Chemokine Ligand 2 Promotes Migration in Osteosarcoma by Regulating the miR-3659/MMP-3 Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Analysis of mRNA Expression Profiles from the Cancer Genome Atlas (TCGA) Database
2.3. Immunohistochemistry (IHC) Staining
2.4. Cell Migration and Invasion Assay
2.5. Quantitative Real-Time PCR (q-PCR)
2.6. Analysis of Clinical Samples
2.7. Cell Transfection
2.8. Western Blot
2.9. Statistics
3. Results
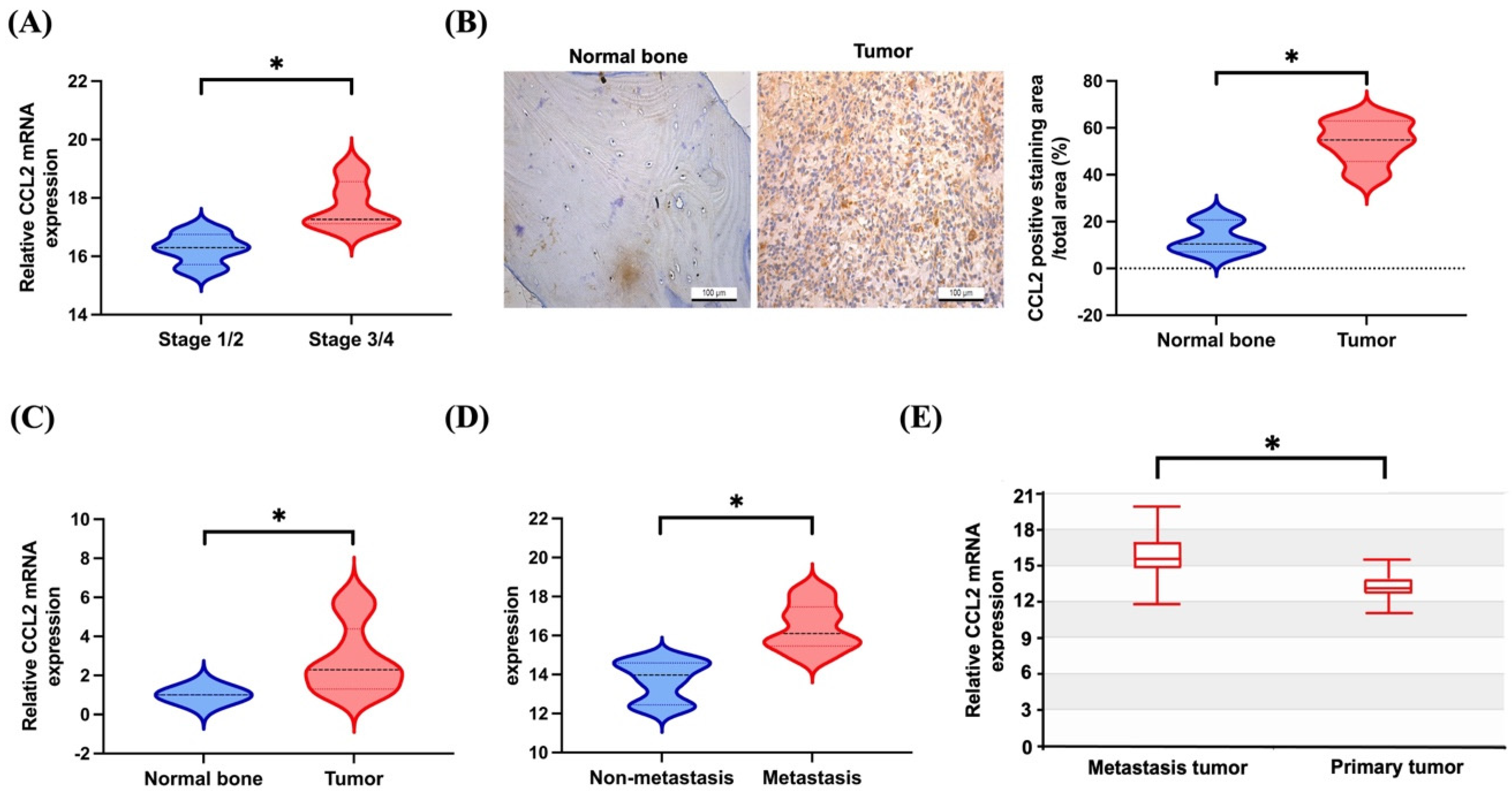
3.1. Elevated Levels of CCL2 Expression in Patients with Metastatic Osteosarcoma
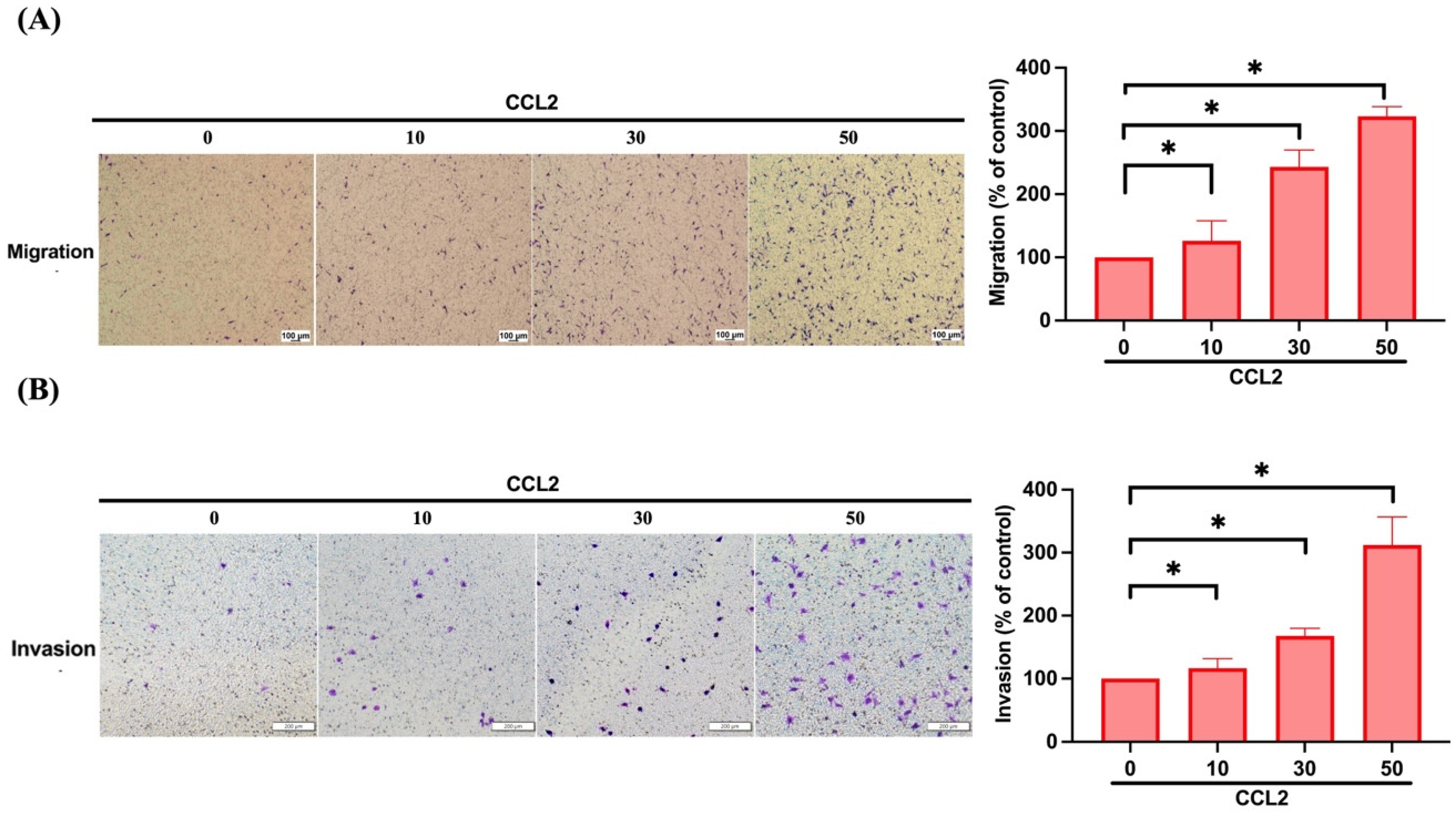
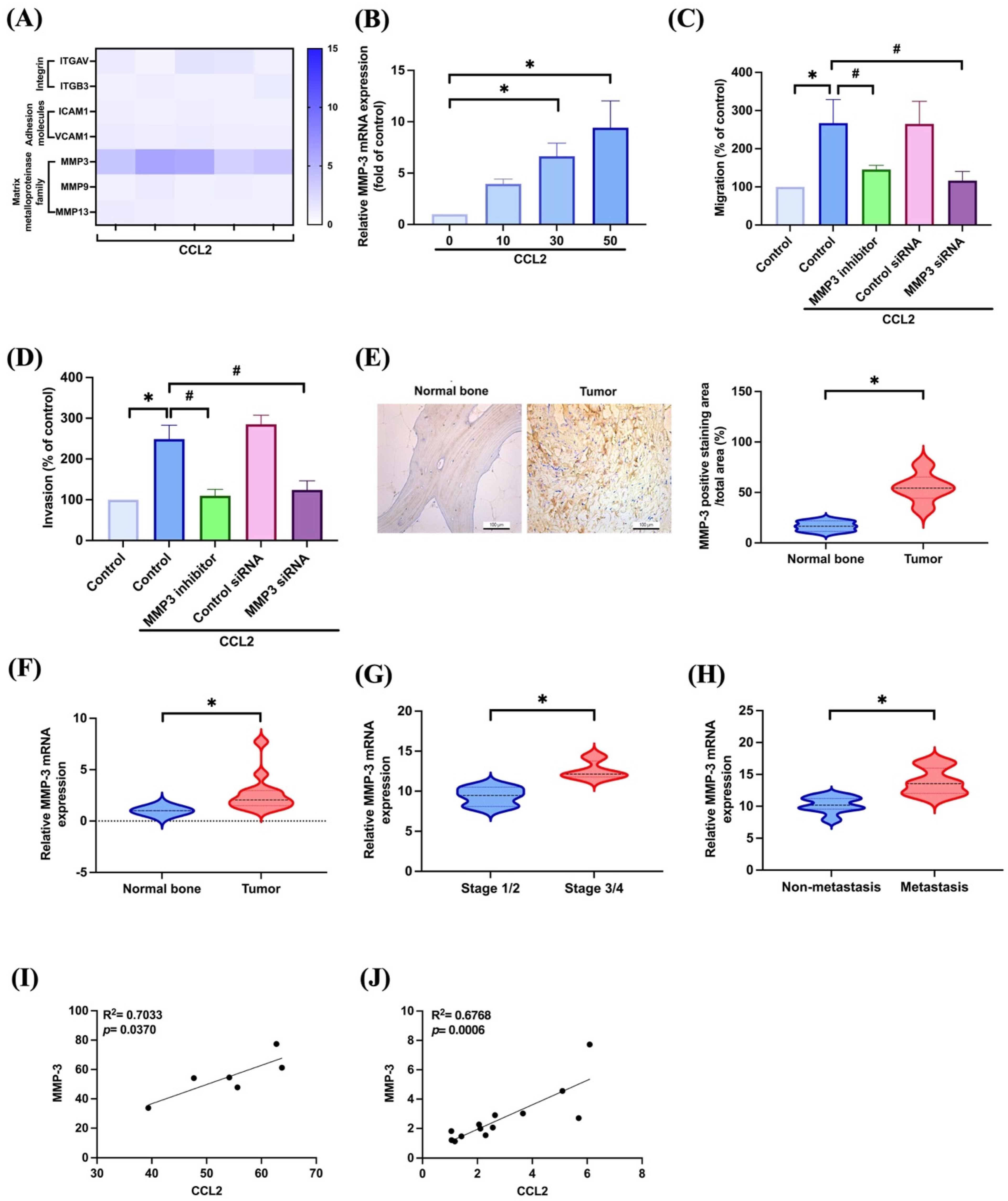
3.2. CCL2 Increases Cell Migration and Invasion in Osteosarcoma by Enhancing MMP-3 Production
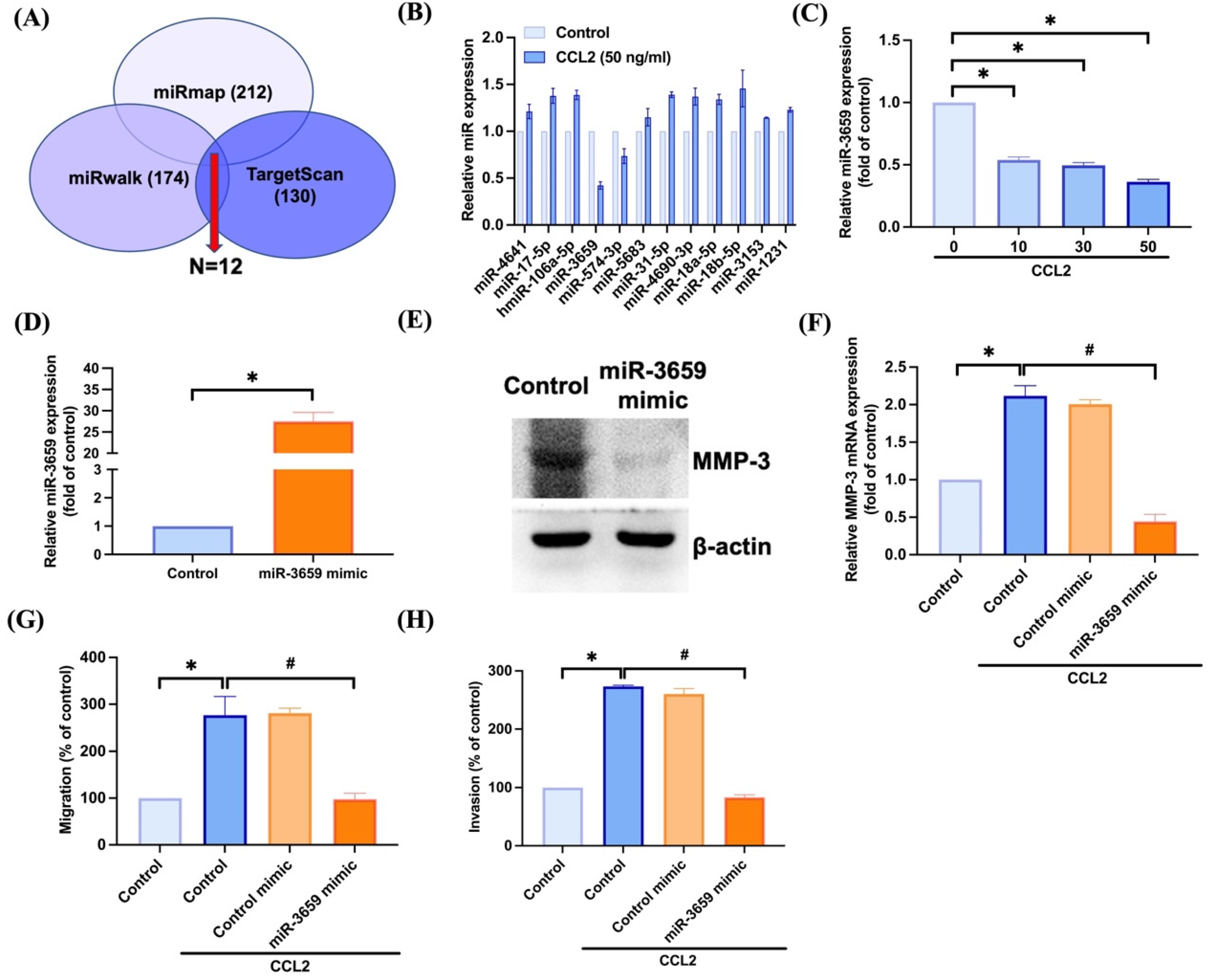
3.3. Inhibiting miR-3659 Regulates CCL2-Induced Promotion of MMP-3 Production and Motility in Osteosarcoma
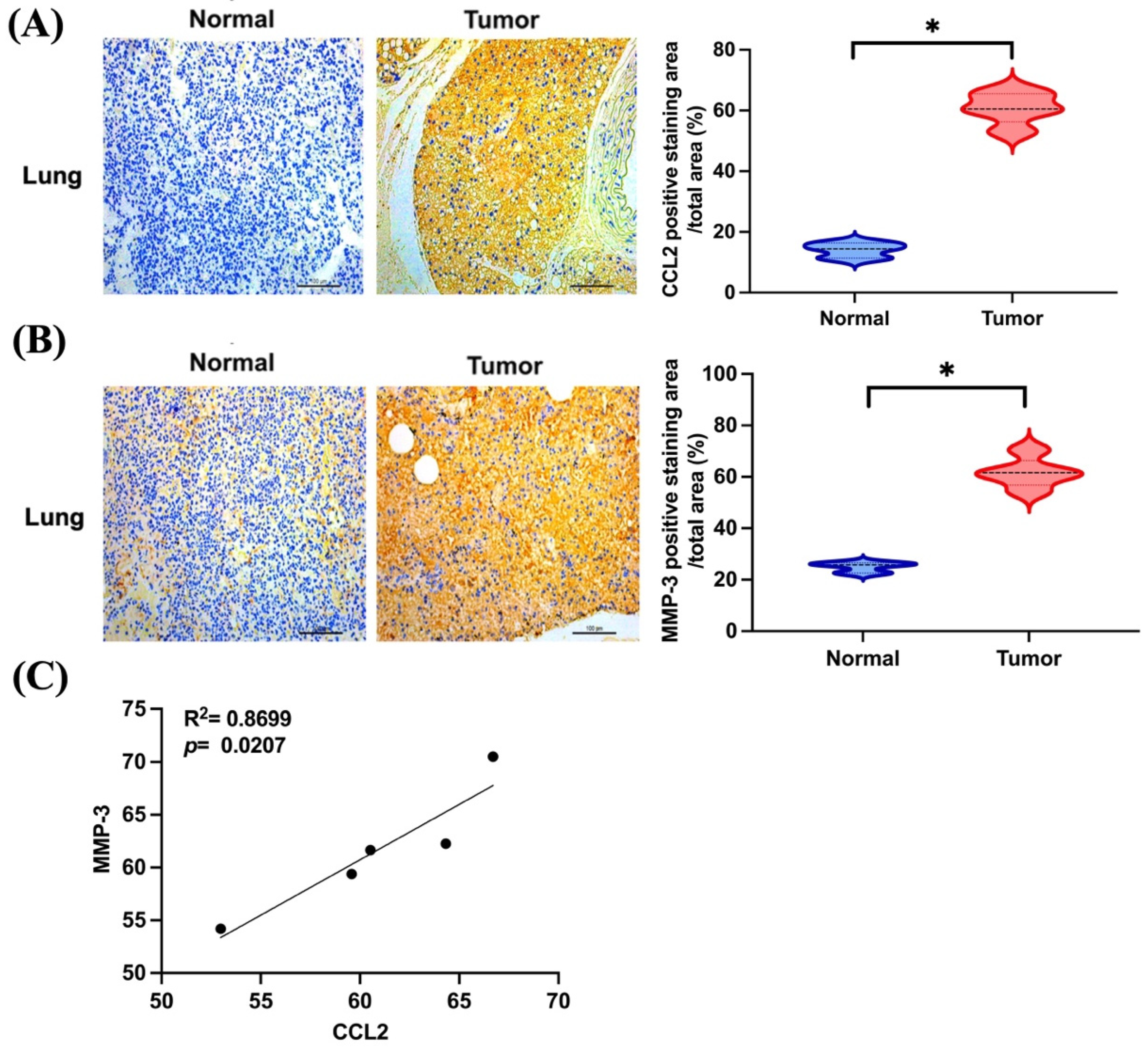
3.4. CCL2 and MMP-3 Are Highly Expressed in Lung Metastatic Osteosarcoma In Vivo
4. Discussion
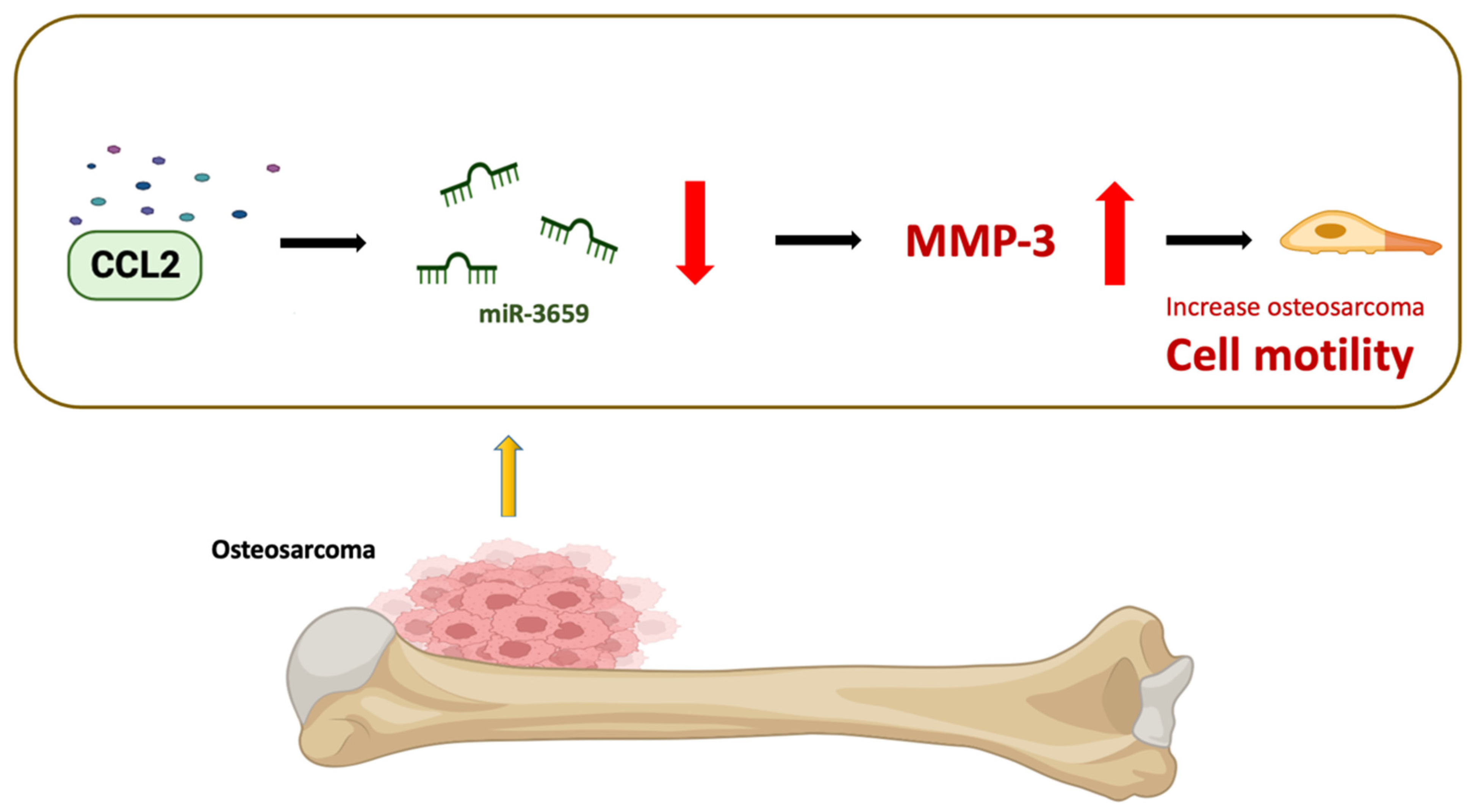
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belayneh, R.; Fourman, M.S.; Bhogal, S.; Weiss, K.R. Update on osteosarcoma. Curr. Oncol. Rep. 2021, 23, 71. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Meltzer, P.S.; Helman, L.J. New horizons in the treatment of osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Lu, S.; Chen, Y.; Shan, L.; Zhao, X.; Xu, Y. The role of notch ligand jagged1 in osteosarcoma proliferation, metastasis, and recurrence. J. Orthop. Surg. Res. 2021, 16, 226. [Google Scholar] [CrossRef]
- Lei, T.; Qian, H.; Lei, P.; Hu, Y. Ferroptosis-related gene signature associates with immunity and predicts prognosis accurately in patients with osteosarcoma. Cancer Sci. 2021, 112, 4785–4798. [Google Scholar] [CrossRef]
