Abstract
Laminin α4 (LAMA4) is one of the main structural adipocyte basement membrane (BM) components that is upregulated during adipogenesis and related to obesity in mice and humans. We conducted RNA-seq-based gene expression analysis of LAMA4 in abdominal subcutaneous (SC) and visceral (VIS) adipose tissue (AT) depots across three human sub-cohorts of the Leipzig Obesity BioBank (LOBB) to explore the relationship between LAMA4 expression and obesity (N = 1479) in the context of weight loss (N = 65) and metabolic health (N = 42). We found significant associations of LAMA4 with body fat mass (p < 0.001) in VIS AT; higher expression in VIS AT compared to SC AT; and significant relation to metabolic health parameters e.g., body fat in VIS AT, waist (p = 0.009) and interleukin 6 (p = 0.002) in male VIS AT, and hemoglobin A1c (p = 0.008) in male SC AT. AT LAMA4 expression was not significantly different between subjects with or without obesity, metabolically healthy versus unhealthy, and obesity before versus after short-term weight loss. Our results support significant associations between obesity related clinical parameters and elevated LAMA4 expression in humans. Our work offers one of the first references for understanding the meaning of LAMA4 expression specifically in relation to obesity based on large-scale RNA-seq data.
1. Introduction
Obesity and overweight remain to be serious challenges to global health, affecting ~60% of adults and one in three children in the European region alone [1]. Obesity is strongly associated with non-communicable diseases (NCD) [2] and thereby contributes to increased NCD mortality worldwide [3]. Thus far, overweight and obesity are risk factors for a variety of NCDs such as type 2 diabetes mellitus, coronary heart disease, and various forms of cancer [4,5,6]. In recent years, obesity has been associated with increased risk of mortality and severe disease causes in patients with COVID-19 [7] and long COVID-19 syndrome [8]. The mechanisms of how adipose tissue (AT) and adipocyte function may link increased fat accumulation in obesity to these diseases or adverse outcomes still needs to be explored.
The outer cell membrane of adipocytes is encapsulated by a basement membrane (BM) which provides cell stability [9] and is involved in cell signaling [10]. Aberrations of BM physiology are suggested to be related to pathological conditions such as disorganized BM structures in obese subjects [11] and stiffening of BM in association with diabetes [12]. One specific substructure of the BM is Laminin isoforms, which form heterotrimeric structures of α, β, and γ subunits [9]. The varying properties of Laminin isoforms allow for a variety of membrane structures and signaling [9], which change with tissue location and disease status [13]. The isoform laminin α4 (LAMA4) is known to be upregulated during adipogenesis [14] and has been proposed as a major molecule in the regulation of AT BM.
Analyses of the adipocyte surface proteome revealed an increased abundance of LAMA4 on the surface of adipocytes, both in diet-induced obesity (DIO) and in ob/ob mice [15]. Also, LAMA4 seems to be expressed in a fat depot-dependent manner as LAMA4 abundance is significantly different between subcutaneous (SC) AT and visceral (VIS) AT in mice [15], in the human secretome [16], as well as in the human transcriptome [17]. Compared to wildtype mice, LAMA4 −/− mice exhibit higher energy expenditure [13], decreased weight gain, and protection against diet-induced obesity and insulin resistance [18]. However, it is unclear whether these effects are due to LAMA4-loss induced expression change of laminin α5 [13,19]. Transcriptome analysis revealed that adipocyte-specific deletion of LAMA4 seems to preserve the transcription patterns of healthy adipose tissue in DIO mice despite weight gain [20]. A more recent study reported an overall upregulation of LAMA4 in SC white AT in both high-fat-diet (HFD)-fed mice and a human cohort of ten women with obesity, but without diabetes, compared to three healthy, lean women at the gene expression level [21]. In this study, SC AT LAMA4 expression did not change with short term weight loss [21]. In addition to its potential relevance in obesity, LAMA4 has been linked to the prognosis of gastric cancer and immune cell infiltration, suggesting LAMA4 as a therapeutic target for these conditions [22].
Previous research has revealed a significant upregulation in LAMA4 expression in obesity [21]. To further investigate the relationship between LAMA4 and clinical markers of obesity, we conducted an analysis of LAMA4 gene expression in SC and VIS AT depots across a total of 1586 individuals from three distinct clinical cohorts of the Leipzig Obesity BioBank (LOBB) using RNA sequencing data. We analyzed a large cross-sectional cohort comprising 1479 subjects, a cohort of 42 obese subjects classified based on their metabolic health (insulin resistance or sensitivity), and a cohort of 65 individuals with morbid obesity who underwent bariatric surgery. Our aim was to explore the relationship between LAMA4 and clinical obesity markers, gender, weight reduction after bariatric surgery, and insulin resistance or sensitivity. Further, we investigated whether LAMA4 expression appeared different among VIS or SC AT between different subgroups of individuals and tested the hypothesis that LAMA4 expression decreases in both VIS and SC AT following bariatric surgery-induced weight loss. Finally, by combining Gene Set Enrichment Analysis (GSEA) with a comprehensive literature review, we identified statistically significant associations between LAMA4 expression and obesity parameters.
