Parkin Promotes Airway Inflammatory Response to Interferon Gamma
Abstract
:1. Background
2. Methods
2.1. Intranasal Administration of Recombinant IFN-γ Protein in Mice
2.2. IFN-γ Stimulation in Cultured Primary Human Airway Epithelial Cells
2.3. Thap11 Overexpression in Primary Human Airway Epithelial Cells
2.4. Parkin Knockout (PKO) in Primary HTBE Cells
2.5. Western Blot
2.6. ELISA
2.7. Statistical Analyses
3. Results
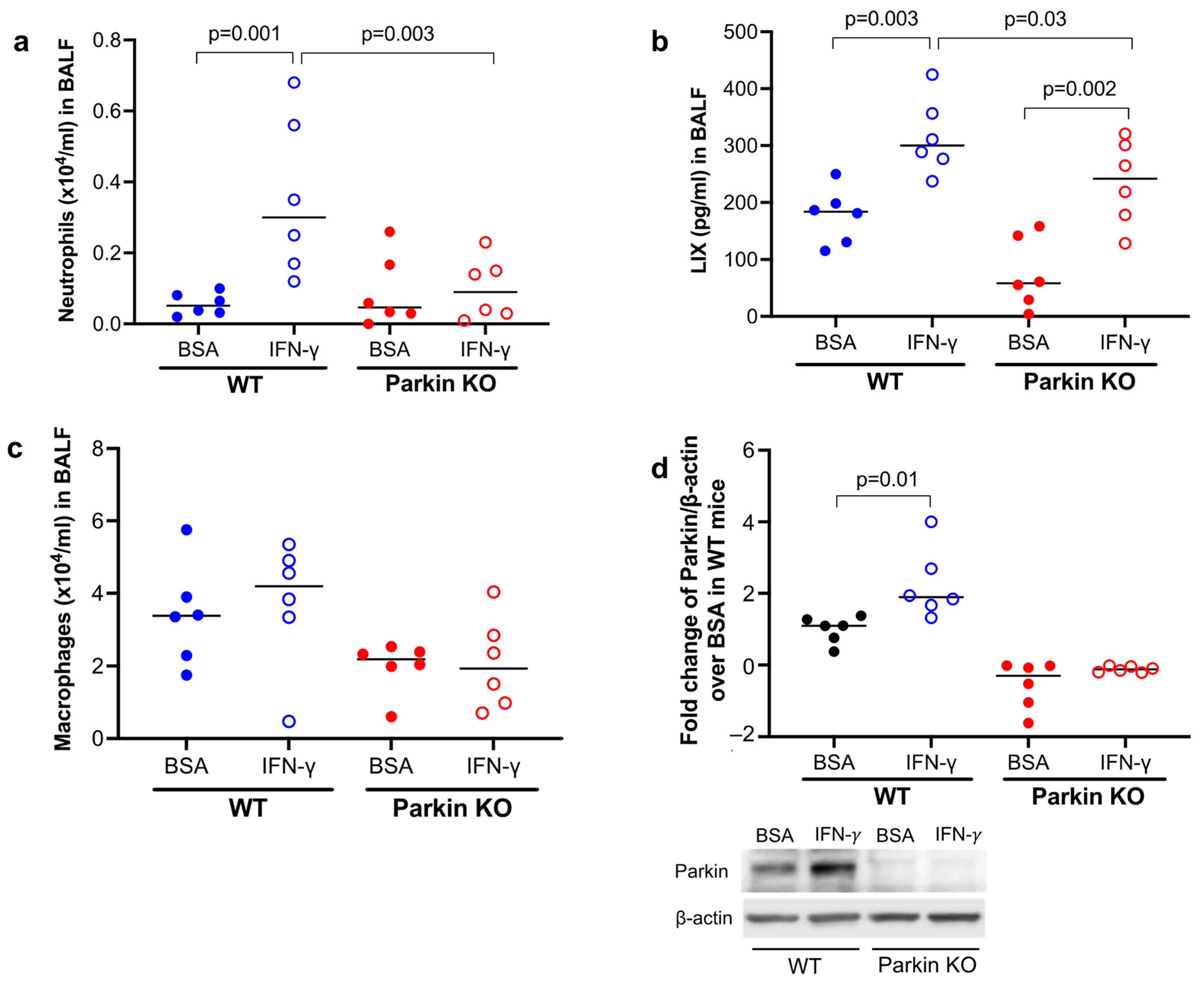
3.1. Parkin Was Essential to Mouse Lung Neutrophilic Inflammation Induced by IFN-γ
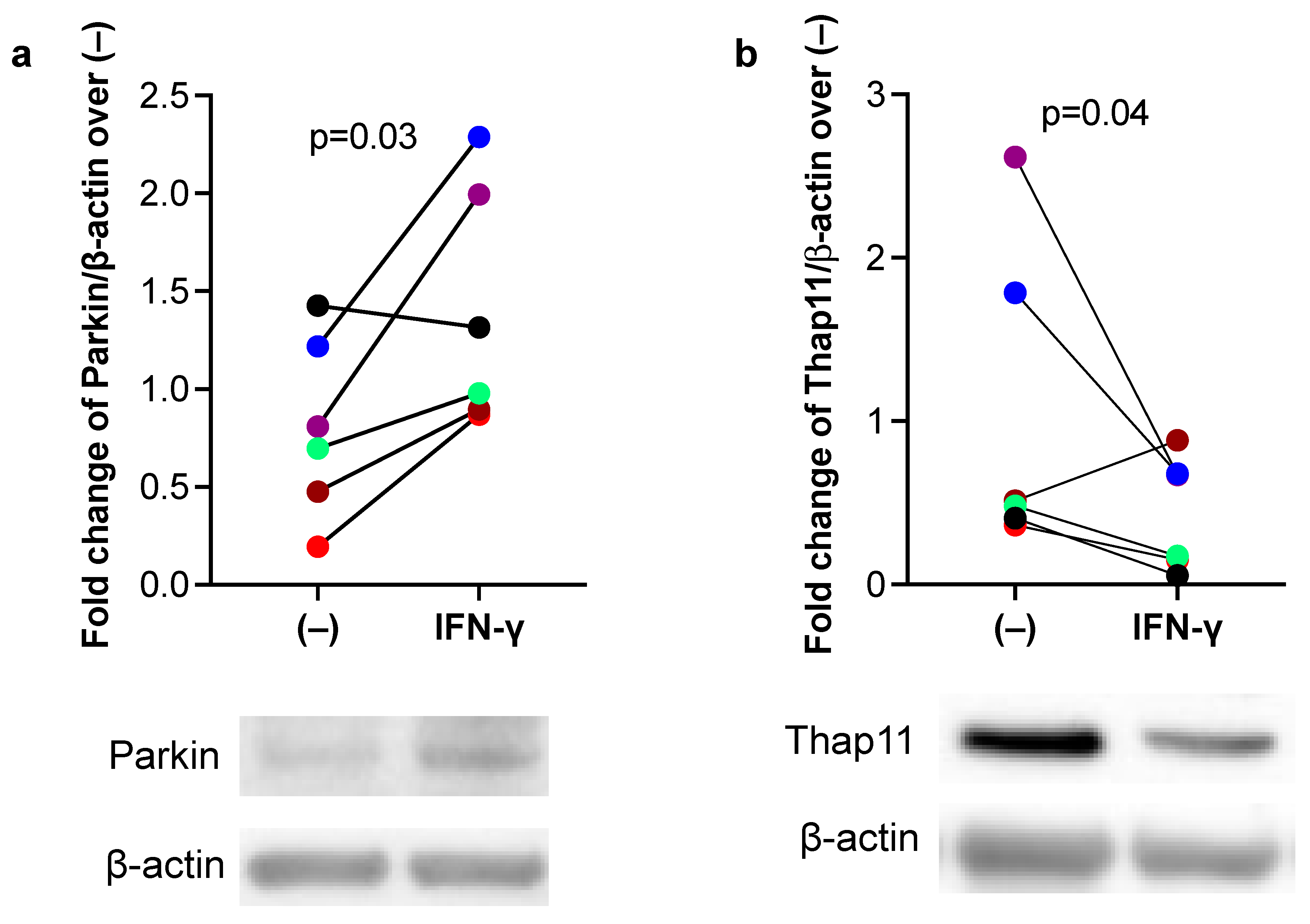
3.2. IFN-γ Increased Parkin, but Inhibited Thap11 Expression in Primary Human Airway Epithelial Cells
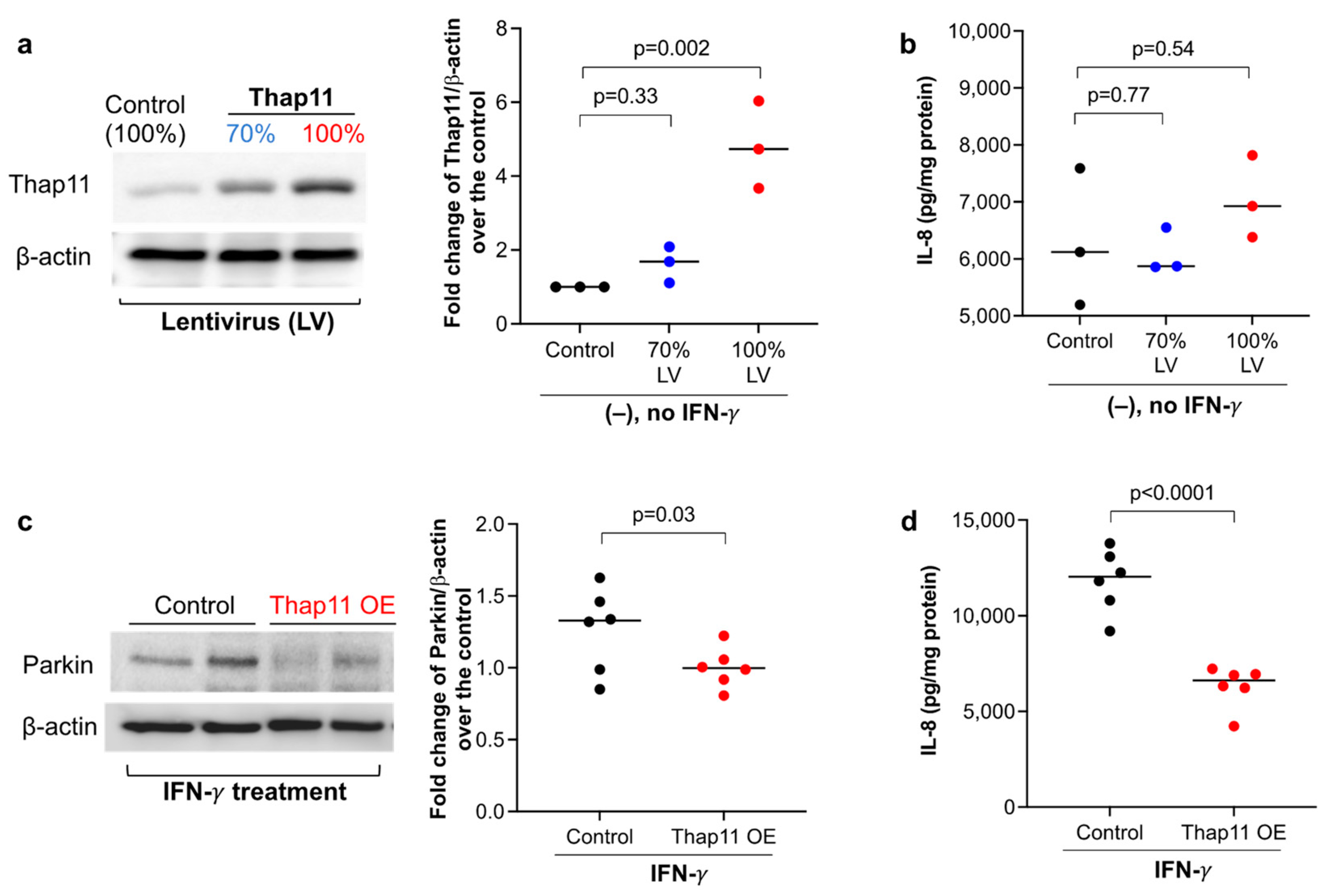
3.3. Overexpression of Thap11 Decreased Parkin Expression in Primary Human Airway Epithelial Cells Treated with IFN-γ
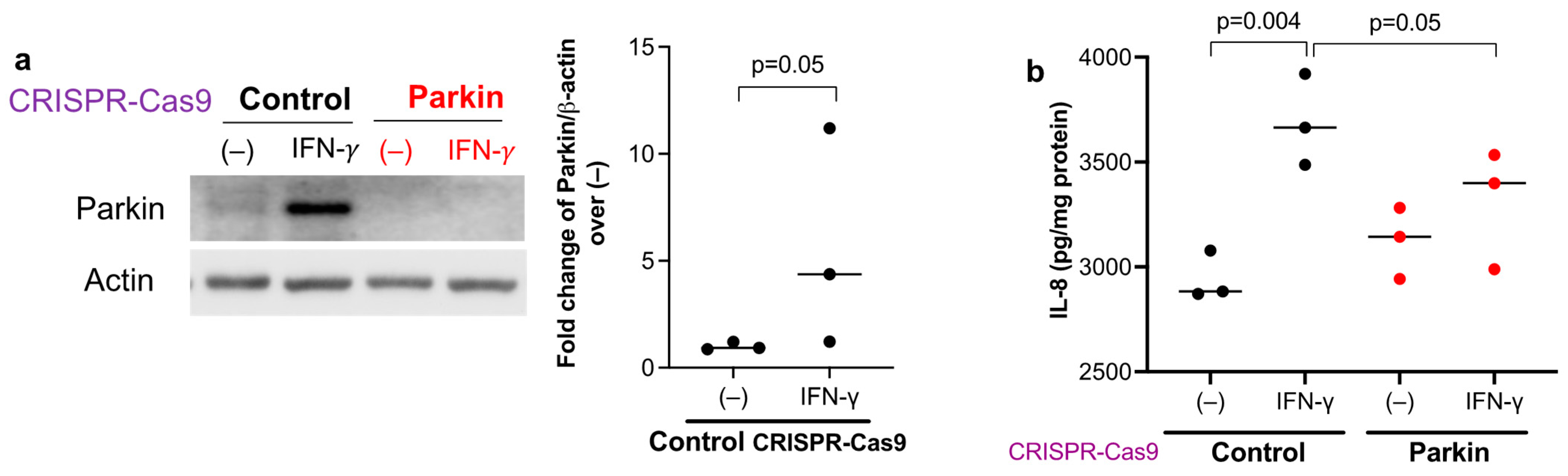
3.4. Parkin Is Essential to Maintain Human Airway Epithelial Pro-Inflammatory Response to IFN-γ
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Robinson, D.; Humbert, M.; Buhl, R.; Cruz, A.A.; Inoue, H.; Korom, S.; Hanania, N.A.; Nair, P. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: Current knowledge and therapeutic implications. Clin. Exp. Allergy 2017, 47, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Hudey, S.N.; Ledford, D.K.; Cardet, J.C. Mechanisms of non-type 2 asthma. Curr. Opin. Immunol. 