su, H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS ONE 2014, 9, e88511. [Google Scholar] [CrossRef]
- Walker, E.J.; Su, H.; Shen, F.; Choi, E.J.; Oh, S.P.; Chen, G.; Lawton, M.T.; Kim, H.; Chen, Y.; Chen, W.; et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann. Neurol. 2011, 69, 954–962. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Z.; Degos, V.; Shen, F.; Choi, E.J.; Sun, Z.; Kang, S.; Wong, M.; Zhu, W.; Zhan, L.; et al. Persistent infiltration and pro-inflammatory differentiation of monocytes cause unresolved inflammation in brain arteriovenous malformation. Angiogenesis 2016, 19, 451–461. [Google Scholar] [CrossRef]
- Guo, Y.; Tihan, T.; Kim, H.; Hess, C.; Lawton, M.T.; Young, W.L.; Zhao, Y.; Su, H. Distinctive distribution of lymphocytes in unruptured and previously untreated brain arteriovenous malformation. Neuroimmunol. Neuroinflamm. 2014, 1, 147–152. [Google Scholar] [CrossRef]
- Aziz, M.M.; Takagi, Y.; Hashimoto, N.; Miyamoto, S. Activation of nuclear factor kappaB in cerebral arteriovenous malformations. Neurosurgery 2010, 67, 1669–1679; discussion 1679–1680. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.M.; Day, A.J. TSG-6: A multifunctional protein associated with inflammation. J. Cell Sci. 2003, 116, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; Li, C.F.; Ke, H.L.; Wei, Y.C.; Shiue, Y.L.; Li, C.C.; Yeh, H.C.; Lee, H.Y.; Huang, S.K.; Wu, W.J.; et al. High TNFAIP6 level is associated with poor prognosis of urothelial carcinomas. Urol. Oncol. 2019, 37, 293.e11–293.e24. [Google Scholar] [CrossRef]
- Beutler, B.A. TLRs and innate immunity. Blood 2009, 113, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, R.; Wang, X.; Xue, X.; Ran, D.; Wang, S. Relevance of IL-6 and MMP-9 to cerebral arteriovenous malformation and hemorrhage. Mol. Med. Rep. 2013, 7, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Pawlikowska, L.; Tran, M.N.; Achrol, A.S.; McCulloch, C.E.; Ha, C.; Lind, D.L.; Hashimoto, T.; Zaroff, J.; Lawton, M.T.; Marchuk, D.A.; et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke 2004, 35, 2294–2300. [Google Scholar] [CrossRef]
- Fishman, D.; Faulds, G.; Jeffery, R.; Mohamed-Ali, V.; Yudkin, J.S.; Humphries, S.; Woo, P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Investig. 1998, 102, 1369–1376. [Google Scholar] [CrossRef]
- Shaftel, S.S.; Griffin, W.S.; O’Banion, M.K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: An evolving perspective. J. Neuroinflammation 2008, 5, 7. [Google Scholar] [CrossRef]
- Kim, H.; Hysi, P.G.; Pawlikowska, L.; Poon, A.; Burchard, E.G.; Zaroff, J.G.; Sidney, S.; Ko, N.U.; Achrol, A.S.; Lawton, M.T.; et al. Common variants in interleukin-1-Beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc. Dis. 2009, 27, 176–182. [Google Scholar] [CrossRef]
- Achrol, A.S.; Pawlikowska, L.; McCulloch, C.E.; Poon, K.Y.; Ha, C.; Zaroff, J.G.; Johnston, S.C.; Lee, C.; Lawton, M.T.; Sidney, S.; et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke 2006, 37, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Eichner, J.E.; Dunn, S.T.; Perveen, G.; Thompson, D.M.; Stewart, K.E.; Stroehla, B.C. Apolipoprotein E polymorphism and cardiovascular disease: A HuGE review. Am. J. Epidemiol. 2002, 155, 487–495. [Google Scholar] [CrossRef]
- Leung, C.H.; Poon, W.S.; Yu, L.M.; Wong, G.K.; Ng, H.K. Apolipoprotein e genotype and outcome in aneurysmal subarachnoid hemorrhage. Stroke 2002, 33, 548–552. [Google Scholar] [CrossRef] [PubMed]
- McCarron, M.O.; Weir, C.J.; Muir, K.W.; Hoffmann, K.L.; Graffagnino, C.; Nicoll, J.A.; Lees, K.R.; Alberts, M.J. Effect of apolipoprotein E genotype on in-hospital mortality following intracerebral haemorrhage. Acta Neurol. Scand. 2003, 107, 106–109. [Google Scholar] [CrossRef]
- O’Donnell, H.C.; Rosand, J.; Knudsen, K.A.; Furie, K.L.; Segal, A.Z.; Chiu, R.I.; Ikeda, D.; Greenberg, S.M. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N. Engl. J. Med. 2000, 342, 240–245. [Google Scholar] [CrossRef]