- Trang, N.T.N.; Lai, C.Y.; Tsai, H.C.; Huang, Y.L.; Liu, S.C.; Tsai, C.H.; Fong, Y.C.; Tzeng, H.E.; Tang, C.H. Apelin promotes osteosarcoma metastasis by upregulating plod2 expression via the hippo signaling pathway and hsa_circ_0000004/mir-1303 axis. Int. J. Biol. Sci. 2023, 19, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-H.; Lin, F.-L.; Tong, K.-B.; Hou, S.-M.; Liu, J.-F. Transforming growth factor alpha promotes osteosarcoma metastasis by icam-1 and pi3k/akt signaling pathway. Biochem. Pharmacol. 2014, 89, 453–463. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lin, C.-W.; Hsieh, Y.-S.; Cheng, H.-L.; Lue, K.-H.; Yang, S.-F.; Lu, K.-H. Selaginella tamariscina (beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing mmp-2 and mmp-9 secretions via p38 and Akt signaling pathways. Food Chem. Toxicol. 2013, 59, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhong, L.; Li, Y.; Xiao, D.; Zhang, R.; Liao, D.; Lv, D.; Wang, X.; Wang, J.; Xie, X.; et al. Up-regulation of pcolce by twist1 promotes metastasis in osteosarcoma. Theranostics 2019, 9, 4342–4353. [Google Scholar] [CrossRef]
- Qin, Q.; Gomez-Salazar, M.; Tower, R.J.; Chang, L.; Morris, C.D.; McCarthy, E.F.; Ting, K.; Zhang, X.; James, A.W. nell1 regulates the matrisome to promote osteosarcoma progression. Cancer Res. 2022, 82, 2734–2747. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yi, Q.; He, J.; Huang, D.; Xiong, J.; Sun, W.; Sun, W. Extracellular vesicles in tumorigenesis, metastasis, chemotherapy resistance and intercellular communication in osteosarcoma. Bioengineered 2023, 14, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Kim, D.-K.; Ki, H.-H.; Kwon, Y.-B.; Lee, Y.-M.; Kim, D.-K. Downregulation of erk signaling impairs u2os osteosarcoma cell migration in collagen matrix by suppressing mmp9 production. Oncol. Lett. 2014, 7, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Chen, P.C.; Chang, T.M.; Hou, C.H. Thrombospondin-2 stimulates mmp-9 production and promotes osteosarcoma metastasis via the plc, pkc, c-src and nf-κb activation. J. Cell Mol. Med. 2020, 24, 12826–12839. [Google Scholar] [CrossRef]
