Multi-Omics Analysis Reveals the Role of Sigma-1 Receptor in a Takotsubo-like Cardiomyopathy Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
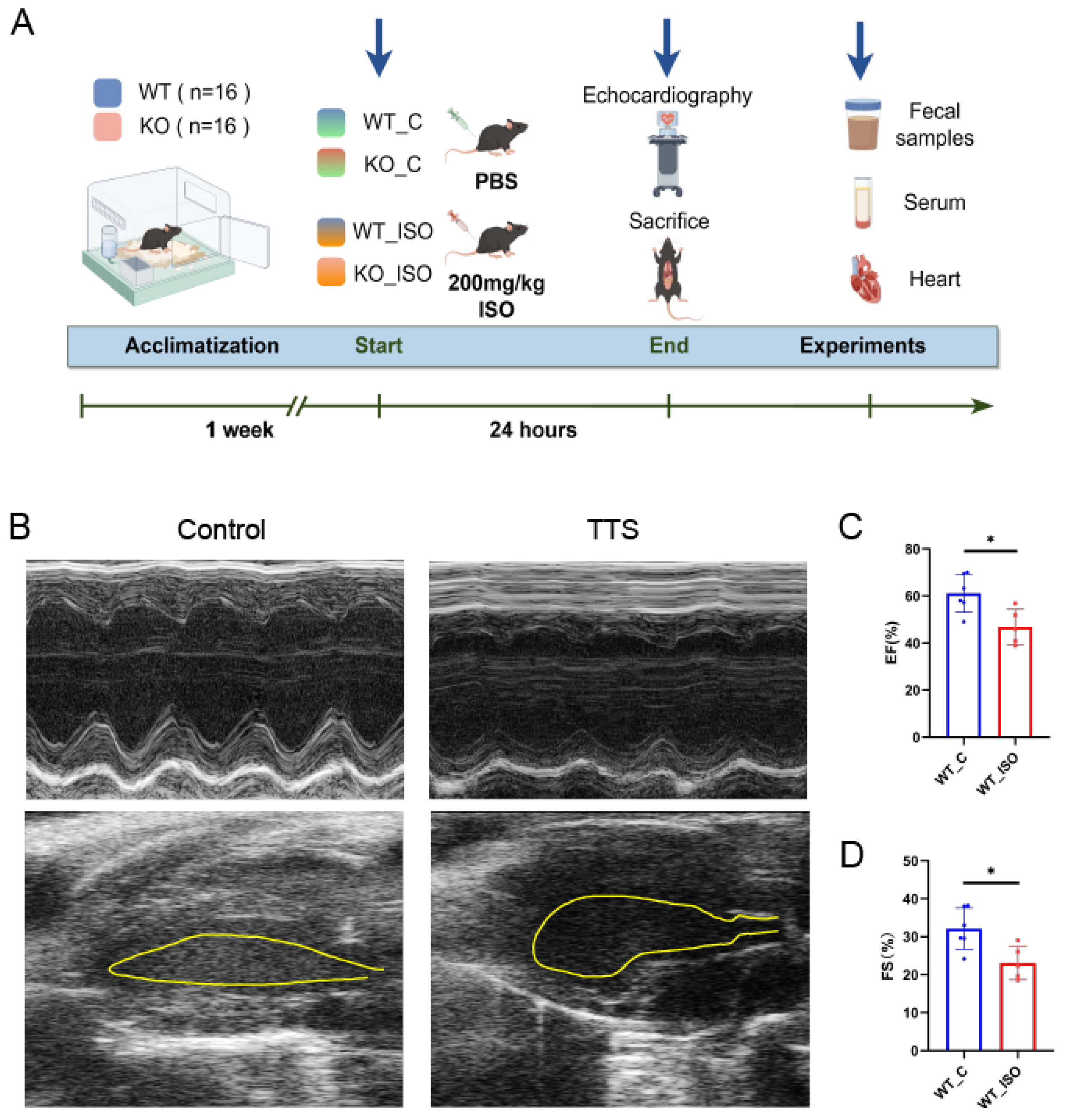
2.2. Mouse TTS Model
2.3. Echocardiography
2.4. Fecal Microbiota Analysis by 16S rRNA Sequencing
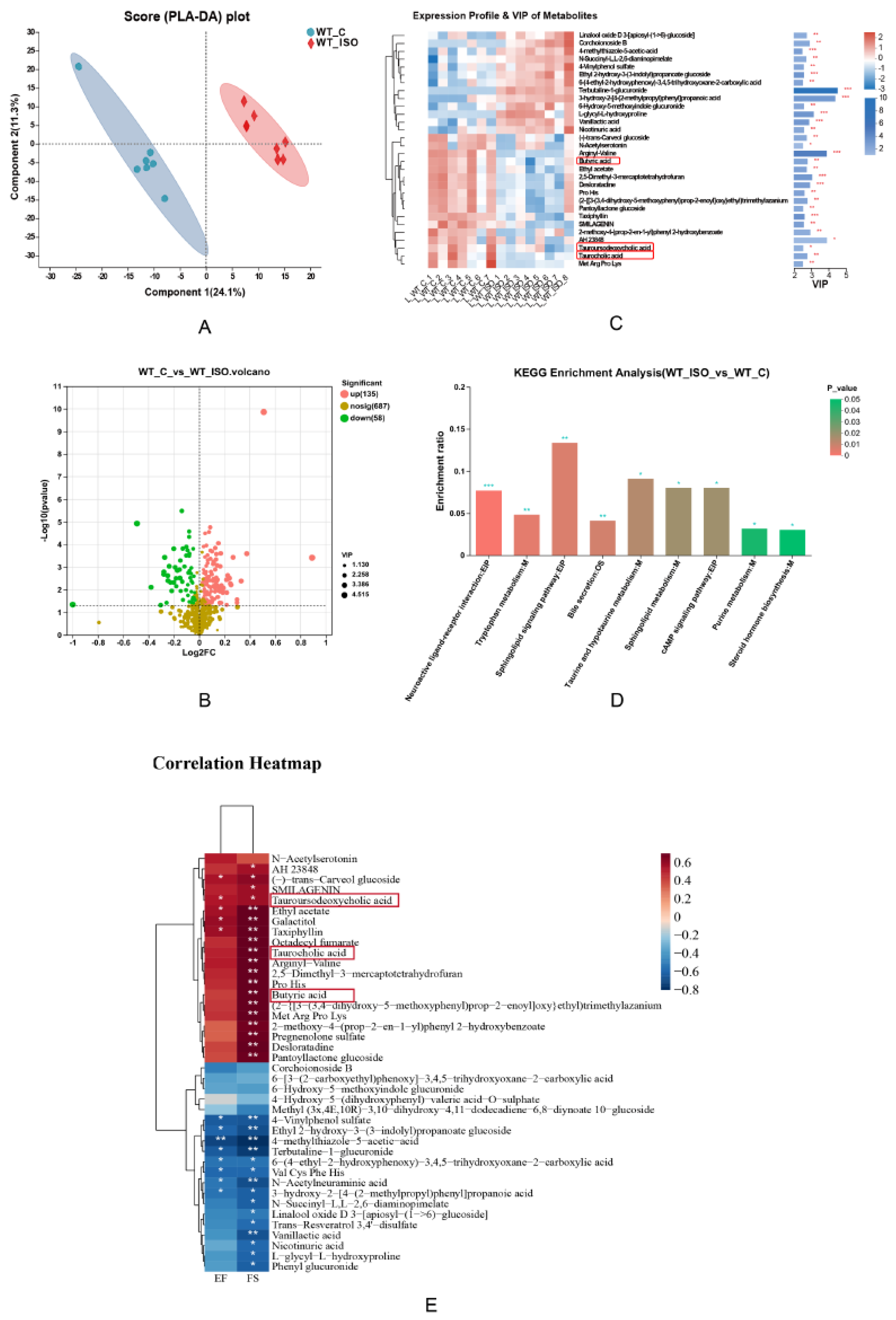
2.5. Metabolic Analysis of Serum by LC–MS/MS
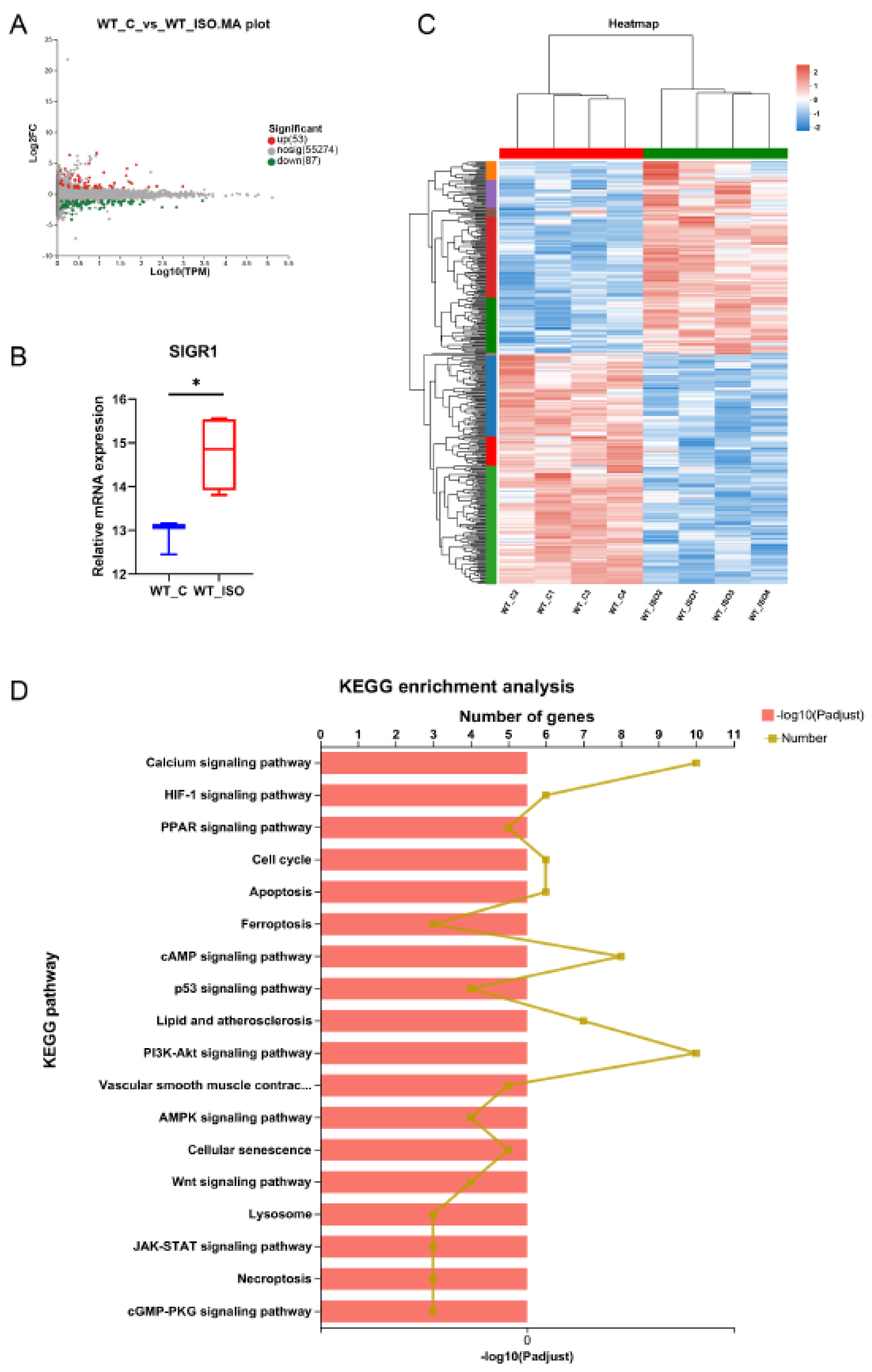
2.6. Transcriptome Analysis of Heart Tissue
2.7. Correlation Analysis
2.8. Statistical Analysis
3. Results
3.1. ISO Led to TTS-like Cardiac Dysfunction
3.2. TTS Induced by ISO Led to Gut Microbial Dysbiosis
3.3. TTS Induced by ISO Led to Serum Metabolic Disturbance
3.4. TTS Induced by ISO Led to Cardiac Transcriptome Variation
3.5. Sigmar1 Knockout Resulted in Cardiac Dysfunction
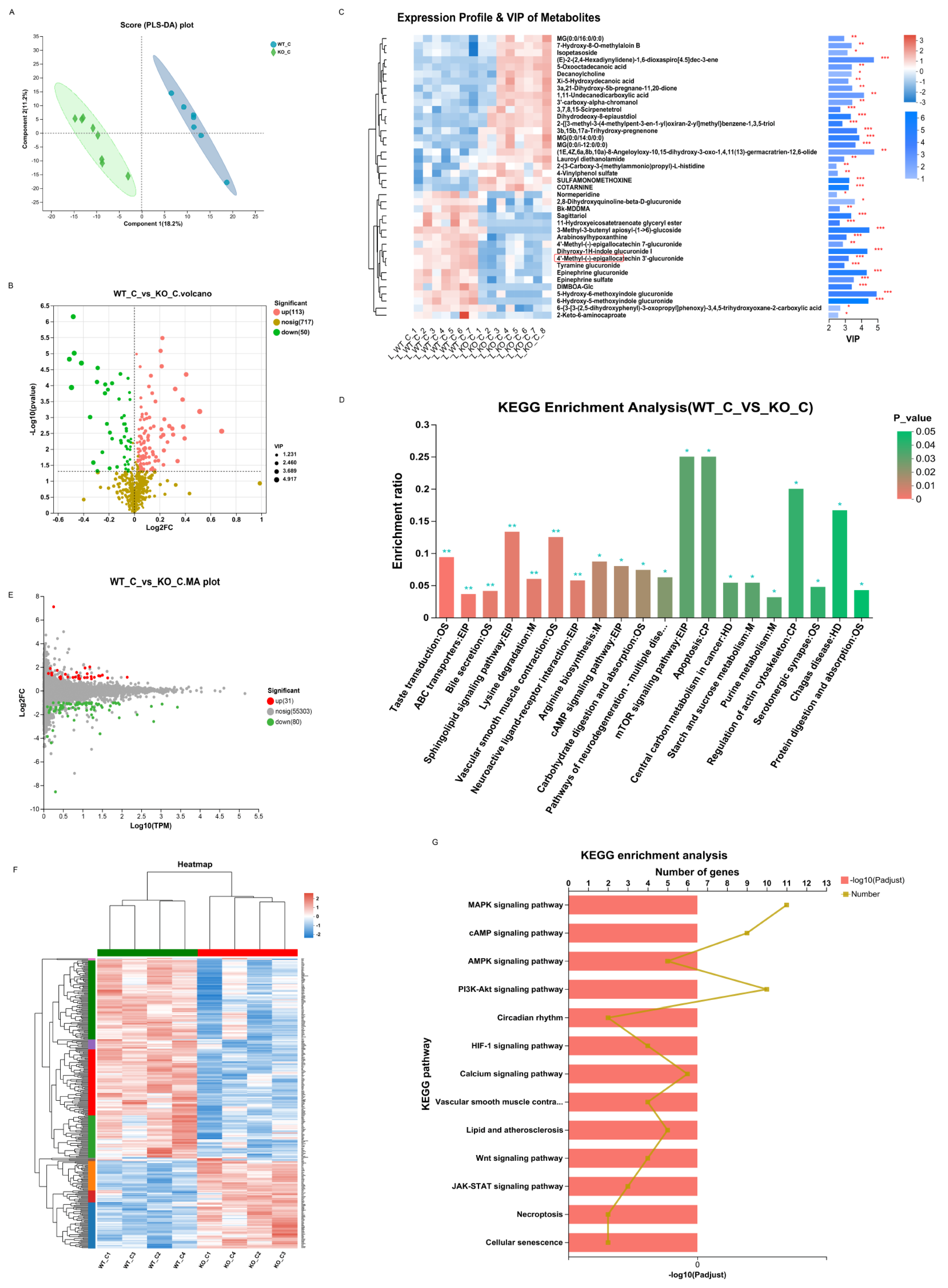
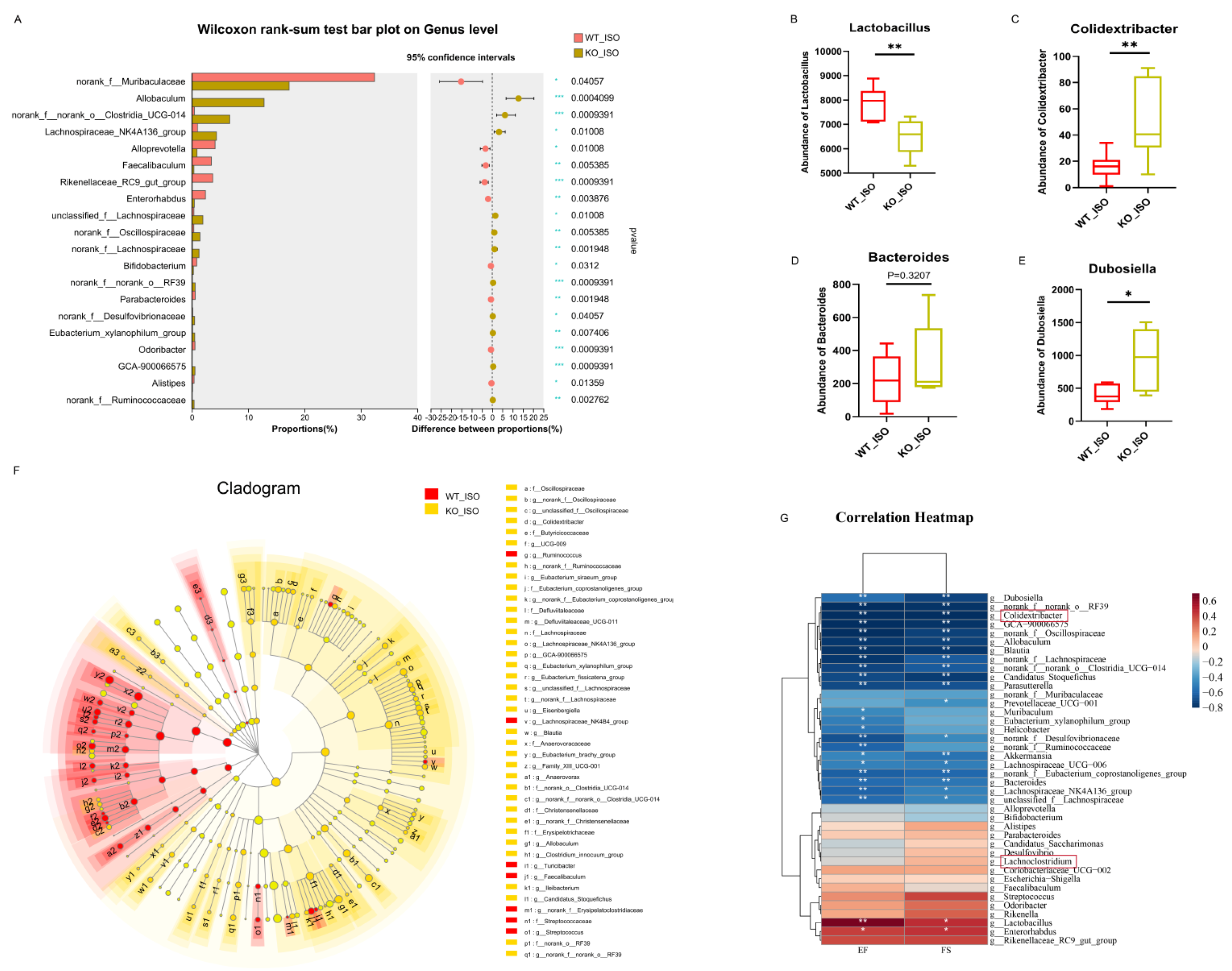
3.6. Sigmar1 Knockout Resulted in Alterations in the Gut Microbial Composition, Serum Metabolites, and Cardiac Transcriptome
3.7. Sigmar1 Knockout Aggravated Cardiac Dysfunction in TTS Mice
3.8. Sigmar1 Knockout Aggravated Gut Microbial Dysbiosis in TTS Mice
3.9. Sigmar1 Knockout Aggravated Serum Metabolic Disturbance in TTS Mice
3.10. Sigmar1 Knockout Changed the Cardiac Transcriptome in TTS Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyd, B.; Solh, T. Takotsubo cardiomyopathy: Review of broken heart syndrome. JAAPA Off. J. Am. Acad. Phys. Assist. 2020, 33, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Cammann, V.L.; Würdinger, M.; Ghadri, J.R.; Templin, C. Takotsubo Syndrome: Uncovering Myths and Misconceptions. Curr. Atheroscleros. Rep. 2021, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, M.; Forte, G.; Favieri, F.; Agostini, F.; Giovannoli, J.; Arcari, L.; Passaseo, I.; Semeraro, R.; Camastra, G.; Langher, V.; et al. The Broken Heart: The Role of Life Events in Takotsubo Syndrome. J. Clin. Med. 2021, 10, 4940. [Google Scholar] [CrossRef]