2. Materials and Methods
2.1. Study Design of the Leipzig Obesity BioBank
The LOBB is a collection of human body fluids, adipose tissue samples, and associated data that was created to extend the knowledge of obesity and related diseases. The LOBB covers paired human samples of omental VIS and abdominal SC AT for three different cohorts. For the purpose of this study, samples have been collected in the period between 2008–2018 during elective laparoscopic abdominal surgery as previously described [23,24] and laboratory measurements of metabolic parameters and body composition were obtained, as detailed before [25,26]. Samples were collected under the following inclusion criteria: men and women at an age >18 years who underwent elective abdominal surgery and provided written informed consent before taking part in the study. Important exclusion criteria included: chronic drug or alcohol abuse, smoking during the last 12 months prior to surgery, acute inflammatory diseases, treatment with any medication affecting adipose tissue directly, end-stage malignant diseases, weight loss > 3% in the last 3 months prior to surgery, uncontrolled thyroid disease, and Cushing’s disease.
The cross-sectional cohort (CSC) covers 1479 individuals which were either normal/overweight (N = 31; 52% women; age: 55.8 ± 13.4 years old; BMI: 25.7 ± 2.7 kg/m2) or had obesity (N = 1448; 71% women; age: 46.9 ± 11.7 years old; BMI: 49.2 ± 8.3 kg/m2).
The two-step bariatric surgery cohort (BSC) was comprised of 65 individuals (66% women) with morbid obesity (BMI > 40 kg/m2) who all completed a two-step bariatric surgery approach with sleeve gastrectomy as a first step and ROUX-en-Y gastric bypass as a second step [23,24]. Patients included in this study had a preoperative BMI of 54.5 ± 9.3 kg/m2 and an age of 44.1 ± 9.2 years. After surgery, the patients average BMI was 40.9 ± 7.2 kg/m2 with an average age of 47.1 ± 9.9 years. On average, the patients lost 40.2 ± 21.2 kg between the two surgeries, and only individuals with a weight loss of more than five kilograms were included in the study. Preoperatively, type 2 diabetes (T2D) was diagnosed in 28 patients. The number of T2D cases dropped to 18 patients after the surgery. All individuals received frequent and structured healthy diet recommendations [27].
The cohort for distinguishing metabolically healthy versus unhealthy obesity (MHU) consisted of 42 individuals who were insulin resistant (IR; 71% female; age: 38.8 ± 11.1 years old; BMI: 45.9 ± 6.9 kg/m2; FPG: 5.2 ± 0.2 mmol/L; FPI: 27.9 ± 13.5 pmol/L) and 31 individuals who were insulin sensitive (IS; 71.43% female; age: 47.2 ± 7.7 years old; BMI: 47.3 ± 8.1 kg/m2; FPG: 5.7 ± 0.3 mmol/L; FPI: 113.7 ± 45.7 pmol/L) classified as previously described.
2.2. Bulk RNA-Sequencing and Analysis
Total RNA was extracted from SC and VIS AT and ribosomal RNA-depleted RNA sequencing data were generated following the SMARTseq protocol [28,29]. In brief, RNA was enriched and reverse-transcribed using Oligo(dT) and TSO primers. In silico PCR primers were employed for cDNA amplification and Tn5 was used to process cDNA with the Nextera DNA Flex kit (Illumina, San Diego, CA, USA). All libraries were sequenced as single-end reads on a Novaseq 6000 instrument (Illumina, San Diego, CA, USA) at the Functional Genomics Center Zurich, Switzerland.
Raw sequencing reads were preprocessed using fastp (v0.20.0) [30], with a minimum read length of 18 nts and a quality cut-off of 20. The pseudoaligner kallisto [31] was used for read alignment against the human reference genome (assembly GRCh38.p13, GENCODE release 32 [32]) and gene-level expression quantification. Samples with more than 20 million read counts were downsampled to 20 million read counts using the R package ezRun (v3.14.1; https://github.com/uzh/ezRun, accessed on 23 March 2022). Homoscedastic normalization with respect to library size was performed using the variance stabilizing transformation from DESeq2 (v1.32.0) [33]. To avoid tissue variation in gene expression, we normalized each tissue separately as LAMA4 expression was also significantly different between tissues.