- Huang, J.-F.; Du, W.-X.; Chen, J.-J. Elevated expression of matrix metalloproteinase-3 in human osteosarcoma and its association with tumor metastasis. J. BUON 2016, 21, 235–243. [Google Scholar]
- Deng, B.; Qiu, B. Shikonin inhibits invasiveness of osteosarcoma through mmp13 suppression. Tumor Biol. 2015, 36, 9311–9317. [Google Scholar] [CrossRef]
- Wang, S.-T.; Li, D.-Z.; Li, J.-M.; Fang, J.; Li, H.-Z.; Tong, P.-J.; Liu, F.-C. Lentivirus-mediated rna interference targeting ubch10 reduces cell growth and invasion of human osteosarcoma cells via inhibition of ki-67 and matrix metalloproteinases. Oncol. Lett. 2015, 9, 2171–2176. [Google Scholar] [CrossRef]
- Chen, C.L.; Zhang, L.; Jiao, Y.R.; Zhou, Y.; Ge, Q.F.; Li, P.C.; Sun, X.J.; Lv, Z. Mir-134 inhibits osteosarcoma cell invasion and metastasis through targeting mmp1 and mmp3 in vitro and in vivo. FEBS Lett. 2019, 593, 1089–1101. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Su, H.-L.; Huang, C.-Y.; Fong, Y.-C.; Hsu, C.-J.; Tang, C.-H. Ctgf increases matrix metalloproteinases expression and subsequently promotes tumor metastasis in human osteosarcoma through down-regulating mir-519d. Oncotarget 2014, 5, 3800–3812. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. In vitro modulation of mmp-2 and mmp-9 in pediatric human sarcoma cell lines by cytokines, inducers and inhibitors. Int. J. Oncol. 2014, 44, 27–34. [Google Scholar] [CrossRef]
- Yang, J.; Lv, X.; Chen, J.; Xie, C.; Xia, W.; Jiang, C.; Zeng, T.; Ye, Y.; Ke, L.; Yu, Y.; et al. Ccl2-ccr2 axis promotes metastasis of nasopharyngeal carcinoma by activating erk1/2-mmp2/9 pathway. Oncotarget 2016, 7, 15632–15647. [Google Scholar] [CrossRef]