- Patel, V.; Levy, S.; Malik, I.; Fertleman, M.B.; Koizia, L.J. Takotsubo cardiomyopathy in elderly female trauma patients: A case series. J. Med. Case Rep. 2021, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Bitigen, A.; Cevik, C.; Biteker, M.; Can, M.; Ozdemir, N. Takotsubo cardiomyopathy in a 52 year old patient. Int. J. Cardiol. 2009, 132, e23–e25. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Alva, D.; Pampa-Quenta, O.D.; Rodríguez-Olivares, R.R.; Gabino-Gonzáles, G. Clinical features and complications of Takotsubo syndrome in a peruvian social security referral center. Rev. Peru. Med. Exp. Salud Publica 2019, 36, 255–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omerovic, E.; Citro, R.; Bossone, E.; Redfors, B.; Backs, J.; Bruns, B.; Ciccarelli, M.; Liam, S.; Dawson, C.D.; Grassi, G.; et al. Pathophysiology of Takotsubo syndrome—A joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—Part 2: Vascular pathophysiology, gender and sex hormones, genetics, chronic cardiovascular problems and clinical implications. Eur. J. Heart Fail. 2022, 24, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Y-Hassan, S.; Holmin, S.; Abdula, G.; Böhm, F. Thrombo-embolic complications in takotsubo syndrome: Review and demonstration of an illustrative case. Clin. Cardiol. 2019, 42, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mrejen-Shakin, K.; Lopez, R.; Shenoy, M.M. Life-threatening Takotsubo Cardiomyopathy. Am. Heart Hosp. J. 2011, 9, 119–121. [Google Scholar] [CrossRef]
- Mitsuma, W.; Kodama, M.; Ito, M.; Kimura, S.; Tanaka, K.; Hoyano, M.; Hirono, S.; Aizawa, Y. Thromboembolism in Takotsubo cardiomyopathy. Int. J. Cardiol. 2010, 139, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Postalian, A.; Hernandez-Vila, E.; Uribe, C.; Costello, B. COVID-19 and the Heart: Could Transient Takotsubo Cardiomyopathy Be Related to the Pandemic by Incidence and Mechanisms? Front. Cardiovasc. Med. 2022, 9, 919715. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Barsness, G.W.; Herrmann, J.; Nandan, S.; Gulati, R.A.; Prasad, A. Comparison of Complications and In-Hospital Mortality in Takotsubo (Apical Ballooning/Stress) Cardiomyopathy Versus Acute Myocardial Infarction. Am. J. Cardiol. 2020, 132, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, G.P. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef] [PubMed]