To effectively mitigate the effects of in vitro RNA degradation, normalized counts were calibrated with respect to their transcript integrity numbers (TINs). TINs were estimated using the R package RSeQC (v4.0.0) [34]. Given the presence of a gender batch effect in the data, we performed an additional adjustment for gender when analyzing the combined dataset, irrespective of gender. However, when we stratified the data by gender, such an adjustment was not applied. Batch effects were adjusted using the removeBatchEffect function from limma (v3.56.2) [35] and prior examined using the R swamp package (v1.5.1) [36]. We did not detect an age-related batch effect, although there was a sometimes very large age difference between patients in the cohorts. Therefore, no adjustment for age was deemed necessary.
To avoid tissue variation in gene expression for correlation and gene set enrichment analyses, we normalized each tissue separately as LAMA4 expression was also significantly different between tissues (Figure 1A). As there are variations in LAMA4 expression observed between female and male individuals, and as we are specifically interested in examining gender-specific effects, we conducted separate normalization and batch correction for male and female subjects when performing correlation analyses.
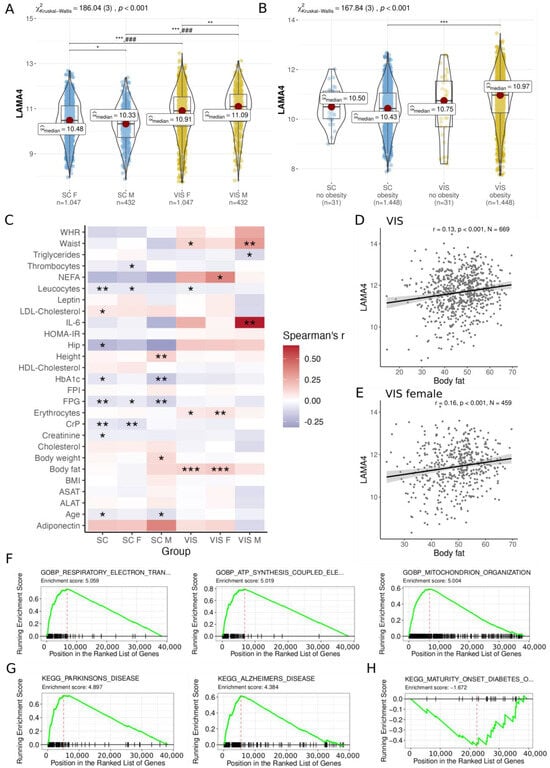
Figure 1.
Analysis of LAMA4 gene expression and correlation within the cross-sectional cohort. LAMA4 expression comparison in SC and VIS AT of (A) male (M) and female (F); (B) individuals without obesity and obesity. Kruskal–Wallis one-way ANOVA and Dunn’s test for pairwise comparisons was conducted, which was corrected for multiple comparisons using the Hommel method. (C) LAMA gene expression correlation in SC and VIS AT of male and female individuals reveals several relationships with clinical parameters (unadjusted p-values). After p-value adjustment, LAMA4 gene expression correlation remains significant with body fat in (D) VIS and (E) female VIS AT. Gene set enrichment analysis (GSEA, using the Gene Ontology database for biological processes) of the co-expressed genes with LAMA4 in VIS AT reveals enrichment for pathways related to (F) metabolism and energy expenditure, (G) neurodegenerative diseases, and (H) a significant underrepresentation of the pathway for maturity onset diabetes. Significance levels are indicated as * < 0.05, ** < 0.01, *** < 0.001. ### comparisons are significant in all groups of the other tissue (p < 0.001). ALAT: Alanine transaminase; ASAT: Aspartate transaminase; AT: adipose tissue; BMI: body mass index; CrP: C-reactive protein; F: female; FPG: fasting plasma glucose; FPI: fasting plasma insulin; HbA1C: hemoglobin A1c; HDL: High density lipoprotein; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; IL-6: interleukin 6; M: male; NEFA: nonesterified fatty acids; SC: subcutaneous; WHR: Waist hip ratio; VIS: visceral.
Gene sets correlated with LAMA4 were determined with genome-wide co-expression correlations using the correlationAnalyzeR (v1.0.0) package [37] to predict LAMA4 gene function based on Gene Set Enrichment Analysis (GSEA) for Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) database annotations.