- Tang, C.-H.; Tsai, C.-C. Ccl2 increases mmp-9 expression and cell motility in human chondrosarcoma cells via the ras/raf/mek/erk/nf-κb signaling pathway. Biochem. Pharmacol. 2012, 83, 335–344. [Google Scholar] [CrossRef]
- Li, S.; Lu, J.; Chen, Y.; Xiong, N.; Li, L.; Zhang, J.; Yang, H.; Wu, C.; Zeng, H.; Liu, Y. Mcp-1-induced erk/gsk-3β/snail signaling facilitates the epithelial–mesenchymal transition and promotes the migration of mcf-7 human breast carcinoma cells. Cell. Mol. Immunol. 2017, 14, 621–630. [Google Scholar] [CrossRef]
- Panee, J. Monocyte chemoattractant protein 1 (mcp-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef]
- Si, M.-Y.; Fan, Z.-C.; Li, Y.-Z.; Chang, X.-L.; Xie, Q.-D.; Jiao, X.-Y. The prognostic significance of serum and cerebrospinal fluid mmp-9, ccl2 and svcam-1 in leukemia cns metastasis. J. Neuro-Oncol. 2015, 122, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-J.; Chao, C.-C.; Chang, A.-C.; Chen, P.-C.; Cheng, F.-J.; Liu, J.-F.; Liu, P.-I.; Huang, C.-L.; Guo, J.-H.; Huang, W.-C.; et al. Cigarette smoke-promoted increases in osteopontin expression attract mesenchymal stem cell recruitment and facilitate lung cancer metastasis. J. Adv. Res. 2022, 41, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Achudhan, D.; Liu, S.; Lin, Y.; Lee, H.; Wang, S.; Huang, W.; Wu, Y.; Kuo, Y.; Tang, C. Antcin K inhibits vegf-dependent angiogenesis in human rheumatoid arthritis synovial fibroblasts. J. Food Biochem. 2022, 46, e14022. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-P.; Chen, P.-C.; Wang, S.-W.; Fong, Y.-C.; Tsai, C.-H.; Tsai, F.-J.; Chung, J.-G.; Huang, C.-Y.; Yang, J.-S.; Hsu, Y.-M.; et al. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J. Funct. Foods 2019, 52, 537–544. [Google Scholar] [CrossRef]
