Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Plant Material and Isolation of Bioactive Components
2.2. Chemical and Reagents
2.3. Preparation and Characterization of LZP-SNEDDS
2.4. Preparation of Solid Dispersion (SD) Formulation
2.4.1. Microwave Method
2.4.2. Lyophilization Method
2.5. Scanning Electron Microscopy (SEM)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Powder X-ray Powder Diffraction (PXRD)
2.8. In Vitro Dissolution Test
2.9. Stability Study
2.10. Quantification of LZP Using the Developed UPLC-UV Method
2.11. Software
2.12. Statistical Analysis
3. Results
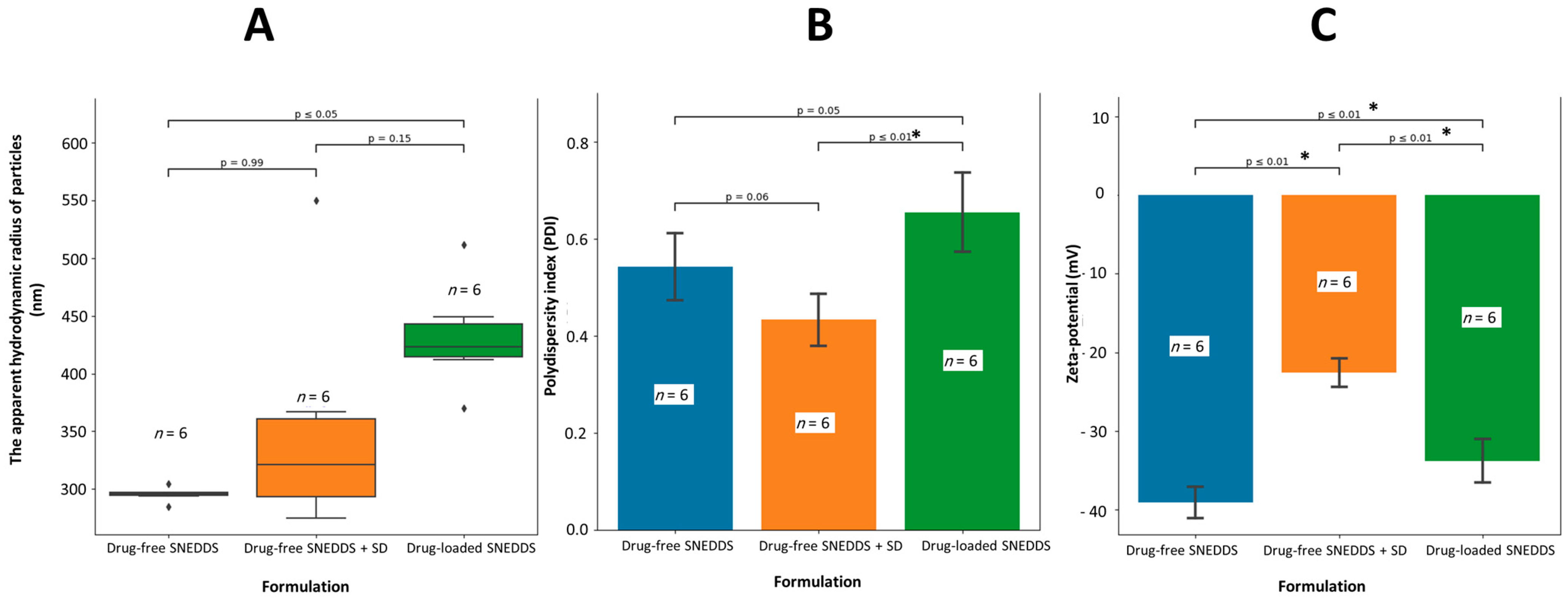
3.1. Characterization of Lansoprazole-Loaded Self-Nanoemulsifying Delivery System
3.2. SEM
3.2.1. Microwave Method
3.2.2. Lyophilization Method
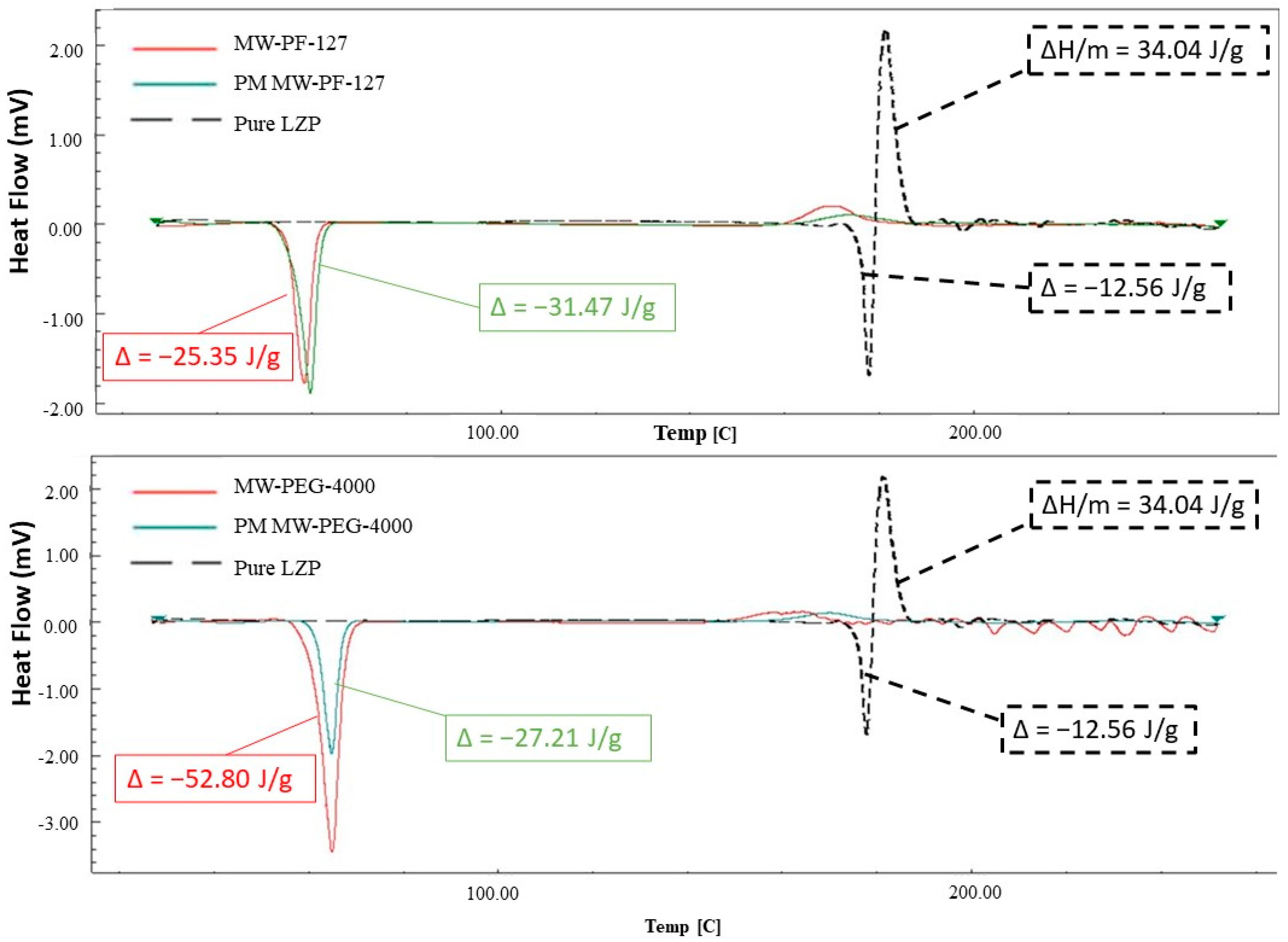
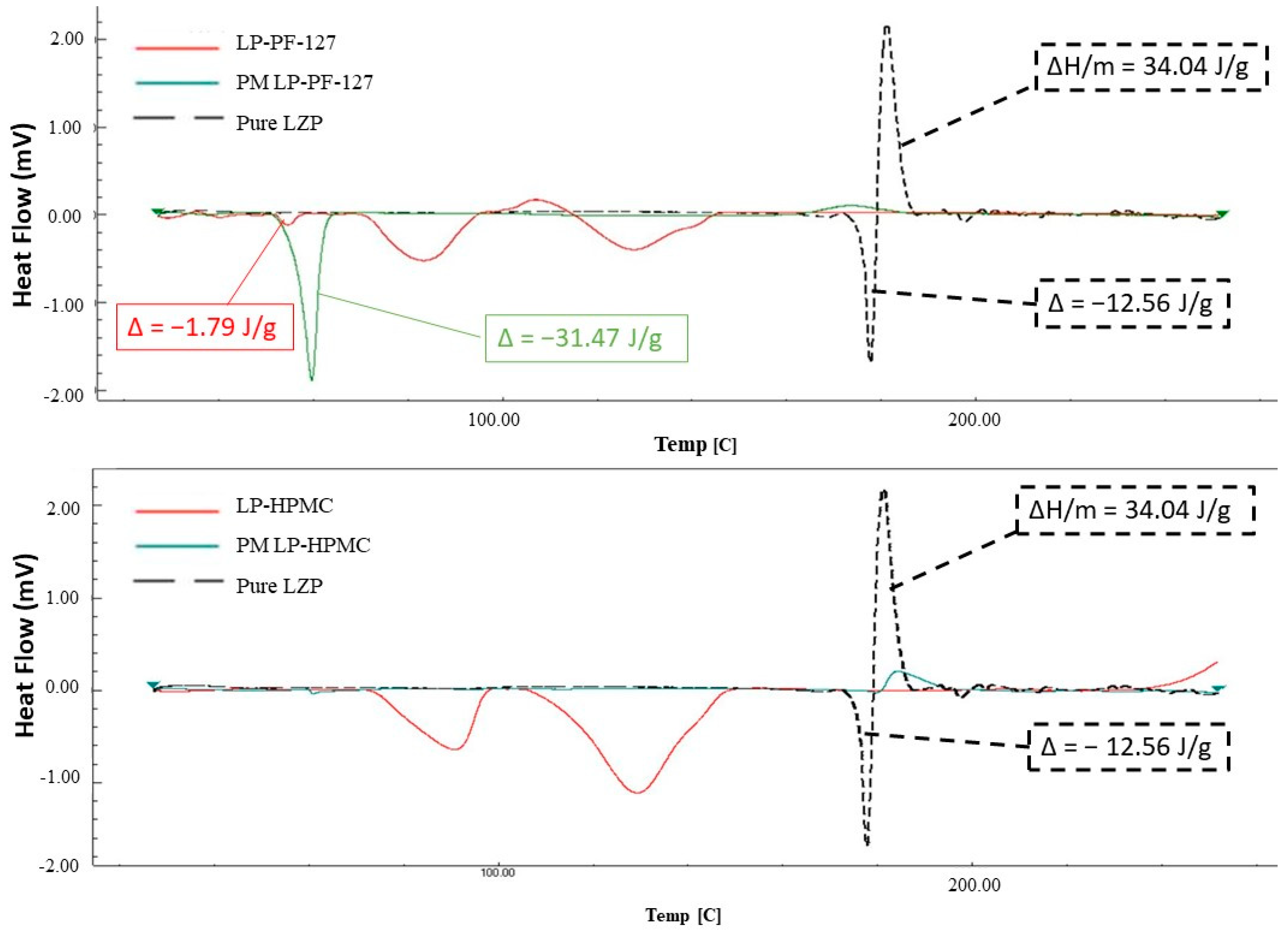
3.3. DSC
3.3.1. Microwave Method
3.3.2. Lyophilization Method
