The Effects of Prenatal Pravastatin Treatment in the Rabbit Fetal Growth Restriction Model
Abstract
:1. Introduction
2. Materials and Methods
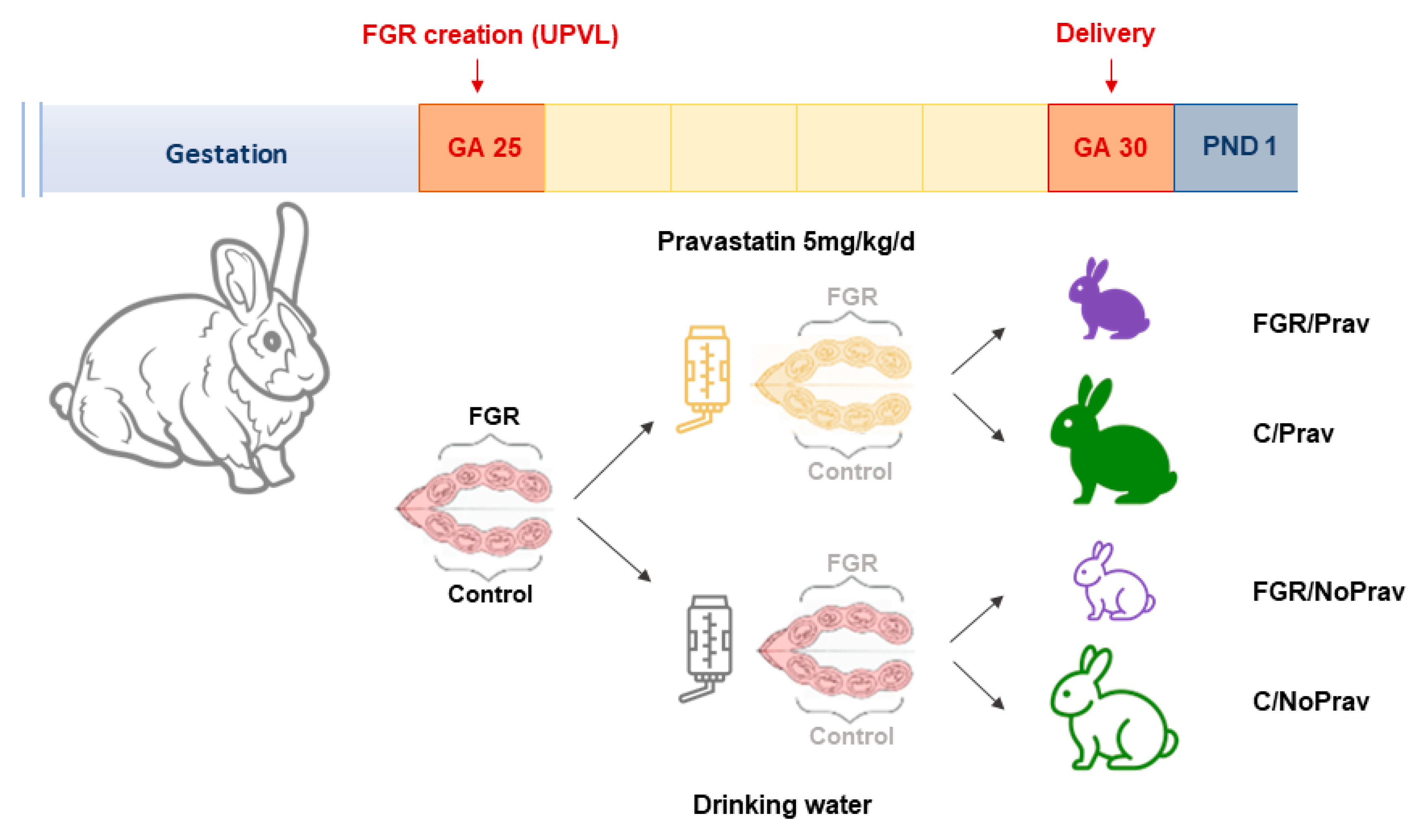
2.1. Animal Model
2.2. UPVL Creation
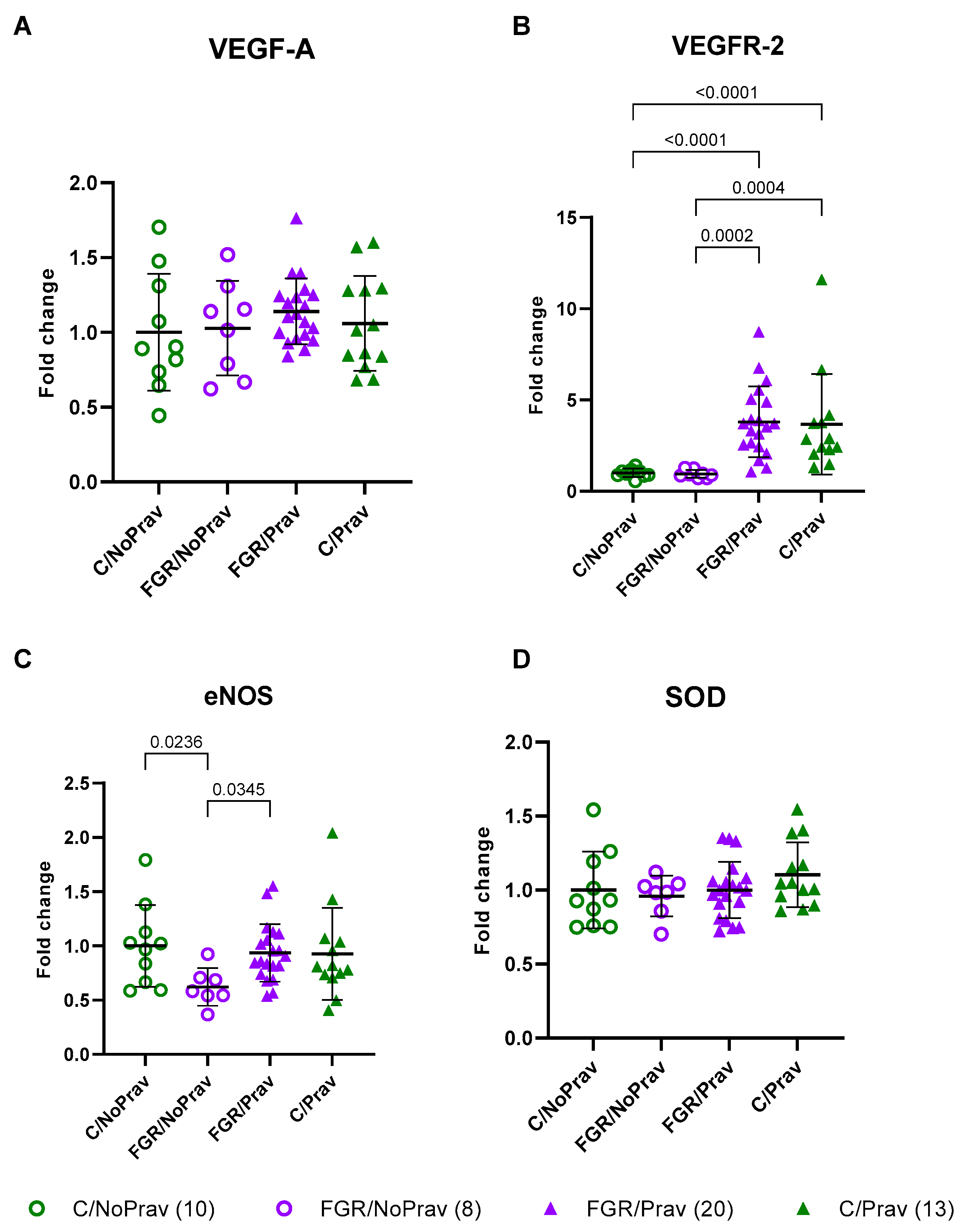
2.3. Placental Gene Expression
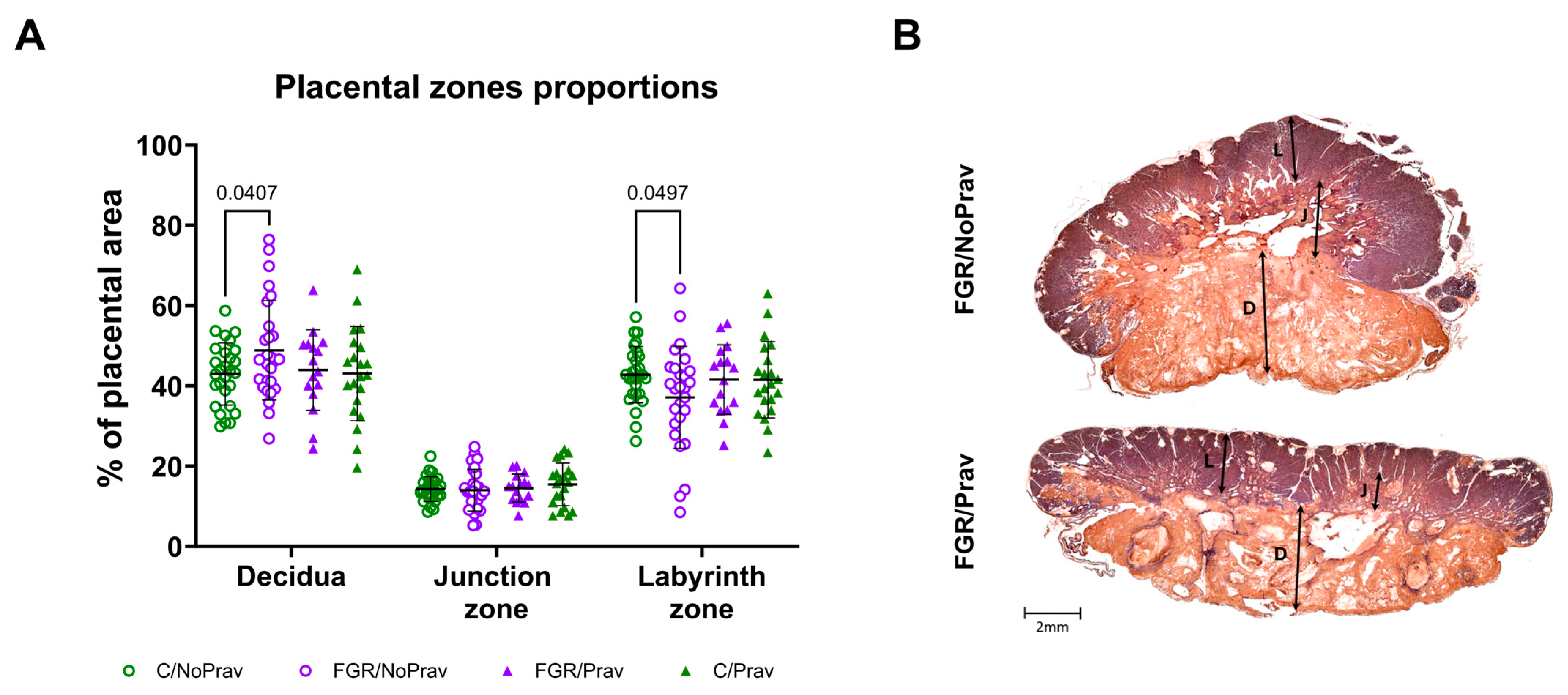
2.4. Placental Histology
2.5. Pulmonary Function Testing (PFT)