2.3. Statistical Analyses
Between-group comparisons were performed using a non-parametric statistical approach with the R package ggstatsplot v0.10.1 [38], based on one-way Kruskal–Wallis ANOVA and pairwise Mann–Whitney U test, corrected for multiple comparisons using the Hommel method. We performed univariate Spearman correlations between LAMA4 expression and clinical phenotypes using the R package RVAideMemoire v0.9-81-2 [39], while accounting for multiple inferences by applying the false discovery rate (FDR) correction appropriate for the sample size. We considered absolute correlations ρ ≥ 0.1 and p < 0.5 as relevant. Analyses were conducted under R version 4.2.2 [40].
3. Results
3.1. Association of LAMA4 Gene Expression with Adiposity and Gene Set Pathway Alterations
In 1479 individuals of the cross-sectional cohort (CSC), we detected significantly higher LAMA4 expression in VIS AT as in SC AT of both male and female individuals (all adj. p < 0.001; Figure 1A) as well as between individuals with and without obesity (adj. p < 0.001; Figure 1B). The difference in LAMA4 expressed between the genders was less pronounced, but still existed. The median expression values of LAMA4 in SC AT were 10.48 in females and 10.33 in males (adj. p = 0.01) and in VIS AT 10.91 in females and 11.09 in males (adj. p = 0.003, Figure 1A). Overall, no significant differences in LAMA4 expression levels were found between individuals with and without obesity (Figure 1B).
Examining the CSC data for obesity-related signals associated with LAMA4 expression we observed several correlations with a p < 0.05 and absolute Spearman’s correlation coefficient |ρ| ≥ 0.1 (not adjusted for FDR; Figure 1C, Supplementary Table S1). For instance, we observed associations of LAMA4 with height (ρ = 0.14, p = 0.004, N = 426; Supplementary Figure S1A), HbA1C (Hemoglobin A1c) (ρ = −0.17, p = 0.008, N = 230; Supplementary Figure S1B), and fasting plasma glucose (FPG; ρ = −0.13, p = 0.008, N = 393; Supplementary Figure S1C) in the SC AT of men. In the VIS AT of men, LAMA4 positively correlated with waist (ρ = 0.31, p = 0.009, N = 69; Supplementary Figure S1D) and interleukin 6 (IL-6; ρ = 0.66, p = 0.002, N = 19; Supplementary Figure S1E). In contrast, LAMA4 positively correlated with body fat in VIS AT when gender was not taken into account (ρ = 0.13, p < 0.001, N = 669, Figure 1D), which was likely driven by the signal in women (ρ = 0.16, p < 0.001, N = 459, Figure 1E). After adjusting for multiple testing, only correlations with body fat in VIS AT (adj. p = 0.014) and the VIS AT of women (adj. p = 0.012) remained significant. Associations of LAMA4 with BMI could not be found, neither in SC AT (ρ = −0.001, p = 0.96, N = 1479; Supplementary Figure S1F) nor in VIS AT (ρ = −0.01, p = 0.578, N = 1479; Supplementary Figure S1G). Due to missing values, N was alternating in the aforementioned correlation analysis.
The gene set enrichment analysis (GSEA, Supplementary Tables S2 and S3) of gene sets correlated with LAMA4 indicate that LAMA4 is associated with the upregulation of pathways related neurodegenerative diseases such as Parkinson’s and Alzheimer’s, as well as respiratory diseases and ATP synthesis coupled electron transport pathways in both VIS (Figure 1F,G) and SC AT (Supplementary Figure S2A,B). In addition, we observed significant negative correlations between LAMA4 and Uncoupling Protein 1 (UCP1), which served as a marker for thermogenic activity in both SAT (ρ = −0.52, p < 0.001, N = 1479) and VIS AT (ρ = −0.45, p < 0.001, N = 1479) (Supplementary Figure S2D,E). Conversely, maturity onset diabetes was found among the underrepresented pathways with regard to LAMA4 expression in both fat depots (VIS AT: Figure 1H, SC AT: Supplementary Figure S2C).
In summary, we found significant correlation between VIS AT LAMA4 expression and body fat, and significantly different expression levels between SC AT and VIS AT as well as between gender but not between obese- and non-obese individuals.
3.2. Extensive Weight Loss Does Not Strongly Affect AT LAMA4 Expression
We analyzed 65 individuals from the LOBB’s two-step bariatric surgery cohort (BSC) who underwent sleeve gastrectomy and gastric bypass intervention (time between surgeries: −2.94 ± 3.84 years, weight loss: 40.2 ± 21.2 kg) as described previously [23,24]. Consistent with the results in the CSC shown above, we found a significantly higher expression of VIS AT LAMA4 compared to SC AT across both surgeries within the BSC (Figure 2A). However, within each tissue our analysis did not reveal any significant LAMA4 expression changes between baseline and second step surgery.