2020, 66, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sprio, A.E.; Baroso, A.; Gallo, F.; Riccardi, E.; Bertolini, F.; Carriero, V.; Arrigo, E.; Ciprandi, G. Characterization of T2-Low and T2-High Asthma Phenotypes in Real-Life. Biomedicines 2021, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Raundhal, M.; Morse, C.; Khare, A.; Oriss, T.B.; Milosevic, J.; Trudeau, J.; Huff, R.; Pilewski, J.; Holguin, F.; Kolls, J.; et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J. Clin. Investig. 2015, 125, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Kale, S.L.; Oriss, T.B.; Scholl, K.; Das, S.; Yuan, H.; Hu, S.; Chen, J.; Camiolo, M.; Ray, P.; et al. Dual role for CXCR3 and CCR5 in asthmatic type 1 inflammation. J. Allergy Clin. Immunol. 2022, 149, 113–124.e7. [Google Scholar] [CrossRef]
- Ghebre, M.A.; Pang, P.H.; Diver, S.; Desai, D.; Bafadhel, M.; Haldar, K.; Kebadze, T.; Cohen, S.; Newbold, P.; Rapley, L.; et al. Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J. Allergy Clin. Immunol. 2018, 141, 2027–2036.e12. [Google Scholar] [CrossRef]