- Veillet-Chowdhury, M.; Hassan, S.F.; Stergiopoulos, K. Takotsubo cardiomyopathy: A review. Acute Card. Care 2014, 16, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Omerovic, E.; Citro, R.; Bossone, E.; Redfors, B.; Backs, J.; Bruns, B.; Ciccarelli, M.; Liam, S.; Dawson, C.D.; Grassi, G.; et al. Pathophysiology of Takotsubo syndrome—A joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—Part 1: Overview and the central role for catecholamines and sympathetic nervous system. Eur. J. Heart Fail. 2022, 24, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Bergamaschi, L.; Rambaldi, P.; Gatta, G.; Foà, A.; Angeli, F.; Fabrizio, M.; Casella, G.; Barbieri, M.; Galiè, N.; et al. Impact of Admission Hyperglycemia on Heart Failure Events and Mortality in Patients with Takotsubo Syndrome at Long-term Follow-up: Data from High-Glucotako Investigators. Diabetes Care 2021, 44, 2158–2161. [Google Scholar] [CrossRef]
- Biolo, A.A.S.; Rosa, N.G.; Mazzotti, S.; Belló-Klein, M.A.; Rohde, L.E.; Clausell, N. The role of adrenergic receptor polymorphisms in heart failure. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Med. Biol. 2006, 39, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Cortesi, P.; Vespignani, R.; Bronico, I.; Gallio, C.; Flospergher, M.; Matteucci, L.; Frassineti, G.L. Takotsubo Syndrome (TTS) in Onco-Hematologic Patients: Retrospective Analysis and Focus on the Correlation or Not with Anticancer Drugs. Case Reports and Review of the Literature. Front. Oncol. 2022, 12, 875391. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Chowdhury, S.; Aishwarya, A.R.; Orr, A.W.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; Pattillo, C.B.; Bhuiyan, S. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. Biosci. Rep. 2017, 37, BSR20170898. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shen, H.; Li, J.; Guo, L.-W. SIGMAR1/Sigma-1 receptor ablation impairs autophagosome clearance. Autophagy 2019, 15, 1539–1557. [Google Scholar] [CrossRef]