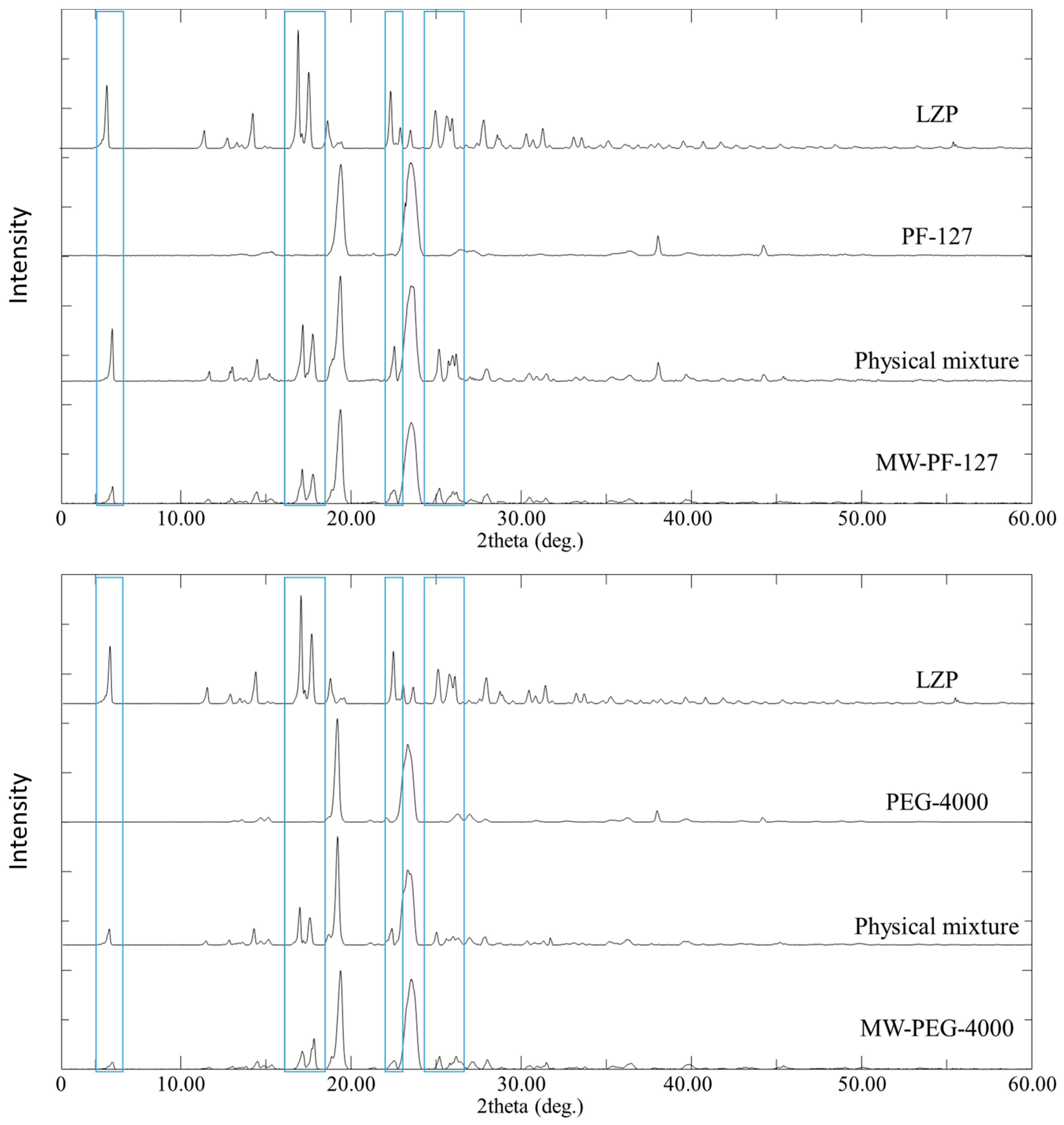
3.4. PXRD
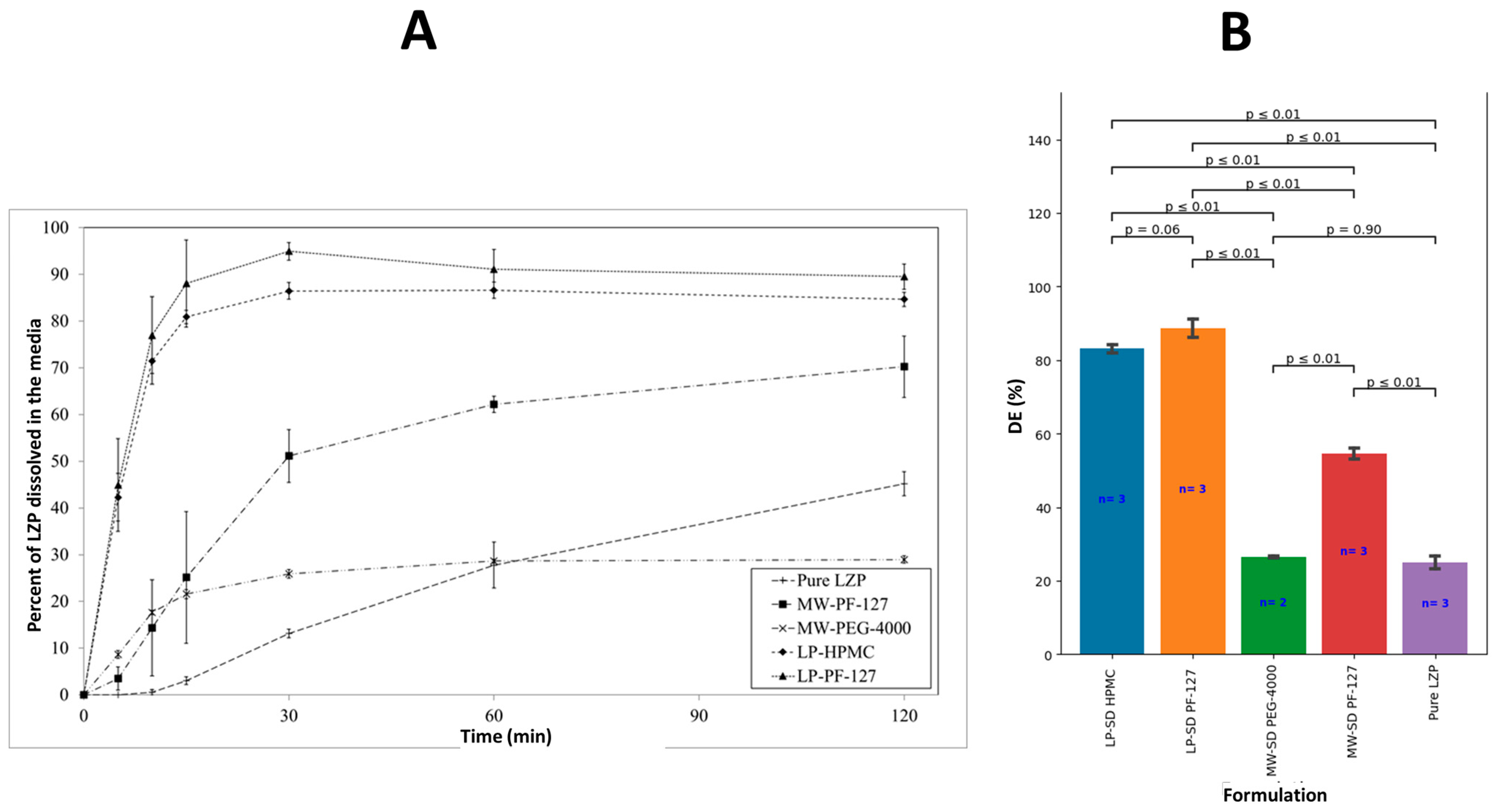
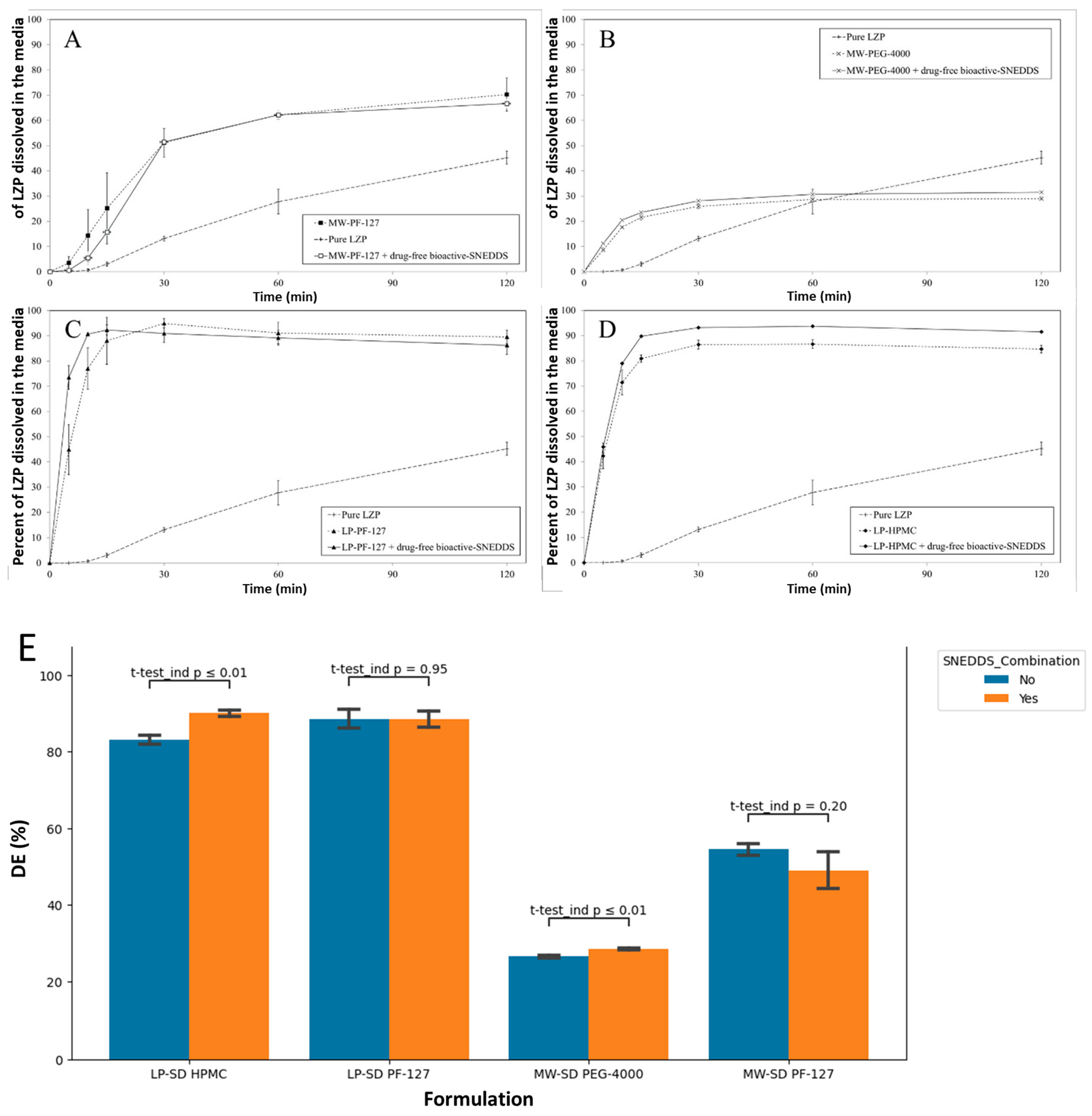
3.5. In Vitro Dissolution Studies
3.5.1. SNEDDS Formulations
3.5.2. SD Formulation
3.5.3. SD Formulation + Drug-Free Bioactive SNEDDS
3.6. Stability Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lien, H.-M.; Wang, Y.-Y.; Huang, M.-Z.; Wu, H.-Y.; Huang, C.-L.; Chen, C.-C.; Hung, S.-W.; Chen, C.-C.; Chiu, C.-H.; Lai, C.-H. Gastroprotective Effect of Anisomeles indica on Aspirin-Induced Gastric Ulcer in Mice. Antioxidants 2022, 11, 2327. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, T.C.; Fossmark, R.; Waldum, H.L. The phylogeny and biological function of gastric juice—Microbiological consequences of removing gastric acid. Int. J. Mol. Sci. 2019, 20, 6031. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, K.; Gould, E. Gastritis and gastric ulceration in dogs and cats. In Clinical Small Animal Internal Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 547–555. [Google Scholar]