- Woo, D.; Sauerbeck, L.R.; Kissela, B.M.; Khoury, J.C.; Szaflarski, J.P.; Gebel, J.; Shukla, R.; Pancioli, A.M.; Jauch, E.C.; Menon, A.G.; et al. Genetic and environmental risk factors for intracerebral hemorrhage: Preliminary results of a population-based study. Stroke 2002, 33, 1190–1195. [Google Scholar] [CrossRef]
- Pawlikowska, L.; Poon, K.Y.; Achrol, A.S.; McCulloch, C.E.; Ha, C.; Lum, K.; Zaroff, J.G.; Ko, N.U.; Johnston, S.C.; Sidney, S.; et al. Apolipoprotein E epsilon 2 is associated with new hemorrhage risk in brain arteriovenous malformations. Neurosurgery 2006, 58, 838–843; discussion 838–843. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Kim, H.; Pawlikowska, L.; Trudy Poon, K.Y.; McCulloch, C.E.; Ko, N.U.; Johnston, S.C.; McDermott, M.W.; Zaroff, J.G.; Lawton, M.T.; et al. Association of tumor necrosis factor-alpha-238G>A and apolipoprotein E2 polymorphisms with intracranial hemorrhage after brain arteriovenous malformation treatment. Neurosurgery 2007, 61, 731–739; discussion 740. [Google Scholar] [CrossRef] [PubMed]
- Goodall, S.; Crowther, M.; Hemingway, D.M.; Bell, P.R.; Thompson, M.M. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation 2001, 104, 304–309. [Google Scholar] [CrossRef]
- Knox, J.B.; Sukhova, G.K.; Whittemore, A.D.; Libby, P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 1997, 95, 205–212. [Google Scholar] [CrossRef]
- Vihinen, P.; Ala-aho, R.; Kahari, V.M. Matrix metalloproteinases as therapeutic targets in cancer. Curr. Cancer Drug Targets 2005, 5, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Wen, G.; Lawton, M.T.; Boudreau, N.J.; Bollen, A.W.; Yang, G.Y.; Barbaro, N.M.; Higashida, R.T.; Dowd, C.F.; Halbach, V.V.; et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke 2003, 34, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Shenkar, R.; Elliott, J.P.; Diener, K.; Gault, J.; Hu, L.J.; Cohrs, R.J.; Phang, T.; Hunter, L.; Breeze, R.E.; Awad, I.A. Differential gene expression in human cerebrovascular malformations. Neurosurgery 2003, 52, 465–477; discussion 477–468. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar] [PubMed]
- Miller, A.M. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Lawton, M.T.; Wen, G.; Yang, G.Y.; Chaly, T., Jr.; Stewart, C.L.; Dressman, H.K.; Barbaro, N.M.; Marchuk, D.A.; Young, W.L. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery 2004, 54, 410–423; discussion 423–425. [Google Scholar] [CrossRef] [PubMed]
- Dingenouts, C.K.; Goumans, M.J.; Bakker, W. Mononuclear cells and vascular repair in HHT. Front. Genet. 2015, 6, 114. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Jonker, L.; Goumans, M.J.; Larsson, J.; Bouwman, P.; Karlsson, S.; Dijke, P.T.; Arthur, H.M.; Mummery, C.L. Defective paracrine signalling by TGFbeta in yolk sac vasculature of endoglin mutant mice: A paradigm for hereditary haemorrhagic telangiectasia. Development 2004, 131, 6237–6247. [Google Scholar] [CrossRef]
- Su, H.; Kim, H.; Pawlikowska, L.; Kitamura, H.; Shen, F.; Cambier, S.; Markovics, J.; Lawton, M.T.; Sidney, S.; Bollen, A.W.; et al. Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am. J. Pathol. 2010, 176, 1018–1027. [Google Scholar] [CrossRef]
- Cambier, S.; Mu, D.Z.; O’Connell, D.; Boylen, K.; Travis, W.; Liu, W.H.; Broaddus, V.C.; Nishimura, S.L. A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth. Cancer Res. 2000, 60, 7084–7093. [Google Scholar]
- Fontanella, M.; Rubino, E.; Crobeddu, E.; Gallone, S.; Gentile, S.; Garbossa, D.; Ducati, A.; Pinessi, L.; Rainero, I. Brain arteriovenous malformations are associated with interleukin-1 cluster gene polymorphisms. Neurosurgery 2012, 70, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, P.; Fan, W.; Chen, D.; Gu, Y.; Lu, D.; Zhao, F.; Hu, J.; Fu, C.; Chen, X.; et al. The rs522616 polymorphism in the matrix metalloproteinase-3 (MMP-3) gene is associated with sporadic brain arteriovenous malformation in a Chinese population. J. Clin. Neurosci. 2010, 17, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Z.; Xue, Z.; Zhu, Y.; Yang, G.Y.; Young, W.L. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke 2007, 38, 2563–2568. [Google Scholar] [CrossRef]