- Chowdhury, S.; Alam, S.A.; Aishwarya, R.; Miriyala, S.; Panchatcharam, M.; Bhuiyan, M.A.N.; Jonette, M.; Wayne Orr, P.A.; James, J.; Osinska, H.; et al. Cardiac Dysfunction in the Sigma 1 Receptor Knockout Mouse Associated with Impaired Mitochondrial Dynamics and Bioenergetics. J. Am. Heart Assoc. 2018, 7, e9775. [Google Scholar] [CrossRef]
- Abdullah, C.S.; Aishwarya, R.; Alam, S.; Morshed, M.; Remex, N.S.; Nitu, S.; Kolluru, G.K.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; et al. Methamphetamine induces cardiomyopathy by Sigmar1 inhibition-dependent impairment of mitochondrial dynamics and function. Commun. Biol. 2020, 3, 682. [Google Scholar] [CrossRef] [PubMed]
- Bartolomaeus, H.; McParland, V.; Wilck, N. Gut-heart axis: How gut bacteria influence cardiovascular diseases. Herz 2020, 45, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Macías, I.; Orenes-Piñero, E.; Camelo-Castillo, A.; Rivera-Caravaca, J.M.; López-García, C.; Marín, F. Novel insights in the relationship of gut microbiota and coronary artery diseases. Crit. Rev. Food Sci. Nutr. 2022, 62, 3738–3750. [Google Scholar] [CrossRef]
- Wu, Z.-X.; Li, S.-F.; Chen, H.; Song, J.-X.; Gao, Y.-F.; Zhang, F.; Cao, C.-F. The changes of gut microbiota after acute myocardial infarction in rats. PLoS ONE 2017, 12, e0180717. [Google Scholar] [CrossRef]
- Lu, D.; Zou, X.; Zhang, H. The Relationship Between Atrial Fibrillation and Intestinal Flora with Its Metabolites. Front. Cardiovasc. Med. 2022, 9, 948755. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wang, Y.; Zhang, W.; Shang, H. Trimethylamine oxide: A potential target for heart failure therapy. Heart 2022, 108, 917–922. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chang, E.; Tang, X.; Watanabe, I.; Zhang, R.; Jeong, H.-W.; Adams, R.H.; Jain, M.K. Cardiac macrophages regulate isoproterenol-induced Takotsubo-like cardiomyopathy. J. Clin. Investig. 2022, 7, 156236. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qian, L.; Li, J.; Ming, H.; Fang, L.; Li, Y.; Zhang, M.; Xu, Y.; Ban, Y.; Zhang, W.; et al. GHSR deficiency exacerbates cardiac fibrosis: Role in macrophage inflammasome activation and myofibroblast differentiation. Cardiovasc. Res. 2020, 116, 2091–2102. [Google Scholar] [CrossRef]