2.6. Histological Lung Assessment
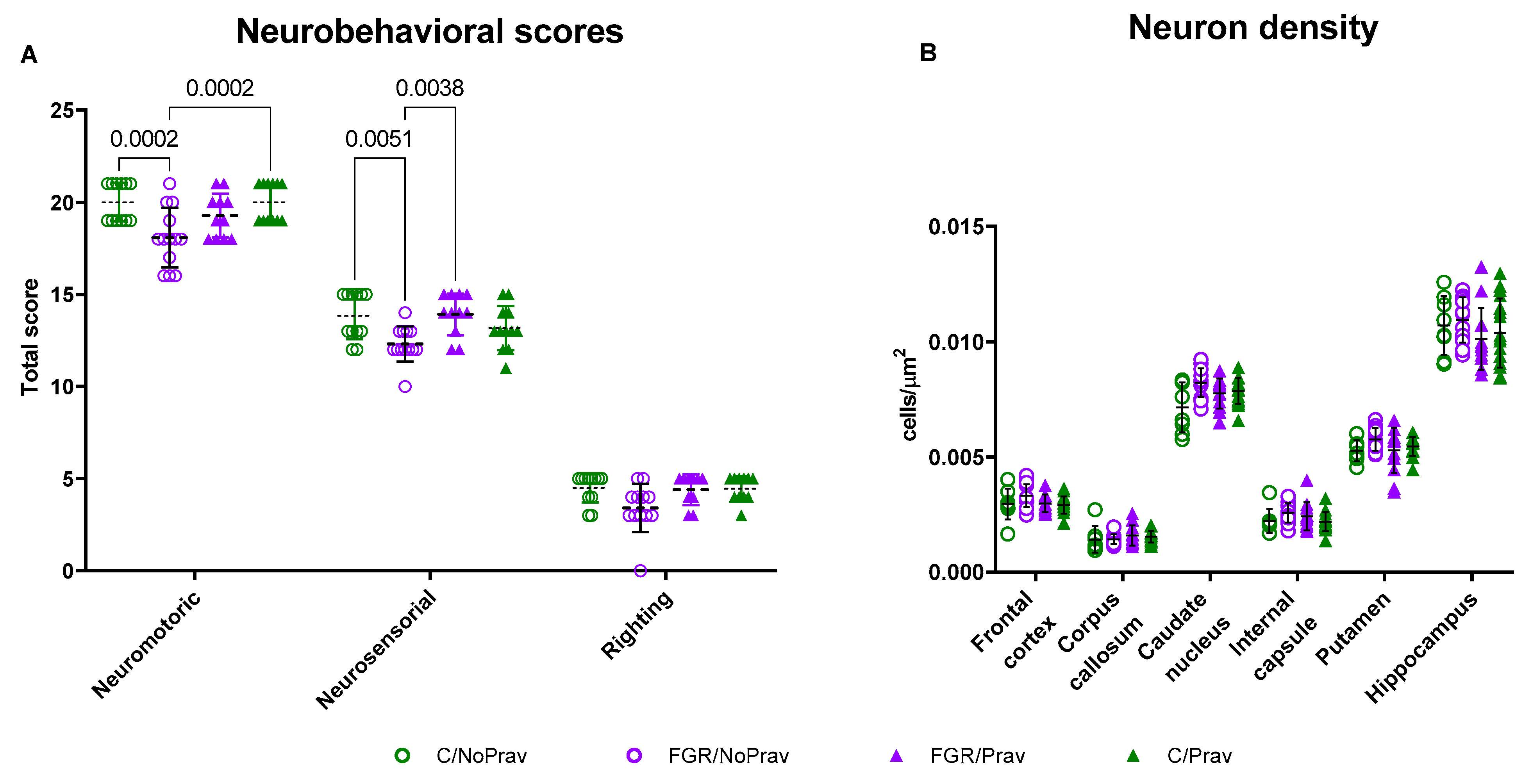
2.7. Neurobehavioral Assessment (NBA)
2.8. Brain Harvesting
2.9. Brain Histology
2.10. Statistical Analysis
3. Results
3.1. Survival and Biometrics
3.2. Placental Histology and Gene Expression
3.3. Pulmonary Function and Structure
3.4. Neurobehavioral and Neuropathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Neurobehavioral Assessment
- Cranial nerves are assessed by testing smell (olfaction is tested by recording time to aversive response to a cotton swab soaked with pure ethanol), sucking, swallowing (by introduction of formula into the kittens’ mouth with a plastic syringe), and head turn to feeding. The responses are graded on a scale of 0 to 3, 0 being the worst response and 3 the best response.