- Vemulapalli, R. Diet and lifestyle modifications in the management of gastroesophageal reflux disease. Nutr. Clin. Pract. 2008, 23, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Miwa, H.; Sanada, K.; Miyata, K.; Haruma, K. Clinical characteristics and effectiveness of lansoprazole in Japanese patients with gastroesophageal reflux disease and dyspepsia. J. Gastroenterol. 2014, 49, 628–637. [Google Scholar] [CrossRef]
- Srebro, J.; Brniak, W.; Mendyk, A. Formulation of dosage forms with proton pump inhibitors: State of the art, challenges and future perspectives. Pharmaceutics 2022, 14, 2043. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, T.; Qi, J.; Zhang, J.; Wu, W. Enhanced dissolution and stability of lansoprazole by cyclodextrin inclusion complexation: Preparation, characterization, and molecular modeling. AAPS PharmSciTech 2012, 13, 1222–1229. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Wu, B.; Lu, Y.; Guan, T.; Wu, W. Physical characterization of lansoprazole/PVP solid dispersion prepared by fluid-bed coating technique. Powder Technol. 2008, 182, 480–485. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, G.; Zhang, R.; Liu, Z.; Liu, Z.; Wu, X.; Cao, D. Eudragit L/HPMCAS blend enteric-coated lansoprazole pellets: Enhanced drug stability and oral bioavailability. AAPS PharmSciTech 2014, 15, 513–521. [Google Scholar] [CrossRef][Green Version]
- Alsulays, B.B.; Kulkarni, V.; Alshehri, S.M.; Almutairy, B.K.; Ashour, E.A.; Morott, J.T.; Alshetaili, A.S.; Park, J.-B.; Tiwari, R.V.; Repka, M.A. Preparation and evaluation of enteric coated tablets of hot-melt extruded lansoprazole. Drug Dev. Ind. Pharm. 2017, 43, 789–796. [Google Scholar] [CrossRef]