- Picard, M.H.; Popp, R.L.; Weyman, A.E. Assessment of Left Ventricular Function by Echocardiography: A Technique in Evolution. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2008, 21, 14–21. [Google Scholar] [CrossRef]
- Keith, A.; Collins, C.; Korcarz, E.; Lang, R.M. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol. Genom. 2003, 13, 227–239. [Google Scholar]
- Chen, L.-J.; Zhi, X.; Zhang, K.-K.; Wang, L.-B.; Li, J.-H.; Liu, J.-L.; Xu, L.-L.; Yoshida, J.S.; Xie, X.-L.; Wang, Q. Escalating dose-multiple binge methamphetamine treatment elicits neurotoxicity, altering gut microbiota and fecal metabolites in mice. Food Chem. Toxicol. 2021, 148, 111946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-K.; Liu, J.-L.; Chen, L.-J.; Li, J.-H.; Yang, J.-Z.; Xu, L.-L.; Chen, Y.-K.; Zhang, Q.-Y.; Li, X.-W.; Liu, Y.; et al. Gut microbiota mediates methamphetamine-induced hepatic inflammation via the impairment of bile acid homeostasis. Food Chem. Toxicol. 2022, 166, 113208. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, S.; Liu, W.; Zou, B.; Lin, L.; Chen, M.; Liu, D.; Wang, M.; Li, L.; Cai, Y.; et al. LC-MS-based metabolomics analysis of Berberine treatment in ulcerative colitis rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1133, 121848. [Google Scholar] [CrossRef]
- Qu, D.; Zhang, K.; Chen, L.; Wang, Q.; Wang, H. RNA-sequencing analysis of the effect of luteolin on methamphetamine-induced hepatotoxicity in rats: A preliminary study. PeerJ 2020, 8, e8529. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, P.; Feng, X.; Ma, C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 3178–3189. [Google Scholar] [CrossRef]
- Gao, G.; Chen, W.; Yan, M.; Liu, J.; Luo, H.; Wang, C.; Yang, P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int. J. Mol. Med. 2020, 45, 195–209. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Delgado, S.; Sánchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules Produced by Probiotics and Intestinal Microorganisms with Immunomodulatory Activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E.; Sun, W.; Hu, Q.; Ji, W.; Wright, G.; Gu, Z.; Gonzalez, L.M.; et al. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.-H.; Xue, M.; Garay, J.A.R.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated with Gut Barrier Dysfunction in Healthy Relatives of Patients with Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, Y. Gut microbiome and its meta-omics perspectives: Profound implications for cardiovascular diseases. Gut Microbes 2021, 13, 1936379. [Google Scholar] [CrossRef]
- Levi, M.; Tarling, E.J.; Ahn, H.; Vallim, T.Q.d.A.; Vickers, K.C.; Rader, D.J.; Lefebvre, P.; Staels, B.; Hageman, J.; Herrema, H.; et al. Role of Bile Acid–Regulated Nuclear Receptor FXR and G Protein–Coupled Receptor TGR5 in Regulation of Cardiorenal Syndrome (Cardiovascular Disease and Chronic Kidney Disease). Hypertension 2016, 67, 1080–1084. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Garmaeva, S.; Kurilshikov, A.; Vila, A.V.; Gacesa, R.; Sinha, T.; Segal, E.; Weersma, R.K.; Wijmenga, C.; et al. The long-term genetic stability and individual specificity of the human gut microbiome. Cell 2021, 184, 2302–2315.e12. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Wang, Y.; Chen, X.; Wang, C.; Chen, X.; Yuan, X.; Liu, L.; Yang, J.; Zhou, X. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 1. [Google Scholar] [CrossRef]
- Aishwarya, R.; Abdullah, C.S.; Morshed, M.; Remex, N.S.; Bhuiyan, S. Sigmar1’s Molecular, Cellular, and Biological Functions in Regulating Cellular Pathophysiology. Front. Physiol. 2021, 12, 705575. [Google Scholar] [CrossRef]
- Lin, H.; Meng, L.; Sun, Z.; Sun, S.; Huang, X.; Lin, N.; Zhang, J.; Lu, W.; Yang, Q.; Chi, J.; et al. Yellow Wine Polyphenolic Compound Protects Against Doxorubicin-Induced Cardiotoxicity by Modulating the Composition and Metabolic Function of the Gut Microbiota. Circ. Heart Fail. 2021, 14, 1136–1150. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, J.; Li, Q.; Zhan, C.; Xu, X.; Zhang, X.; Ai, D.; Zhu, Y.; Wen, Z.; Wang, D.W. CYP2J2-derived EETs attenuated ethanol-induced myocardial dysfunction through inducing autophagy and reducing apoptosis. Free. Radic. Biol. Med. 2018, 117, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.A.; Wang, D.W. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv. Drug Deliv. Rev. 2011, 63, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-Inducible Factor 1 and Cardiovascular Disease. Annu. Rev. Physiol. 2014, 76, 39–56. [Google Scholar] [CrossRef]