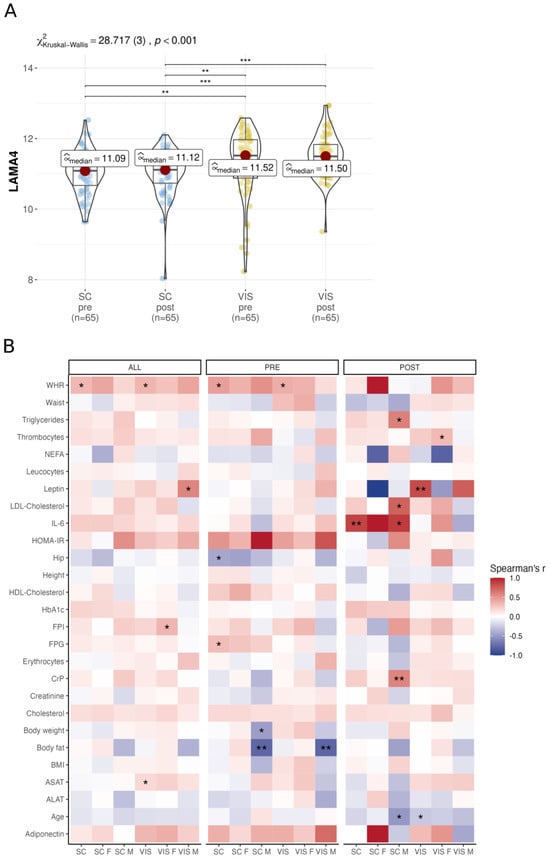
Figure 2.
Analysis of LAMA4 gene expression and correlation within the bariatric surgery cohort. (A) Comparison of LAMA4 expression pre- and post-surgery in SC AT and VIS AT, analyzed with Kruskal–Wallis one-way ANOVA and Dunn’s test for pairwise comparisons, corrected for multiple comparisons using the Hommel method. (B) Correlation analysis with Spearman correlation coefficient pre- and post-surgery and in both timepoints separated by gender and tissue (unadjusted p-values). Significance levels are indicated as * < 0.05, ** < 0.01, *** < 0.001 respectively.
When analyzing the BSC RNA sequencing data to identify obesity-related signals associated with LAMA4 expression, we found several correlations that met the statistical criteria of p < 0.05 and |ρ| ≥ 0.1 (not adjusted for FDR; Figure 2B, Supplementary Table S4). Before surgery, we found negative correlations of LAMA4 with body fat in VIS AT (ρ = −0.78, p = 0.005, N = 12) and SC AT (ρ = −0.78, p = 0.005, N = 12) obtained from men, but positive correlations with waist-to-hip ratio (WHR) in both SC (ρ = 0.45, p = 0.019, N = 25) and VIS AT (ρ = 0.38, p = 0.049, N = 25) when gender was not considered. Furthermore, pre-surgery LAMA4 correlated positively with FPG (ρ = 0.31, p = 0.017, N = 56) and negatively correlated with hip circumference (ρ = −0.43, p = 0.024, N = 25). Post-surgery, we identified positive associations of LAMA4 with IL-6 in SC AT (ρ = 0.87, p = 0.002, N = 8), C-reactive Protein (CrP) in male’s SC AT (ρ = 0.57, p = 0.005, N = 22) and serum Leptin in VIS AT (ρ = 0.84, p = 0.005, N = 8). At both time points, LAMA4 expression correlated positively with WHR in SC AT (ρ = 0.33, p = 0.047, N = 33) and VIS AT (ρ = 0.34, p = 0.039, N = 33) (Figure 2B). No correlation remained significant after p adjustment.
In conclusion, we confirm higher LAMA4 expression in VIS AT compared to SC AT and found correlations of LAMA4 expression with WHR in both AT among others but did not find changes in LAMA4 expression before and after surgery treatment.
3.3. AT LAMA4 Expression Is Not Related to Metabolically Healthy Obesity
To test the hypothesis that LAMA4 expression is related to parameters of metabolic health, including preserved insulin sensitivity despite obesity, we performed group comparisons of AT LAMA4 expression between IR and IS individuals [25,26] within the cohort, distinguishing metabolically healthy from unhealthy obesity (MHU). Our analysis did not reveal significant differences in LAMA4 expression between IR and IS subjects between both fat depots (Supplementary Figure S3A). However, we observed a correlation between LAMA4 expression and alanine transaminase (ALAT; ρ = −0.32, p = 0.039, N = 42) in IR SC AT, and positive correlations with HbA1c of male IR individuals in SC AT (ρ = 0.68, p = 0.016, N = 12) and VIS AT (ρ = 0.60, p = 0.041, N = 12). In the SC AT of IS individuals, we found a negative correlation between LAMA4 expression and mean adipocyte size (ρ = −0.41, p = 0.021, N = 31) as well as positive correlation with serum adiponectin (ρ = 0.46, p = 0.009, N = 31). After p adjustment, no correlation remained significant. Correlations are summarized in Supplementary Figure S3B and can be further explored in Supplementary Table S5.