- Ubgade, S.; Bapat, A.; Kilor, V. Effect of various stabilizers on the stability of lansoprazole nanosuspension prepared using high shear homogenization: Preliminary investigation. J. Appl. Pharm. Sci. 2021, 11, 085–092. [Google Scholar]
- Morakul, B. Self-nanoemulsifying drug delivery systems (SNEDDS): An advancement technology for oral drug delivery. Pharm. Sci. Asia 2020, 47, 205–220. [Google Scholar] [CrossRef]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Coskun, O.; Uysal, H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch. Toxicol. 2006, 80, 217–224. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Zielińska, M.; Helma, K.; Sokal, A.; Filip, R. Effect of Nigella sativa on Selected Gastrointestinal Diseases. Curr. Issues Mol. Biol. 2023, 45, 3016–3034. [Google Scholar] [CrossRef] [PubMed]
- Zeren, S.; Bayhan, Z.; Kocak, F.E.; Kocak, C.; Akcılar, R.; Bayat, Z.; Simsek, H.; Duzgun, S.A. Gastroprotective effects of sulforaphane and thymoquinone against acetylsalicylic acid–induced gastric ulcer in rats. J. Surg. Res. 2016, 203, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.F.; El-Moselhy, M.A.; Alarif, W.M.; Orif, M.; Alruwaili, N.K.; Alhakamy, N.A. Optimization of Thymoquinone-Loaded Self-Nanoemulsion for Management of Indomethacin-Induced Ulcer. Dose-Response 2021, 19, 15593258211013655. [Google Scholar] [CrossRef]
- Paseban, M.; Niazmand, S.; Soukhtanloo, M.; Meibodi, N.T.; Abbasnezhad, A.; Mousavi, S.M.; Niazmand, M.J. The therapeutic effect of Nigella sativa seed on indomethacin-induced gastric ulcer in Rats. Curr. Nutr. Food Sci. 2020, 16, 276–283. [Google Scholar] [CrossRef]
- Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Black Cumin Seed. [Updated 2023 Apr 27]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK591552/ (accessed on 1 October 2023).