- Jain, T.; Nikolopoulou, E.A.; Xu, Q.; Qu, A. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol. Ther. 2018, 183, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Clerk, A.; Sugden, H.P. Inflame my heart (by p38-MAPK). Circ. Res. 2006, 99, 455–458. [Google Scholar] [CrossRef]
- Sugden, P.H.; Clerk, A. “Stress-Responsive” Mitogen-Activated Protein Kinases (c-Jun N-Terminal Kinases and p38 Mitogen-Activated Protein Kinases) in the Myocardium. Circ. Res. 1998, 83, 345–352. [Google Scholar] [CrossRef]
- Page, C.; Doubell, A.F. Mitogen-activated protein kinase (MAPK) in cardiac tissues. Mol. Cell. Biochem. 1996, 157, 49–57. [Google Scholar] [CrossRef]
- Men, H.; Cai, H.; Cheng, Q.; Zhou, W.; Wang, X.; Huang, S.; Zheng, Y.; Cai, L. The regulatory roles of p53 in cardiovascular health and disease. Cell. Mol. Life Sci. 2021, 78, 2001–2018. [Google Scholar] [CrossRef]
- Pan, J.-A.; Tang, Y.; Yu, J.-Y.; Zhang, H.; Zhang, J.-F.; Wang, C.-Q.; Gu, J. miR-146a attenuates apoptosis and modulates autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2019, 10, 668. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.-Y.; Liu, Z.-Q.; Jiang, D.; Wu, S.-Y.; Guo, Y.-Q.; Tao, H.-M.; Sun, M.; You, L.-N.; Qin, S.; et al. TNAP inhibition attenuates cardiac fibrosis induced by myocardial infarction through deactivating TGF-β1/Smads and activating P53 signaling pathways. Cell Death Dis. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhai, P.; Hu, C.; Mukai, R.; Sciarretta, S.; Del Re, D.; Sadoshima, J. Lats2 promotes heart failure by stimulating p53-mediated apoptosis during pressure overload. Sci. Rep. 2021, 11, 23469. [Google Scholar] [CrossRef]