- Motor examination includes tone, motor activity, locomotion on a flat surface, righting reflex, and gait. The righting reflex is assessed when the kittens are placed on their backs, and the number of times turned prone (within 2 s) from supine position in 5 tries is registered. Gait is examined based on a modification by Georgiadis et al. [46]. Locomotion is assessed as described by Kannan et al. [47].
- Sensory examination is limited to touch on the face (touching the face with cotton swab on both sides) and extremities as well as pain on limbs (mild pin prick).
Appendix A.2. Sample Size Calculation
| Input: | Tail (s) | = | Two |
| Effect size d | = | 0.7243715 | |
| α err prob | = | 0.05 | |
| Power (1-β err prob) | = | 0.8 | |
| Allocation ratio N2/N1 | = | 1 | |
| Output: | Noncentrality parameter δ | = | 2.8518534 |
| Critical t | = | 2.0002978 | |
| Df | = | 60 | |
| Sample size group 1 | = | 31 | |
| Sample size group 2 | = | 31 | |
| Total sample size | = | 62 | |
| Actual power | = | 0.80121 |
| Parameter | Control/NoPrav (n = 14) | FGR/NoPrav (n = 17) | FGR/Prav (n = 13) | Control/Prav (n = 17) |
|---|---|---|---|---|
| Neuron density (cells/μm2) | ||||
| Frontal cortex | 0.0029 ± 0.0001 | 0.0032 ± 0.0002 | 0.0030 ± 0.0.0003 | 0.0029 ± 0.0002 |
| Corpus calosum | 0.0014 ± 0.0002 | 0.0014 ± 0.0002 | 0.0015 ± 0.0002 | 0.0015 ± 0.0002 |
| Caudate nucleus | 0.0070 ± 0.0002 | 0.0081 ± 0.0003 | 0.0077 ± 0.0004 | 0.0060 ± 0.0002 |
| Internal capsule | 0.0021 ± 0.0003 | 0.0024 ± 0.0002 | 0.0024 ± 0.0004 | 0.0021 ± 0.0002 |
| Putamen | 0.0051 ± 0.001 | 0.0056 ± 0.001 | 0.0080 ± 0.002 | 0.0071 ± 0.002 |
| Hippocampus | 0.011 ± 0.0004 | 0.011 ± 0.0005 | 0.010 ± 0.0006 | 0.010 ± 0.0006 |
| GFAP (+) cells (%) | ||||
| Frontal cortex | 0.18 ± 0.17 | 0.37 ± 0.17 | 0.21 ± 0.25 | 0.22 ± 0.25 |
| Corpus calosum | 26.66 ± 9.7 | 28.88 ± 3.85 | 30.79 ± 13.99 | 28.75 ± 5.78 |
| Caudate nucleus | 0.20 ± 0.06 | 0.09 ± 0.08 | 0.06 ± 0.12 | 0.09 ± 0.10 |
| Internal capsule | 0.24 ± 021 | 0.22 ± 0.22 | 0.41 ± 0.32 | 0.50 ± 0.31 |
| Putamen | 0.051 ± 0.09 | 0.31 ± 0.11 | 0.086 ± 0.16 | 0.12 ± 0.12 |
| Hippocampus | 1.04 ± 1.60 | 1.78 ± 0.79 | 2.10 ± 1.18 | 1.92 ± 2.31 |
| TUNEL (+) cells (%) | ||||
| Frontal cortex | 0.12 ± 0.06 | 0.14 ± 0.04 | 0.033 ± 0.05 | 0.078 ± 0.06 |
| Corpus calosum | 1.06 ± 0.36 | 1.21 ± 0.24 | 0.93 ± 0.33 | 0.83 ± 0.46 |
| Caudate nucleus | 0.32 ± 0.13 | 0.58 ± 0.15 | 0.25 ± 0.21 | 0.33 ± 0.16 |
| Internal capsule | 0.26 ± 0.010 | 0.33 ± 0.13 | 0.35 ± 0.17 | 0.26 ± 0.12 |
| Putamen | 0.73 ± 0.35 | 0.93 ± 0.30 | 0.35 ± 0.4 | 0.42 ± 0.44 |
| Hippocampus | 0.057 ± 0.07 | 0.289 ± 0.09 | 0.12 ± 0.12 | 0.074 ± 0.09 |
References
- Gardosi, J.; Madurasinghe, V.; Williams, M.; Malik, A.; Francis, A. Maternal and fetal risk factors for stillbirth: Population based study. BMJ 2013, 346, f108. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Hediger, M.; Levine, R.; Naficy, A.; Vik, T. Effect of breastfeeding on cognitive development of infants born small for gestational age. Acta Paediatr. 2002, 91, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Als, H.; Duffy, F.H.; McAnulty, G.; Butler, S.C.; Lightbody, L.; Kosta, S.; Weisenfeld, N.I.; Robertson, R.; Parad, R.B.; Ringer, S.A.; et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J. Perinatol. 2012, 32, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, I.; Kinoshita, M.; van der Merwe, J.; Marsal, K.; Deprest, J. Prenatal interventions for fetal growth restriction in animal models: A systematic review. Placenta 2022, 126, 90–113. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Arias-Alvarez, M.; Gonzalez-Bulnes, A.; Sferuzzi-Perri, A.N. Models of Intrauterine growth restriction and fetal programming in rabbits. Mol. Reprod. Dev. 2019, 86, 1781–1809. [Google Scholar] [CrossRef]