- Ashfaq, M.; Shah, S.; Rasul, A.; Hanif, M.; Khan, H.U.; Khames, A.; Abdelgawad, M.A.; Ghoneim, M.M.; Ali, M.Y.; Abourehab, M.A. Enhancement of the solubility and bioavailability of pitavastatin through a self-nanoemulsifying drug delivery system (SNEDDS). Pharmaceutics 2022, 14, 482. [Google Scholar] [CrossRef]
- Shahba, A.A.; Tashish, A.Y.; Alanazi, F.K.; Kazi, M. Combined Self-Nanoemulsifying and Solid Dispersion Systems Showed Enhanced Cinnarizine Release in Hypochlorhydria/Achlorhydria Dissolution Model. Pharmaceutics 2021, 13, 627. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Ahmed, A.R.; Alanazi, F.K.; Mohsin, K.; Abdel-Rahman, S.I. Multi-layer self-nanoemulsifying pellets: An innovative drug delivery system for the poorly water-soluble drug cinnarizine. AAPS PharmSciTech 2018, 19, 2087–2102. [Google Scholar] [CrossRef]
- Nair, A.R.; Lakshman, Y.D.; Anand, V.S.K.; Sree, K.N.; Bhat, K.; Dengale, S.J. Overview of extensively employed polymeric carriers in solid dispersion technology. AAPS PharmSciTech 2020, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Alshadidi, A.; Shahba, A.A.; Sales, I.; Rashid, M.A.; Kazi, M. Combined Curcumin and Lansoprazole-Loaded Bioactive Solid Self-Nanoemulsifying Drug Delivery Systems (Bio-SSNEDDS). Pharmaceutics 2021, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Shahba, A.A.-W.; Sherif, A.Y.; Elzayat, E.M.; Kazi, M. Combined Ramipril and Black Seed Oil Dosage Forms Using Bioactive Self-Nanoemulsifying Drug Delivery Systems (BIO-SNEDDSs). Pharmaceuticals 2022, 15, 1120. [Google Scholar] [CrossRef] [PubMed]
- Aboutaleb, A.E.; Abdel-Rahman, S.I.; Ahmed, M.O.; Younis, M.A. Improvement of domperidone solubility and dissolution rate by dispersion in various hydrophilic carriers. J. Appl. Pharm. Sci. 2016, 6, 133–139. [Google Scholar] [CrossRef]
- Alshehri, S.; Shakeel, F.; Ibrahim, M.; Elzayat, E.; Altamimi, M.; Shazly, G.; Mohsin, K.; Alkholief, M.; Alsulays, B.; Alshetaili, A.; et al. Influence of the microwave technology on solid dispersions of mefenamic acid and flufenamic acid. PLoS ONE 2017, 12, e0182011. [Google Scholar] [CrossRef] [PubMed]
- Tashish, A.Y.; Shahba, A.A.-W.; Alanazi, F.K.; Kazi, M. Adsorbent Precoating by Lyophilization: A Novel Green Solvent Technique to Enhance Cinnarizine Release from Solid Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDS). Pharmaceutics 2023, 15, 134. [Google Scholar] [CrossRef]
- Tian, Z.; Yi, Y.; Yuan, H.; Han, J.; Zhang, X.; Xie, Y.; Lu, Y.; Qi, J.; Wu, W. Solidification of nanostructured lipid carriers (NLCs) onto pellets by fluid-bed coating: Preparation, in vitro characterization and bioavailability in dogs. Powder Technol. 2013, 247, 120–127. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Elzayat, E.M.; Alanazi, F.K. Development of modified in situ gelling oral liquid sustained release formulation of dextromethorphan. Drug Dev. Ind. Pharm. 2012, 38, 971–978. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Alanazi, F.K.; Abdel-Rahman, S.I. Stabilization benefits of single and multi-layer self-nanoemulsifying pellets: A poorly-water soluble model drug with hydrolytic susceptibility. PLoS ONE 2018, 13, e0198469. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Nahm, F.S. Nonparametric statistical tests for the continuous data: The basic concept and the practical use. Korean J Anesth. 2016, 69, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.U.; Shah, K.U.; Shah, S.U.; Khan, I.U.; Khan, G.M.; Khan, A. From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS). Expert Opin. Drug Deliv. 2017, 14, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Krstić, M.; Medarević, Đ.; Đuriš, J.; Ibrić, S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In Lipid Nanocarriers for Drug Targeting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 473–508. [Google Scholar]
- Kazi, M.; Nasr, F.A.; Noman, O.; Alharbi, A.; Alqahtani, M.S.; Alanazi, F.K. Development, characterization optimization, and assessment of curcumin-loaded bioactive self-nanoemulsifying formulations and their inhibitory effects on human breast cancer MCF-7 cells. Pharmaceutics 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Alwadei, M.; Kazi, M.; Alanazi, F.K. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals–curcumin and thymoquinone. Saudi Pharm. J. 2019, 27, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing oral bioavailability of apigenin using a bioactive self-nanoemulsifying drug delivery system (Bio-SNEDDS): In vitro, in vivo and stability evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef]