In summary, we are not able to confirm differential LAMA4 expression between IS and IR individuals but found LAMA4 expression correlation with HbA1c in males, among others.
4. Discussion
To the best of our knowledge, this study is one of the first investigations relating LAMA4 expression to obesity-related health impairments with RNA sequencing technology. This LAMA4 expression analysis of previously unprecedented scale provides a good overview of LAMA4 interaction with clinical parameters.
In this study, we aimed at investigating the expression of LAMA4 in SC and VIS AT depots across three distinct cohorts consisting of 1586 tissue donors from the LOBB. Specifically, we sought to explore the overall association between LAMA4 expression and clinical parameters in a cross-sectional cohort primarily comprised of individuals with obesity. Moreover, we focused on investigating the potential differences in LAMA4 expression between metabolically healthy and unhealthy individuals with obesity, as well as changes in LAMA4 expression in response to extensive weight loss following bariatric surgery.
In both the cross-sectional and bariatric surgery cohorts, we found a significantly higher LAMA4 expression in VIS AT compared to SC AT (Figure 1A,B and Figure 2A). This observation is comparable to similar results from a previous study by Goddi et al. [21]. While Goddi et al. report correlation between LAMA4 in SC AT and body weight in HFD mice [21], we found significant associations between LAMA4 and body fat in VIS AT (Figure 1C,D); WHR in both SC and VIS AT; and hip circumference in SC AT (Figure 2B). Also, it is worth noting that RNA sequencing data from Goddi et al. demonstrated higher expression of LAMA4 in obese women when compared to lean women [21]. We were unable to confirm this finding in our cohort (Figure 1D), possibly due to our cohort’s structure comprising of individuals predominantly with obesity and a low number of individuals with normal or overweight. Nevertheless, our findings support previous reports [18,20,21] which associated LAMA4 expression with obesity and increased fat mass.
Several studies have shown that LAMA4 knock-out in mice resulted in healthier phenotypes and metabolisms [13,18,20]. Contrasting these results, we did not find associations of low LAMA4 expression with metabolically healthy obesity. In accordance with previous results [21], we did not find changes in LAMA4 expression after weight loss induced by bariatric surgery (Figure 2A). The absence of correlations between LAMA4 and post-surgery WHR (Figure 2B) suggests that LAMA4 expression is not affected by weight loss induced changes in adipose tissue function or cellular composition. Notably, we investigated whole AT biopsies and therefore cannot know whether or not LAMA4 expression is altered at the adipocyte level.
Interestingly, gene enrichment analyses identified an overrepresentation of pathways relating LAMA4 to mitochondrial activity and energy expenditure, (Figure 1F, Supplementary Figure S2B) supporting recent observations in LAMA4 −/− mice [13]. Additionally, we found a strong correlation between LAMA4 and UCP1 (Supplementary Figure S2C,D), an indicator of thermogenic activity in (brown) adipocytes [41] suggesting that LAMA4 is connected to energy expenditure signatures at the tissue level.
LAMA4 knock-out in mice has been shown to preserve insulin sensitivity under DIO conditions [13], but we could not find significant expression differences between insulin-sensitive and insulin-resistant individuals in our MHU cohort (Supplementary Figure S3A). LAMA4 was previously reported to be upregulated in adipogenesis [14] and might therefore alter AT function at early stages of AT formation, while changes in phenotype during adulthood might not affect LAMA4 expression.
We need to acknowledge some limitations of our study. Although we did not include patients that reported smoking in the 12 months prior to surgery, we were not able to evaluate alcohol consumption as a potential confounding factor. Our cohort was composed of individuals predominantly with obesity and only a low number of individuals with normal or overweight could be included (N = 31). Despite the small sample size in the subgroups of metabolically healthy obesity with or without insulin resistance (N = 73) and in the 2-step surgery longitudinal cohorts (N = 65), these subgroups represent very well-characterized and, for the purpose of our study, relatively large groups. However, the lower patient numbers in these subgroups may be considered a limitation.