- van der Merwe, J.; van der Veeken, L.; Ferraris, S.; Gsell, W.; Himmelreich, U.; Toelen, J.; Ourselin, S.; Melbourne, A.; Vercauteren, T.; Deprest, J. Early neuropathological and neurobehavioral consequences of preterm birth in a rabbit model. Sci. Rep. 2019, 9, 3506. [Google Scholar] [CrossRef]
- Eixarch, E.; Figueras, F.; Hernandez-Andrade, E.; Crispi, F.; Nadal, A.; Torre, I.; Oliveira, S.; Gratacos, E. An experimental model of fetal growth restriction based on selective ligature of uteroplacental vessels in the pregnant rabbit. Fetal Diagn. Ther. 2009, 26, 203–211. [Google Scholar] [CrossRef]
- Dobierzewska, A.; Palominos, M.; Irarrazabal, C.E.; Sanchez, M.; Lozano, M.; Perez-Sepulveda, A.; Monteiro, L.J.; Burmeister, Y.; Figueroa-Diesel, H.; Rice, G.E.; et al. NFAT5 Is Up-Regulated by Hypoxia: Possible Implications in Preeclampsia and Intrauterine Growth Restriction. Biol. Reprod. 2015, 93, 14. [Google Scholar] [CrossRef]
- Bassan, H.; Trejo, L.L.; Kariv, N.; Bassan, M.; Berger, E.; Fattal, A.; Gozes, I.; Harel, S. Experimental intrauterine growth retardation alters renal development. Pediatr. Nephrol. 2000, 15, 192–195. [Google Scholar] [CrossRef]
- Basilious, A. Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: A systematic review. Dev. Med. Child. Neurol. 2014, 57, 420–430. [Google Scholar] [CrossRef]
- Valenzuela, I.; Zapletalova, K.; Greyling, M.; Regin, Y.; Gie, A.; Basurto, D.; Deprest, J.; van der Merwe, J. Fetal Growth Restriction Impairs Lung Function and Neurodevelopment in an Early Preterm Rabbit Model. Biomedicines 2023, 11, 139. [Google Scholar] [CrossRef]
- Melchiorre, K.; Giorgione, V.; Thilaganathan, B. The placenta and preeclampsia: Villain or victim? Am. J. Obstet. Gynecol. 2022, 226, S954–S962. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.; Longo, M.; Tamayo, E.; Kechichian, T.; Bytautiene, E.; Hankins, G.D.; Saade, G.R.; Costantine, M.M. Effects of pravastatin on mediators of vascular function in a mouse model of soluble Fms-like tyrosine kinase-1-induced preeclampsia. Am. J. Obstet. Gynecol. 2011, 205, 366.e1–366.e5. [Google Scholar] [CrossRef] [PubMed]
- de Alwis, N.; Beard, S.; Mangwiro, Y.T.; Binder, N.K.; Kaitu’u-Lino, T.J.; Brownfoot, F.C.; Tong, S.; Hannan, N.J. Pravastatin as the statin of choice for reducing pre-eclampsia-associated endothelial dysfunction. Pregnancy Hypertens. 2020, 20, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Vahedian-Azimi, A.; Karimi, L.; Reiner, Z.; Makvandi, S.; Sahebkar, A. Effects of statins on preeclampsia: A systematic review. Pregnancy Hypertens. 2021, 23, 123–130. [Google Scholar] [CrossRef]
- Wyrwoll, C.S.; Noble, J.; Thomson, A.; Tesic, D.; Miller, M.R.; Rog-Zielinska, E.A.; Moran, C.M.; Seckl, J.R.; Chapman, K.E.; Holmes, M.C. Pravastatin ameliorates placental vascular defects, fetal growth, and cardiac function in a model of glucocorticoid excess. Proc. Natl. Acad. Sci. USA 2016, 113, 6265–6270. [Google Scholar] [CrossRef]
- Kumasawa, K.; Ikawa, M.; Kidoya, H.; Hasuwa, H.; Saito-Fujita, T.; Morioka, Y.; Takakura, N.; Kimura, T.; Okabe, M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl. Acad. Sci. USA 2011, 108, 1451–1455. [Google Scholar] [CrossRef]