- Hansel, T.T.; Tunstall, T.; Trujillo-Torralbo, M.B.; Shamji, B.; Del-Rosario, A.; Dhariwal, J.; Kirk, P.D.W.; Stumpf, M.P.H.; Koopmann, J.; Telcian, A.; et al. A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-gamma and IFN-lambda) and Type 2 Inflammation (IL-5 and IL-13). EBioMedicine 2017, 19, 128–138. [Google Scholar] [CrossRef]
- Hu, X.; Li, W.P.; Meng, C.; Ivashkiv, L.B. Inhibition of IFN-gamma signaling by glucocorticoids. J. Immunol. 2003, 170, 4833–4839. [Google Scholar] [CrossRef]
- Hu, C.; Li, Z.; Feng, J.; Tang, Y.; Qin, L.; Hu, X.; Zhang, Y.; He, R. Glucocorticoids Modulate Th1 and Th2 Responses in Asthmatic Mouse Models by Inhibition of Notch1 Signaling. Int. Arch. Allergy Immunol. 2018, 175, 44–52. [Google Scholar] [CrossRef]
- Bayne, A.N.; Trempe, J.F. Mechanisms of PINK1, ubiquitin and Parkin interactions in mitochondrial quality control and beyond. Cell. Mol. Life Sci. 2019, 76, 4589–4611. [Google Scholar] [CrossRef]
- Kim, K.Y.; Sack, M.N. Parkin in the regulation of fat uptake and mitochondrial biology: Emerging links in the pathophysiology of Parkinson’s disease. Curr. Opin. Lipidol. 2012, 23, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Alves da Costa, C.; Duplan, E.; Rouland, L.; Checler, F. The Transcription Factor Function of Parkin: Breaking the Dogma. Front. Neurosci. 