In conclusion, we show an association of LAMA4 expression with parameters of fat accumulation and distribution in patients with obesity. However, at least in adults, LAMA4 expression does not seem to be directly regulated by alterations in fat mass, obesity status, or metabolic health. Since we did not find differences in LAMA4 expression between insulin-sensitive and insulin-resistant individuals with obesity, we propose that the previously suggested association of LAMA4 with maintained metabolic health regardless of obesity requires altered LAMA4 expression during early AT developmental stages. Our cohorts do not cover AT developmental stages, which is why we cannot comment on the developmental impact of LAMA4 in AT within this study’s scope. Our data are indicative of associations between LAMA4, obesity, and adipose tissue function, but further studies are required to substantiate whether LAMA4 is mechanistically linked to AT dysfunction and fat mass.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11102806/s1. Figure S1. Correlation of LAMA4 expression with clinical parameters within the cross-sectional cohort. Figure S2. Gene set enrichment analysis and correlation of LAMA4 within the cross-sectional cohort. Figure S3. Analysis of LAMA4 gene expression and correlation within the metabolic healthy and unhealthy obesity cohort. Table S1: Correlation analysis of LAMA4 gene expression within the cross-sectional cohort. Table S2: Gene ontology gene set enrichment analysis within the cross-sectional cohort. Table S3: Kyoto Encyclopedia of Genes and Genomes gene set enrichment analysis within the cross-sectional cohort. Table S4: Correlation analysis of LAMA4 gene expression within the bariatric surgery cohort. Table S5: Correlation analysis of LAMA4 gene expression within the cohort for distinguishing metabolically healthy versus unhealthy obesity.
Author Contributions
Conceptualization, M.B. and A.H.; Methodology, A.H., A.G., P.C. and T.H.; Formal Analysis, T.H., A.H., A.G. and F.N.; Resources, M.B. and C.W.; Data Curation, C.W., W.S., A.G. and H.D.; Writing—Original Draft Preparation T.H., A.H. and M.B; Writing—Review and Editing, T.H., P.C., A.G., F.N., W.S., H.D., C.W., A.H. and M.B.; Visualization, A.H. and T.H.; Supervision, M.B.; Project Administration, A.H.; Funding Acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
M.B. received funding from grants from the DFG (German Research Foundation)—Projektnummer 209933838—SFB 1052 (project B1) and by Deutsches Zentrum für Diabetesforschung (DZD, Grant: 82DZD00601).
Institutional Review Board Statement
The study was approved by the Ethics Committee of the University of Leipzig (approval no: 159-12-21052012) and performed in accordance with the Declaration of Helsinki.
Informed Consent Statement
All subjects gave written informed consent before participating in the study.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The human RNA sequencing data from the LOBB have not been deposited in a public repository due to restriction by patient consent but are available from the corresponding author on request.
Acknowledgments
We thank all patients and their families for participating in this study.
Conflicts of Interest
M.B. received honoraria as a consultant and speaker from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Lilly, Novo Nordisk, Novartis, and Sanofi. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization. WHO European Regional Obesity Report 2022; Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases Progress Monitor. 2017. Available online: https://www.who.int/publications-detail-redirect/WHO-NMH-NVI-17.9 (accessed on 23 March 2023).
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C. Multiple risk factors for cardiovascular disease and diabetes mellitus. Am. J. Med. 2007, 120 (Suppl. 1), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Yurchenco, P.D. Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef]
- LeBleu, V.S.; MacDonald, B.; Kalluri, R. Structure and Function of Basement Membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef]
- Reggio, S.; Rouault, C.; Poitou, C.; Bichet, J.-C.; Prifti, E.; Bouillot, J.-L.; Rizkalla, S.; Lacasa, D.; Tordjman, J.; Clément, K. Increased Basement Membrane Components in Adipose Tissue During Obesity: Links with TGFβ and Metabolic Phenotypes. J. Clin. Endocrinol. Metab. 2016, 101, 2578–2587. [Google Scholar] [CrossRef]
- Yang, X.; Scott, H.A.; Monickaraj, F.; Xu, J.; Ardekani, S.; Nitta, C.F.; Cabrera, A.; McGuire, P.G.; Mohideen, U.; Das, A.; et al. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J. 2016, 30, 601–611. [Google Scholar] [CrossRef]