- Redecha, P.; van Rooijen, N.; Torry, D.; Girardi, G. Pravastatin prevents miscarriages in mice: Role of tissue factor in placental and fetal injury. Blood 2009, 113, 4101–4109. [Google Scholar] [CrossRef]
- Mendoza, M.; Ferrer-Oliveras, R.; Bonacina, E.; Garcia-Manau, P.; Rodo, C.; Carreras, E.; Alijotas-Reig, J. Evaluating the Effect of Pravastatin in Early-Onset Fetal Growth Restriction: A Nonrandomized and Historically Controlled Pilot Study. Am. J. Perinatol. 2021, 38, 1472–1479. [Google Scholar]
- Dobert, M.; Varouxaki, A.N.; Mu, A.C.; Syngelaki, A.; Ciobanu, A.; Akolekar, R.; De Paco Matallana, C.; Cicero, S.; Greco, E.; Singh, M.; et al. Pravastatin Versus Placebo in Pregnancies at High Risk of Term Preeclampsia. Circulation 2021, 144, 670–679. [Google Scholar] [CrossRef]
- Valenzuela, I.; Basurto, D.; Regin, Y.; Gie, A.; van der Veeken, L.; Vergote, S.; Munoz-Moreno, E.; Leszczynski, B.; Tielemans, B.; Velde, G.V.; et al. Placental vascular alterations are associated with early neurodevelopmental and pulmonary impairment in the rabbit fetal growth restriction model. Sci. Rep. 2022, 12, 19720. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.M. Effects of single-dose morning and evening administration of pravastatin on antioxidant markers in cholesterol-fed rabbits. J. Exp. Pharmacol. 2011, 3, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Toelen, J.; Vanoirbeek, J.; Kakigano, A.; Dekoninck, P.; Verbeken, E.; Deprest, J. Functional assessment of hyperoxia-induced lung injury after preterm birth in the rabbit. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L277–L283. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Salaets, T.; Tack, B.; Gie, A.; Pavie, B.; Sindhwani, N.; Jimenez, J.; Regin, Y.; Allegaert, K.; Deprest, J.; Toelen, J. A semi-automated method for unbiased alveolar morphometry: Validation in a bronchopulmonary dysplasia model. PLoS ONE 2020, 15, e0239562. [Google Scholar] [CrossRef]
- Derrick, M.; Luo, N.L.; Bregman, J.C.; Jilling, T.; Ji, X.; Fisher, K.; Gladson, C.L.; Beardsley, D.J.; Murdoch, G.; Back, S.A.; et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: A model for human cerebral palsy? J. Neurosci. 2004, 24, 24–34. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Sladek, S.M.; Magness, R.R.; Conrad, K.P. Nitric oxide and pregnancy. Am. J. Physiol. 1997, 272 Pt 2, R441–R463. [Google Scholar] [CrossRef]
- George, H.; Steeves, K.L.; Mercer, G.V.; Aghaei, Z.; Schneider, C.M.; Cahill, L.S. Endothelial nitric oxide deficiency results in abnormal placental metabolism. Placenta 2022, 128, 36–38. [Google Scholar] [CrossRef]
- Shaheen, G.; Jahan, S.; Ain, Q.U.; Ullah, A.; Afsar, T.; Almajwal, A.; Alam, I.; Razak, S. Placental endothelial nitric oxide synthase expression and role of oxidative stress in susceptibility to preeclampsia in Pakistani women. Mol. Genet. Genom. Med. 2020, 8, e1019. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, B.; Mylonas, I.; Hantschmann, P.; Kuhn, C.; Schulze, S.; Kunze, S.; Friese, K.; Jeschke, U. Expression of Endothelial NO Synthase, Inducible NO Synthase, and Estrogen Receptors Alpha and Beta in Placental Tissue of Normal, Preeclamptic, and Intrauterine Growth-restricted Pregnancies. J. Histochem. Cytochem. 2005, 53, 1441–1449. [Google Scholar] [CrossRef]