- Lee, H.-P.; Wu, Y.-C.; Chen, B.-C.; Liu, S.-C.; Li, T.-M.; Huang, W.-C.; Hsu, C.-J.; Tang, C.-H. soya-cerebroside reduces interleukin production in human rheumatoid arthritis synovial fibroblasts by inhibiting the erk, nf-κb and ap-1 signalling pathways. Food Agric. Immunol. 2020, 31, 740–750. [Google Scholar] [CrossRef]
- Lee, H.-P.; Liu, S.-C.; Wang, Y.-H.; Chen, B.-C.; Chen, H.-T.; Li, T.-M.; Huang, W.-C.; Hsu, C.-J.; Wu, Y.-C.; Tang, C.-H. Cordycerebroside a suppresses vcam-dependent monocyte adhesion in osteoarthritis synovial fibroblasts by inhibiting mek/erk/ap-1 signaling. J. Funct. Foods 2021, 86, 104712. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Roles of ccl2-ccr2 axis in the tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 8530. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Mizokami, A. Is the c-c motif ligand 2-c-c chemokine receptor 2 axis a promising target for cancer therapy and diagnosis? Int. J. Mol. Sci. 2020, 21, 9328. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Zhao, S.; Xuan, M.; Zhu, G.; Zhang, Z.; Guo, W. Ccl2 promotes lymphatic metastasis via activating rhoa and rac1 pathway and predict prognosis to some extent in tongue cancer. Cancer Biol. Ther. 2023, 24, 2205342. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y.; Pienta, K.J. Multiple roles of chemokine (c-c motif) ligand 2 in promoting prostate cancer growth. J. Natl. Cancer Inst. 2010, 102, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Knopfová, L.; Biglieri, E.; Volodko, N.; Masařík, M.; Hermanová, M.; Garzón, J.F.G.; Dúcka, M.; Kučírková, T.; Souček, K.; Šmarda, J.; et al. Transcription factor c-myb inhibits breast cancer lung metastasis by suppression of tumor cell seeding. Oncogene 2017, 37, 1020–1030. [Google Scholar] [CrossRef]