- Vaicik, M.K.; Blagajcevic, A.; Ye, H.; Morse, M.C.; Yang, F.; Goddi, A.; Brey, E.M.; Cohen, R.N. The Absence of Laminin α4 in Male Mice Results in Enhanced Energy Expenditure and Increased Beige Subcutaneous Adipose Tissue. Endocrinology 2018, 159, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Noro, A.; Sillat, T.; Virtanen, I.; Ingerpuu, S.; Bäck, N.; Konttinen, Y.T.; Korhonen, M. Laminin Production and Basement Membrane Deposition by Mesenchymal Stem Cells upon Adipogenic Differentiation. J. Histochem. Cytochem. 2013, 61, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Moest, H.; Frei, A.P.; Bhattacharya, I.; Geiger, M.; Wollscheid, B.; Wolfrum, C. Malfunctioning of adipocytes in obesity is linked to quantitative surfaceome changes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 1208–1216. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Belen Bravo, S.; Pérez-Sotelo, D.; Alonso, J.; Isabel Castro, A.; Baamonde, I.; Baltar, J.; Casanueva, F.F.; Pardo, M. CILAIR-Based Secretome Analysis of Obese Visceral and Subcutaneous Adipose Tissues Reveals Distinctive ECM Remodeling and Inflammation Mediators. Sci. Rep. 2015, 5, 12214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, X.; Sun, A.; Zhang, L.; Zhang, J.; Yan, Y. Adipose extracellular matrix deposition is an indicator of obesity and metabolic disorders. J. Nutr. Biochem. 2023, 111, 109159. [Google Scholar] [CrossRef] [PubMed]
- Vaicik, M.K.; Thyboll Kortesmaa, J.; Movérare-Skrtic, S.; Kortesmaa, J.; Soininen, R.; Bergström, G.; Ohlsson, C.; Chong, L.Y.; Rozell, B.; Emont, M.; et al. Laminin α4 Deficient Mice Exhibit Decreased Capacity for Adipose Tissue Expansion and Weight Gain. PLoS ONE 2014, 9, e109854. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y. Laminin: Loss-of-function studies. Cell. Mol. Life Sci. 2017, 74, 1095–1115. [Google Scholar] [CrossRef]
- Bailey, J.L.; Burk, D.H.; Burke, S.J.; Reed, S.D.; Ghosh, S.; Elks, C.M. Adipocyte-Specific Laminin Alpha 4 Deletion Preserves Adipose Tissue Health despite Increasing Adiposity. Biomedicines 2022, 10, 2077. [Google Scholar] [CrossRef]
- Goddi, A.; Carmona, A.; Schroedl, L.; White, J.M.; Piron, M.J.; De Leon, A.; Casimiro, I.; Hoffman, A.; Porras, M.A.G.; Brey, E.M.; et al. Laminin-α4 Is Upregulated in Both Human and Murine Models of Obesity. Front. Endocrinol. 2021, 12, 698621. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Liu, Y.; Wang, Z. Effect of LAMA4 on Prognosis and Its Correlation with Immune Infiltration in Gastric Cancer. BioMed Res. Int. 2021, 2021, 6428873. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Heiker, J.T.; Gärtner, D.; Björnson, E.; Schön, M.R.; Flehmig, G.; Klöting, N.; Krohn, K.; Fasshauer, M.; Stumvoll, M.; et al. Extensive weight loss reveals distinct gene expression changes in human subcutaneous and visceral adipose tissue. Sci. Rep. 2015, 5, 14841. [Google Scholar] [CrossRef] [PubMed]
- Langhardt, J.; Flehmig, G.; Klöting, N.; Lehmann, S.; Ebert, T.; Kern, M.; Schön, M.R.; Gärtner, D.; Lohmann, T.; Dressler, M.; et al. Effects of Weight Loss on Glutathione Peroxidase 3 Serum Concentrations and Adipose Tissue Expression in Human Obesity. Obes. Facts 2018, 11, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-sensitive obesity. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef] [PubMed]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Picelli, S.; Faridani, O.R.; Björklund, Å.K.; Winberg, G.; Sagasser, S.; Sandberg, R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014, 9, 171–181. [Google Scholar] [CrossRef]
- Song, Y.; Milon, B.; Ott, S.; Zhao, X.; Sadzewicz, L.; Shetty, A.; Boger, E.T.; Tallon, L.J.; Morell, R.J.; Mahurkar, A.; et al. A comparative analysis of library prep approaches for sequencing low input translatome samples. BMC Genom. 2018, 19, 696. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Ferreira, A.-M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nie, J.; Sicotte, H.; Li, Y.; Eckel-Passow, J.E.; Dasari, S.; Vedell, P.T.; Barman, P.; Wang, L.; Weinshiboum, R.; et al. Measure transcript integrity using RNA-seq data. BMC Bioinform. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.I.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Lauss, M. swamp: Visualization, Analysis and Adjustment of High-Dimensional Data in Respect to Sample Annotations. R Package Version 1.5.1. 2019. Available online: https://CRAN.R-project.org/package=swamp (accessed on 23 March 2022).
- Miller, H.E.; Bishop, A.J.R. Correlation AnalyzeR: Functional predictions from gene co-expression correlations. BMC Bioinform. 2021, 22, 206. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with statistical details: The “ggstatsplot” approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Herve, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-81-2. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 23 March 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).