2018, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Son, D.J.; Lee, H.L.; Kim, D.H.; Song, M.J.; Ham, Y.W.; Kim, Y.; Han, S.B.; Park, M.H.; Hong, J.T. Loss of Parkin reduces inflammatory arthritis by inhibiting p53 degradation. Redox Biol. 2017, 12, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Schaunaman, N.; Martin, R.J.; Pavelka, N.; Kolakowski, C.; Gottlieb, R.A.; Holguin, F.; Chu, H.W. Parkin, an E3 ubiquitin ligase, enhances airway mitochondrial DNA release and inflammation. Thorax 2020, 75, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, C.; Long, F.; Yang, D.; Liu, X.; Hu, Y.; Wu, C.; Wang, B.; Wang, M.; Chen, Y.; et al. Parkin Impairs Antiviral Immunity by Suppressing the Mitochondrial Reactive Oxygen Species-Nlrp3 Axis and Antiviral Inflammation. iScience 2019, 16, 468–484. [Google Scholar] [CrossRef]
- Potting, C.; Crochemore, C.; Moretti, F.; Nigsch, F.; Schmidt, I.; Manneville, C.; Carbone, W.; Knehr, J.; DeJesus, R.; Lindeman, A.; et al. Genome-wide CRISPR screen for PARKIN regulators reveals transcriptional repression as a determinant of mitophagy. Proc. Natl. Acad. Sci. USA 2018, 115, E180–E189. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, D.; Song, Y.; Wang, X. Interferon gamma induces inflammatory responses through the interaction of CEACAM1 and PI3K in airway epithelial cells. J. Transl. Med. 2019, 17, 147. [Google Scholar] [CrossRef]