- Yoshimura, T.; Li, C.; Wang, Y.; Matsukawa, A. The chemokine monocyte chemoattractant protein-1/ccl2 is a promoter of breast cancer metastasis. Cell Mol. Immunol. 2023, 20, 714–738. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, A.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Rodríguez-Fernández, J.L.; et al. Ccl2 shapes macrophage polarization by gm-csf and m-csf: Identification of ccl2/ccr2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Lazennec, G.; Richmond, A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol. Med. 2010, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Lamhamedi-Cherradi, S.-E.; Mohiuddin, S.; Mishra, D.K.; Krishnan, S.; Velasco, A.R.; Vetter, A.M.; Pence, K.; McCall, D.; Truong, D.D.; Cuglievan, B.; et al. Transcriptional activators yap/taz and axl orchestrate dedifferentiation, cell fate, and metastasis in human osteosarcoma. Cancer Gene Ther. 2021, 28, 1325–1338. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.P.; Kuang, J.M.; Liu, Y.H. Proteomic investigation of resistance to chemotherapy drugs in osteosarcoma. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2017, 26, 145–153. [Google Scholar] [CrossRef]
- Biazzo, A.; De Paolis, M. Multidisciplinary approach to osteosarcoma. Acta Orthop. Belg. 2016, 82, 690–698. [Google Scholar]
- Bajpai, M.; Pardhe, N.; Chandolia, B.; Arora, M. Osteogenic sarcoma of mandible. J. Coll. Physicians Surg. Pak. JCPSP 2017, 27, S26–S27. [Google Scholar]
- Gebhard, C.; Miller, I.; Hummel, K.; Ondrovics, M.N.N.; Schlosser, S.; Walter, I. Comparative proteome analysis of monolayer and spheroid culture of canine osteosarcoma cells. J. Proteom. 2018, 177, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Rocca, M.; Salone, M.; Balladelli, A.; Ferrari, S.; Palmerini, E.; Forni, C.; Briccoli, A. High grade osteosarcoma of the extremities with lung metastases at presentation: Treatment with neoadjuvant chemotherapy and simultaneous resection of primary and metastatic lesions. J. Surg. Oncol. 2008, 98, 415–420. [Google Scholar] [CrossRef]