- Buhimschi, I.; Yallampalli, C.; Chwalisz, K.; Garfield, R.E. Pre-eclampsia-like conditions produced by nitric oxide inhibition: Effects of L-arginine, D-arginine and steroid hormones. Hum. Reprod. 1995, 10, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Thaete, L.; Kushner, D.; Dewey, E.; Neerhof, M. Endothelin and the regulation of uteroplacental perfusion in nitric oxide synthase inhibition-induced fetal growth restriction. Placenta 2005, 26, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Renshall, L.J.; Morgan, H.L.; Moens, H.; Cansfield, D.; Finn-Sell, S.L.; Tropea, T.; Cottrell, E.C.; Greenwood, S.; Sibley, C.P.; Wareing, M.; et al. Melatonin Increases Fetal Weight in Wild-Type Mice but Not in Mouse Models of Fetal Growth Restriction. Front. Physiol. 2018, 9, 1141. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Banach, M.; Penson, P.E.; Jamialahmadi, T.; Sahebkar, A. Statin-Induced Nitric Oxide Signaling: Mechanisms and Therapeutic Implications. J. Clin. Med. 2019, 8, 2051. [Google Scholar] [CrossRef]
- Meyer, N.; Brodowski, L.; Richter, K.; von Kaisenberg, C.S.; Schröder-Heurich, B.; von Versen-Höynck, F. Pravastatin Promotes Endothelial Colony-Forming Cell Function, Angiogenic Signaling and Protein Expression In Vitro. J. Clin. Med. 2021, 10, 183. [Google Scholar] [CrossRef]
- Herraiz, I.; Quezada, M.S.; Rodriguez-Calvo, J.; Gomez-Montes, E.; Villalain, C.; Galindo, A. Longitudinal change of sFlt-1/PlGF ratio in singleton pregnancy with early-onset fetal growth restriction. Ultrasound Obstet. Gynecol. 2018, 52, 631–638. [Google Scholar] [CrossRef]
- Shinohara, S.; Uchida, Y.; Kasai, M.; Sunami, R. Association between the high soluble fms-like tyrosine kinase-1 to placental growth factor ratio and adverse outcomes in asymptomatic women with early-onset fetal growth restriction. Hypertens. Pregnancy 2017, 36, 269–275. [Google Scholar] [CrossRef]
- Gaccioli, F.; Sovio, U.; Cook, E.; Hund, M.; Charnock-Jones, D.S.; Smith, G.C.S. Screening for fetal growth restriction using ultrasound and the sFLT1/PlGF ratio in nulliparous women: A prospective cohort study. Lancet Child. Adolesc. Health 2018, 2, 569–581. [Google Scholar] [CrossRef]
- Sharp, A.; Jackson, R.; Cornforth, C.; Harrold, J.; Turner, M.A.; Kenny, L.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P.; et al. A prediction model for short-term neonatal outcomes in severe early-onset fetal growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 241, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Helske, S.; Vuorela, P.; Carpén, O.; Hornig, C.; Weich, H.; Halmesmäki, E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol. Hum. Reprod. 2001, 7, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Nanovskaya, T.N.; Patrikeeva, S.L.; Paul, J.; Costantine, M.M.; Hankins, G.D.; Ahmed, M.S. Transplacental transfer and distribution of pravastatin. Am. J. Obstet. Gynecol. 2013, 209, 373.e1–373.e5. [Google Scholar] [CrossRef] [PubMed]
- Zarek, J.; DeGorter, M.K.; Lubetsky, A.; Kim, R.B.; Laskin, C.A.; Berger, H.; Koren, G. The transfer of pravastatin in the dually perfused human placenta. Placenta 2013, 34, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, P.; Xu, H.; Chua, C.; Hu, F.; Collins, L.; Huynh, C.; Lagamma, E.F.; Ballabh, P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke 2008, 39, 3378–3388. [Google Scholar] [CrossRef]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci. Transl. Med. 2012, 4, 130ra146. [Google Scholar] [CrossRef]


| Gene | Position | Primer Sequence |
|---|---|---|
| CAT | I3 | ACCCCCATTGCAGTTCGATT |
| I4 | CCGGGTCCTTTAGGTGTGTC | |
| eNOS | C1 | ACAGTTACCAGCTCGCCAAA |
| C2 | GCTCATTCTCCAGGTGCTTC | |
| SOD | H3 | GACGCATAACAGGACTGACCG |
| H4 | AACACATCAGCGACACCATTG | |
| VEGF-A | F1 | CTTGCTGCTCTACCTCCACC |
| F2 | CTTTGGTCTGCATTCACATTTG | |
| VEGFR-2 | G1 | CCCCTGATTACACTACGCCC |
| G2 | TGTAGTCTTTGCCACCCTGC | |