- Oras, B.J.; Redfors, A.; Ali, J.; Lundgren, C.; Sihlbom, A.; Seeman-Lodding, T.H.; Omerovic, E.; Ricksten, S.-E. Anaes-thetic-induced cardioprotection in an experimental model of the Takotsubo syndrome—Isoflurane vs. propofol. Acta Anaesthesiol. Scand. 2017, 61, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M.; Cheyne, L.; Brown, P.A.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Quagliariello, V.; Berretta, M.; Buccolo, S.; Iovine, M.; Paccone, A.; Cavalcanti, E.; Taibi, R.; Montopoli, M.; Botti, G.; Maurea, N. Polydatin Reduces Cardiotoxicity and Enhances the Anticancer Effects of Sunitinib by Decreasing Pro-Oxidative Stress, Pro-Inflammatory Cytokines, and NLRP3 Inflammasome Expression. Front. Oncol. 2021, 11, 680758. [Google Scholar] [CrossRef]
- Marfella, R.; Barbieri, M.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Paolisso, P.; Ambrosino, M.; Fava, I.; Materazzi, C.; Cinquegrana, G.; et al. Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy. J. Cardiol. 2016, 67, 153–161. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Q.; Yang, J.-Z.; Li, X.-W.; Chen, L.-J.; Zhang, K.-K.; Liu, J.-L.; Li, J.-H.; Hsu, C.; Chen, L.; et al. Multi-Omics Analysis Reveals the Role of Sigma-1 Receptor in a Takotsubo-like Cardiomyopathy Model. Biomedicines 2023, 11, 2766. https://doi.org/10.3390/biomedicines11102766
Liu Y, Chen Q, Yang J-Z, Li X-W, Chen L-J, Zhang K-K, Liu J-L, Li J-H, Hsu C, Chen L, et al. Multi-Omics Analysis Reveals the Role of Sigma-1 Receptor in a Takotsubo-like Cardiomyopathy Model. Biomedicines. 2023; 11(10):2766. https://doi.org/10.3390/biomedicines11102766
Chicago/Turabian StyleLiu, Yi, Qing Chen, Jian-Zheng Yang, Xiu-Wen Li, Li-Jian Chen, Kai-Kai Zhang, Jia-Li Liu, Jia-Hao Li, Clare Hsu, Long Chen, and et al. 2023. "Multi-Omics Analysis Reveals the Role of Sigma-1 Receptor in a Takotsubo-like Cardiomyopathy Model" Biomedicines 11, no. 10: 2766. https://doi.org/10.3390/biomedicines11102766
APA StyleLiu, Y., Chen, Q., Yang, J.-Z., Li, X.-W., Chen, L.-J., Zhang, K.-K., Liu, J.-L., Li, J.-H., Hsu, C., Chen, L., Zeng, J.-H., Wang, Q., Zhao, D., & Xu, J.-T. (2023). Multi-Omics Analysis Reveals the Role of Sigma-1 Receptor in a Takotsubo-like Cardiomyopathy Model. Biomedicines, 11(10), 2766. https://doi.org/10.3390/biomedicines11102766