- Chao, C.-C.; Lee, W.-F.; Yang, W.-H.; Lin, C.-Y.; Han, C.-K.; Huang, Y.-L.; Fong, Y.-C.; Wu, M.-H.; Lee, I.-T.; Tsai, Y.-H.; et al. Igfbp-3 stimulates human osteosarcoma cell migration by upregulating vcam-1 expression. Life Sci. 2021, 265, 118758. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Chiang, Y.-C.; Yu, P.-A.; Peng, K.-T.; Chi, M.-C.; Lee, M.-H.; Fang, M.-L.; Lee, K.-H.; Hsu, L.-F.; Liu, J.-F. A role of cxcl1 drives osteosarcoma lung metastasis via vcam-1 production. Front. Oncol. 2021, 11, 735277. [Google Scholar] [CrossRef]
- Wang, S.-W.; Wu, H.-H.; Liu, S.-C.; Wang, P.-C.; Ou, W.-C.; Chou, W.-Y.; Shen, Y.-S.; Tang, C.-H. Ccl5 and ccr5 interaction promotes cell motility in human osteosarcoma. PLoS ONE 2012, 7, e35101. [Google Scholar]
- Levinson, H.; Hopper, J.E.; Ehrlich, H.P. Overexpression of integrin alphav promotes human osteosarcoma cell populated collagen lattice contraction and cell migration. J. Cell. Physiol. 2002, 193, 219–224. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Lai, Y.-Y.; Hsu, H.-C.; Fong, Y.-C.; Lien, M.-Y.; Tang, C.-H. Ccl4 stimulates cell migration in human osteosarcoma via the mir-3927-3p/integrin αvβ3 axis. Int. J. Mol. Sci. 2021, 22, 12737. [Google Scholar] [CrossRef]
- Wu, C.-L.; Tsai, H.-C.; Chen, Z.-W.; Wu, C.-M.; Li, T.-M.; Fong, Y.-C.; Tang, C.-H. Ras activation mediates wisp-1-induced increases in cell motility and matrix metalloproteinase expression in human osteosarcoma. Cell. Signal. 2013, 25, 2812–2822. [Google Scholar] [CrossRef]
- Yang, M.-D.; Lin, K.-C.; Lu, M.-C.; Jeng, L.-B.; Hsiao, C.-L.; Yueh, T.-C.; Fu, C.-K.; Li, H.-T.; Yen, S.-T.; Lin, C.-W.; et al. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. BioMedicine 2017, 7, 10. [Google Scholar] [CrossRef]