| YWHAZ | H8 | GGTCTGGCCCTTAACTTCTCTGTGTTCTA |
| H9 | GCGTGCTGTCTTTGTATGATTCTTCACTT |
| Group | C/NoPrav | FGR/NoPrav | FGR/Prav | C/Prav |
|---|---|---|---|---|
| Survival at birth | 39/40 (98%) | 38/43 (88%) | 34/41 (83%) | 35/39 (90%) |
| Survival at PND 1 | 33/39 (84%) | 36/43 (83%) | 30/41 (73%) | 32/39 (82%) |
| Birth weight (g) | 42.73 ± 1.48 | 35.99 ± 3.16 ab | 37.50 ± 6.67 b | 43.09 ± 3.57 |
| Placental weight (g) | 6.04 ± 0.33 | 5.63 ± 0.54 | 5.44 ± 1.3 | 5.60 ± 0.81 |
| BBWR | 0.045 ± 0.003 | 0.049 ± 0.002 | 0.046 ± 0.003 | 0.048 ± 0.004 |
| FPWR | 7.23 ± 0.30 | 6.52 ± 0.52 c | 7.06 ± 1.2 b | 7.75 ± 0.73 |
| Parameter | Control/NoPrav (n = 17) | FGR/NoPrav (n = 16) | FGR/Prav (n = 18) | Control/Prav (n = 15) |
|---|---|---|---|---|
| Inspiratory capacity, mL/kg | 32.07 ± 2.68 | 27.96 ± 4.72 | 24.70 ± 7.45 b | 31.7 ± 6.52 |
| Static compliance mL/(cm H2O·kg) | 2.58 ± 0.23 | 2.12 ± 0.38 | 1.87 ± 0.61 | 2.27 ± 0.55 |
| Hysteresis (A), mL·cm H2O | 1.44 ± 0.13 | 1.11 ± 0.23 c | 1.02 ± 0.54 b | 1.37 ± 0.30 |
| Tissue elastance (H), cm H2O/mL | 8.35 ± 1.19 | 12.39 ± 2.18 d | 10.58 ± 3.56 a | 7.62 ± 2.91 |
| Tissue damping (G), cm H2O/mL | 2.31 ± 0.26 | 2.95 ± 0.51 | 2.89 ± 0.86 a | 2.17 ± 0.65 |
| Respiratory system resistance, cmH2O·s/mL | 0.297 ± 0.034 | 0.380 ± 0.071 | 0.357 ± 0.121 | 0.302 ± 0.083 |
| Central airway resistance, cmH2O·s/mL | 0.099 ± 0.028 | 0.085 ± 0.055 | 0.055 ± 0.13 | 0.035 ± 0.068 |
| Dynamic compliance, mL/cmH2O·kg | 2.15 ± 0.23 | 1.70 ± 0.38 a | 1.55 ± 0.91 | 1.94 ± 0.55 |
| Inspiratory capacity, mL/kg | 32.07 ± 2.68 | 27.96 ± 4.72 | 24.70 ± 7.45 b | 31.7 ± 6.52 |
| Static compliance mL/(cmH20·kg) | 2.58 ± 0.23 | 2.12 ± 0.38 | 1.87 ± 0.61 | 2.27 ± 0.55 |
| Hysteresis (A), mL·cmH2O | 1.44 ± 0.13 | 1.11 ± 0.23 c | 1.02 ± 0.54 b | 1.37 ± 0.30 |
| Tissue elastance (H), cmH2O/mL | 8.35 ± 1.19 | 12.39 ± 2.18 d | 10.58 ± 3.56 a | 7.62 ± 2.91 |
| Tissue damping (G), cmH2O/mL | 2.31 ± 0.26 | 2.95 ± 0.51 | 2.89 ± 0.86 a | 2.17 ± 0.65 |
| Respiratory system resistance, cmH2O·s/mL | 0.297 ± 0.034 | 0.380 ± 0.071 | 0.357 ± 0.121 | 0.302 ± 0.083 |
| Central airway resistance, cmH2O·s/mL | 0.099 ± 0.028 | 0.085 ± 0.055 | 0.055 ± 0.13 | 0.035 ± 0.068 |
| Dynamic compliance, mL/cmH2O·kg | 2.15 ± 0.23 | 1.70 ± 0.38 a | 1.55 ± 0.91 | 1.94 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapletalova, K.; Valenzuela, I.; Greyling, M.; Regin, Y.; Frigolett, C.; Krofta, L.; Deprest, J.; van der Merwe, J. The Effects of Prenatal Pravastatin Treatment in the Rabbit Fetal Growth Restriction Model. Biomedicines 2023, 11, 2685. https://doi.org/10.3390/biomedicines11102685
Zapletalova K, Valenzuela I, Greyling M, Regin Y, Frigolett C, Krofta L, Deprest J, van der Merwe J. The Effects of Prenatal Pravastatin Treatment in the Rabbit Fetal Growth Restriction Model. Biomedicines. 2023; 11(10):2685. https://doi.org/10.3390/biomedicines11102685
Chicago/Turabian StyleZapletalova, Katerina, Ignacio Valenzuela, Marnel Greyling, Yannick Regin, Cristian Frigolett, Ladislav Krofta, Jan Deprest, and Johannes van der Merwe. 2023. "The Effects of Prenatal Pravastatin Treatment in the Rabbit Fetal Growth Restriction Model" Biomedicines 11, no. 10: 2685. https://doi.org/10.3390/biomedicines11102685
APA StyleZapletalova, K., Valenzuela, I., Greyling, M., Regin, Y., Frigolett, C., Krofta, L., Deprest, J., & van der Merwe, J. (2023). The Effects of Prenatal Pravastatin Treatment in the Rabbit Fetal Growth Restriction Model. Biomedicines, 11(10), 2685. https://doi.org/10.3390/biomedicines11102685