- Lee, C.Z.; Xu, B.; Hashimoto, T.; McCulloch, C.E.; Yang, G.Y.; Young, W.L. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 2004, 35, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Matsumoto, M.M.; Li, J.F.; Lawton, M.T.; Young, W.L.; University of California, S.F.B.S.G. Suppression of MMP-9 by doxycycline in brain arteriovenous malformations. BMC Neurol. 2005, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.J.; Su, H.; Shen, F.; Degos, V.; Amend, G.; Jun, K.; Young, W.L. Bevacizumab attenuates VEGF-induced angiogenesis and vascular malformations in the adult mouse brain. Stroke 2012, 43, 1925–1930. [Google Scholar] [CrossRef]
- Kurkjian, C.; Kim, E.S. Risks and benefits with bevacizumab: Evidence and clinical implications. Ther. Adv. Drug Saf. 2012, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Diez, R.; Gonzalez-Guerrero, C.; Ocana-Salceda, C.; Rodrigues-Diez, R.R.; Egido, J.; Ortiz, A.; Ruiz-Ortega, M.; Ramos, A.M. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. Sci. Rep. 2016, 6, 27915. [Google Scholar] [CrossRef]
- Meyer, N.; Brodowski, L.; von Kaisenberg, C.; Schroder-Heurich, B.; von Versen-Hoynck, F. Cyclosporine A and Tacrolimus Induce Functional Impairment and Inflammatory Reactions in Endothelial Progenitor Cells. Int. J. Mol. Sci. 2021, 22, 9696. [Google Scholar] [CrossRef]
- Lucas, R.M.; Luo, L.; Stow, J.L. ERK1/2 in immune signalling. Biochem. Soc. Trans. 2022, 50, 1341–1352. [Google Scholar] [CrossRef]
- Wu, M.H.; Yuan, S.Y.; Granger, H.J. The protein kinase MEK1/2 mediate vascular endothelial growth factor- and histamine-induced hyperpermeability in porcine coronary venules. J. Physiol. 2005, 563, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Pintus, G.; Tadolini, B.; Posadino, A.M.; Sanna, B.; Debidda, M.; Carru, C.; Deiana, L.; Ventura, C. PKC/Raf/MEK/ERK signaling pathway modulates native-LDL-induced E2F-1 gene expression and endothelial cell proliferation. Cardiovasc. Res. 2003, 59, 934–944. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Nakagawa, S.; Morofuji, Y.; Dohgu, S.; Watanabe, D.; Horie, N.; Izumo, T.; Niwa, M.; Walter, F.R.; Santa-Maria, A.R.; et al. MAP Kinase Pathways in Brain Endothelial Cells and Crosstalk with Pericytes and Astrocytes Mediate Contrast-Induced Blood-Brain Barrier Disruption. Pharmaceutics 2021, 13, 1272. [Google Scholar] [CrossRef]
- Narasimhan, P.; Liu, J.; Song, Y.S.; Massengale, J.L.; Chan, P.H. VEGF Stimulates the ERK 1/2 Signaling Pathway and Apoptosis in Cerebral Endothelial Cells After Ischemic Conditions. Stroke 2009, 40, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Boon, L.M.; Vikkula, M. Trametinib as a promising therapeutic option in alleviating vascular defects in an endothelial KRAS-induced mouse model. Hum. Mol. Genet. 2023, 32, 276–289. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardelli, A.R.; Robledo, A.; Fish, J.E.; Kan, P.T.; Harris, T.H.; Wythe, J.D. The Role and Therapeutic Implications of Inflammation in the Pathogenesis of Brain Arteriovenous Malformations. Biomedicines 2023, 11, 2876. https://doi.org/10.3390/biomedicines11112876
Ricciardelli AR, Robledo A, Fish JE, Kan PT, Harris TH, Wythe JD. The Role and Therapeutic Implications of Inflammation in the Pathogenesis of Brain Arteriovenous Malformations. Biomedicines. 2023; 11(11):2876. https://doi.org/10.3390/biomedicines11112876
Chicago/Turabian StyleRicciardelli, Ashley R., Ariadna Robledo, Jason E. Fish, Peter T. Kan, Tajie H. Harris, and Joshua D. Wythe. 2023. "The Role and Therapeutic Implications of Inflammation in the Pathogenesis of Brain Arteriovenous Malformations" Biomedicines 11, no. 11: 2876. https://doi.org/10.3390/biomedicines11112876
APA StyleRicciardelli, A. R., Robledo, A., Fish, J. E., Kan, P. T., Harris, T. H., & Wythe, J. D. (2023). The Role and Therapeutic Implications of Inflammation in the Pathogenesis of Brain Arteriovenous Malformations. Biomedicines, 11(11), 2876. https://doi.org/10.3390/biomedicines11112876