- Puppo, M.; Valluru, M.K.; Clézardin, P. Micrornas and their roles in breast cancer bone metastasis. Curr. Osteoporos. Rep. 2021, 19, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Zhao, H.; Gu, A.; Wu, Y.; Weng, Y.; Li, S.; Song, J.; Gu, X.; Qiu, J.; Zhao, W. Mirna-10a-5p inhibits cell metastasis in hepatocellular carcinoma via targeting ska1. Kaohsiung J. Med. Sci. 2021, 37, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, J.; Wang, Y.; Zhou, S. The role of mirna-433 in malignant tumors of digestive tract as tumor suppressor. Cancer Rep. 2022, 5, e1694. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of micrornas in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Azimi, M.; Totonchi, M.; Ebrahimi, M. Determining the role of micrornas in self-renewal, metastasis and resistance to drugs in human gastric cancer based on data mining approaches: A systematic review. Cell J. 2022, 24, 1. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Huang, Y.-L.; Tsai, H.-C.; Chang, A.-C.; Ko, C.-Y.; Fong, Y.-C.; Tang, C.-H. Chemokine Ligand 2 Promotes Migration in Osteosarcoma by Regulating the miR-3659/MMP-3 Axis. Biomedicines 2023, 11, 2768. https://doi.org/10.3390/biomedicines11102768
Chang Y-H, Huang Y-L, Tsai H-C, Chang A-C, Ko C-Y, Fong Y-C, Tang C-H. Chemokine Ligand 2 Promotes Migration in Osteosarcoma by Regulating the miR-3659/MMP-3 Axis. Biomedicines. 2023; 11(10):2768. https://doi.org/10.3390/biomedicines11102768
Chicago/Turabian StyleChang, Yu-Hsiang, Yuan-Li Huang, Hsiao-Chi Tsai, An-Chen Chang, Chih-Yuan Ko, Yi-Chin Fong, and Chih-Hsin Tang. 2023. "Chemokine Ligand 2 Promotes Migration in Osteosarcoma by Regulating the miR-3659/MMP-3 Axis" Biomedicines 11, no. 10: 2768. https://doi.org/10.3390/biomedicines11102768
APA StyleChang, Y.-H., Huang, Y.-L., Tsai, H.-C., Chang, A.-C., Ko, C.-Y., Fong, Y.-C., & Tang, C.-H. (2023). Chemokine Ligand 2 Promotes Migration in Osteosarcoma by Regulating the miR-3659/MMP-3 Axis. Biomedicines, 11(10), 2768. https://doi.org/10.3390/biomedicines11102768