- Heller, N.M.; Matsukura, S.; Georas, S.N.; Boothby, M.R.; Rothman, P.B.; Stellato, C.; Schleimer, R.P. Interferon-gamma inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004, 31, 573–582. [Google Scholar] [CrossRef]
- Letsiou, E.; Sammani, S.; Wang, H.; Belvitch, P.; Dudek, S.M. Parkin regulates lipopolysaccharide-induced proinflammatory responses in acute lung injury. Transl. Res. 2017, 181, 71–82. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Lin, Y.; Jamison, S.; Lin, W. Interferon-gamma activates nuclear factor-kappa B in oligodendrocytes through a process mediated by the unfolded protein response. PLoS ONE 2012, 7, e36408. [Google Scholar] [CrossRef]
- Lawrence, D.W.; Kornbluth, J. E3 ubiquitin ligase NKLAM ubiquitinates STAT1 and positively regulates STAT1-mediated transcriptional activity. Cell Signal. 2016, 28, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.E.; Lougheed, J.C.; Callaway, K.; Velasquez, M.; Brecht, E.; Nguyen, L.; Shaler, T.; Walker, D.; Yang, Y.; Regnstrom, K.; et al. Structure and function of Parkin E3 ubiquitin ligase reveals aspects of RING and HECT ligases. Nat. Commun. 2013, 4, 1982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, B.; Liang, Y.; Wei, H.; Yuan, J. Parkin regulates NF-kappaB by mediating site-specific ubiquitination of RIPK1. Cell Death Dis. 