- Pathak, K.; Raghuvanshi, S. Oral bioavailability: Issues and solutions via nanoformulations. Clin. Pharmacokinet. 2015, 54, 325–357. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; El-Badry, M.; Alanazi, F.K.; Alsarra, I.A. Ultra fine super self-nanoemulsifying drug delivery system (SNEDDS) enhanced solubility and dissolution of indomethacin. J. Mol. Liq. 2013, 180, 89–94. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Chow, A.H.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Zhang, W. Influence of Ionic Surfactants on the Properties of Nanoemulsions Emulsified by Nonionic Surfactants Span 80/Tween 80. J. Dispers. Sci. Technol. 2016, 37, 1511–1517. [Google Scholar] [CrossRef]
- He, W.; Yang, M.; Fan, J.H.; Feng, C.X.; Zhang, S.J.; Wang, J.X.; Guan, P.P.; Wu, W. Influences of sodium carbonate on physicochemical properties of lansoprazole in designed multiple coating pellets. AAPS PharmSciTech 2010, 11, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Nora, G.-I.; Venkatasubramanian, R.; Strindberg, S.; Siqueira-Jørgensen, S.D.; Pagano, L.; Romanski, F.S.; Swarnakar, N.K.; Rades, T.; Müllertz, A. Combining lipid based drug delivery and amorphous solid dispersions for improved oral drug absorption of a poorly water-soluble drug. J. Control. Release 2022, 349, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Spray drying, freeze drying and related processes for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2012; pp. 73–109. [Google Scholar]
- Tabatar, T.; Makino, T.; Kashihara, T.; Hirai, S.; Kitamori, N.; Toguchi, H. Stabilization of a new antiulcer drug (lansoprazole) in the solid dosage forms. Drug Dev. Ind. Pharm. 1992, 18, 1437–1447. [Google Scholar] [CrossRef]
- Pasic, M. Study to Design Stable Lansoprazole Pellets. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2008. [Google Scholar]
- Yu, M.; Sun, L.; Li, W.; Lan, Z.; Li, B.; Tan, L.; Li, M.; Yang, X. Investigation of structure and dissolution properties of a solid dispersion of lansoprazole in polyvinylpyrrolidone. J. Mol. Struct. 2011, 1005, 70–77. [Google Scholar] [CrossRef]
- Harabor, A.; Rotaru, P.; Harabor, N. Two phases in a commercial anhydrous sodium carbonate by air contact. Phys. AUC 2013, 23, 79–88. [Google Scholar]
- Leemsuthep, A.; Mohd Nayan, N.A.; Zakaria, Z.; Uy Lan, D.N. Effect of sodium bicarbonate in fabrication of carbon black-filled epoxy porous for conductive application. In Proceedings of the Macromolecular Symposia—Special Issue: 4th Federation of Asian Polymer Societies International Polymer Congress, Kuala Lumpur, Malaysia, 5–8 October 2015; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 44–49. [Google Scholar]
- Uysal, D.; Dogan, Ö.M.; Uysal, B.Z. Kinetics of absorption of carbon dioxide into sodium metaborate solution. Int. J. Chem. Kinet. 2017, 49, 377–386. [Google Scholar] [CrossRef]
- Reddy, V.V.; Rao, H.S.; Jayaveera, K. Influence of strong alkaline substances (sodium carbonate and sodium bicarbonate) in mixing water on strength and setting properties of concrete. Indian J. Eng. Mater. Sci. 2006, 13, 123–128. [Google Scholar]
- Alshehri, S.; Alanazi, A.; Elzayat, E.M.; Altamimi, M.A.; Imam, S.S.; Hussain, A.; Alqahtani, F.; Shakeel, F. Formulation, in vitro and in vivo evaluation of gefitinib solid dispersions prepared using different techniques. Processes 2021, 9, 1210. [Google Scholar] [CrossRef]
- Moneghini, M.; De Zordi, N.; Grassi, M.; Zingone, G. Sustained-release solid dispersions of ibuprofen prepared by microwave irradiation. J. Drug Deliv. Sci. Technol. 2008, 18, 327–333. [Google Scholar] [CrossRef]
- Tafu, N.N.; Jideani, V.A. Proximate, elemental, and functional properties of novel solid dispersions of Moringa oleifera leaf powder. Molecules 2022, 27, 4935. [Google Scholar] [CrossRef]
- Hempel, N.-J.; Knopp, M.M.; Zeitler, J.A.; Berthelsen, R.; Löbmann, K. Microwave-induced in situ drug amorphization using a mixture of polyethylene glycol and polyvinylpyrrolidone. J. Pharm. Sci. 2021, 110, 3221–3229. [Google Scholar] [CrossRef] [PubMed]





| Formulation Code | LZP | PF-127 | PEG-4000 | HPMC | Sodium Hydrogen Carbonate (NaHCO3) | Disodium Carbonate (Na2CO3) |
|---|---|---|---|---|---|---|
| MW-PF-127 | 1.0 | 4.0 | - | - | - | - |
| MW-PEG 4000 | 1.0 | - | 4.0 | - | - | - |
| LP-PF-127 | 1.0 | 4.0 | - | - | 1.4 | 12.6 |
| LP-HPMC | 1.0 | - | - | 4.0 | 1.4 | 12.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherif, A.Y.; Shahba, A.A.-W. Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System. Biomedicines 2023, 11, 2733. https://doi.org/10.3390/biomedicines11102733
Sherif AY, Shahba AA-W. Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System. Biomedicines. 2023; 11(10):2733. https://doi.org/10.3390/biomedicines11102733
Chicago/Turabian StyleSherif, Abdelrahman Y., and Ahmad Abdul-Wahhab Shahba. 2023. "Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System" Biomedicines 11, no. 10: 2733. https://doi.org/10.3390/biomedicines11102733
APA StyleSherif, A. Y., & Shahba, A. A.-W. (2023). Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System. Biomedicines, 11(10), 2733. https://doi.org/10.3390/biomedicines11102733