2018, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Bouman, L.; Schlierf, A.; Lutz, A.K.; Shan, J.; Deinlein, A.; Kast, J.; Galehdar, Z.; Palmisano, V.; Patenge, N.; Berg, D.; et al. Parkin is transcriptionally regulated by ATF4: Evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ. 2011, 18, 769–782. [Google Scholar] [CrossRef]
- Pirot, P.; Eizirik, D.L.; Cardozo, A.K. Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia 2006, 49, 1229–1236. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimasuay, K.G.; Schaunaman, N.; Berg, B.; Nichols, T.; Chu, H.W. Parkin Promotes Airway Inflammatory Response to Interferon Gamma. Biomedicines 2023, 11, 2850. https://doi.org/10.3390/biomedicines11102850
Dimasuay KG, Schaunaman N, Berg B, Nichols T, Chu HW. Parkin Promotes Airway Inflammatory Response to Interferon Gamma. Biomedicines. 2023; 11(10):2850. https://doi.org/10.3390/biomedicines11102850
Chicago/Turabian StyleDimasuay, Kris Genelyn, Niccolette Schaunaman, Bruce Berg, Taylor Nichols, and Hong Wei Chu. 2023. "Parkin Promotes Airway Inflammatory Response to Interferon Gamma" Biomedicines 11, no. 10: 2850. https://doi.org/10.3390/biomedicines11102850
APA StyleDimasuay, K. G., Schaunaman, N., Berg, B., Nichols, T., & Chu, H. W. (2023). Parkin Promotes Airway Inflammatory Response to Interferon Gamma. Biomedicines, 11(10), 2850. https://doi.org/10.3390/biomedicines11102850




