Genes Involved by Dexamethasone in Prevention of Long-Term Memory Impairment Caused by Lipopolysaccharide-Induced Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocols
2.3. Neurological Test
2.4. Novel Object Recognition (NOR) Test
2.5. RNA-Sequencing and Data Analysis
2.6. Real-Time PCR (RT-PCR)
2.7. Immunofluorescence Assay
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Ecute Effects of LPS on Striatum and Hippocampus
3.1.1. Striatum
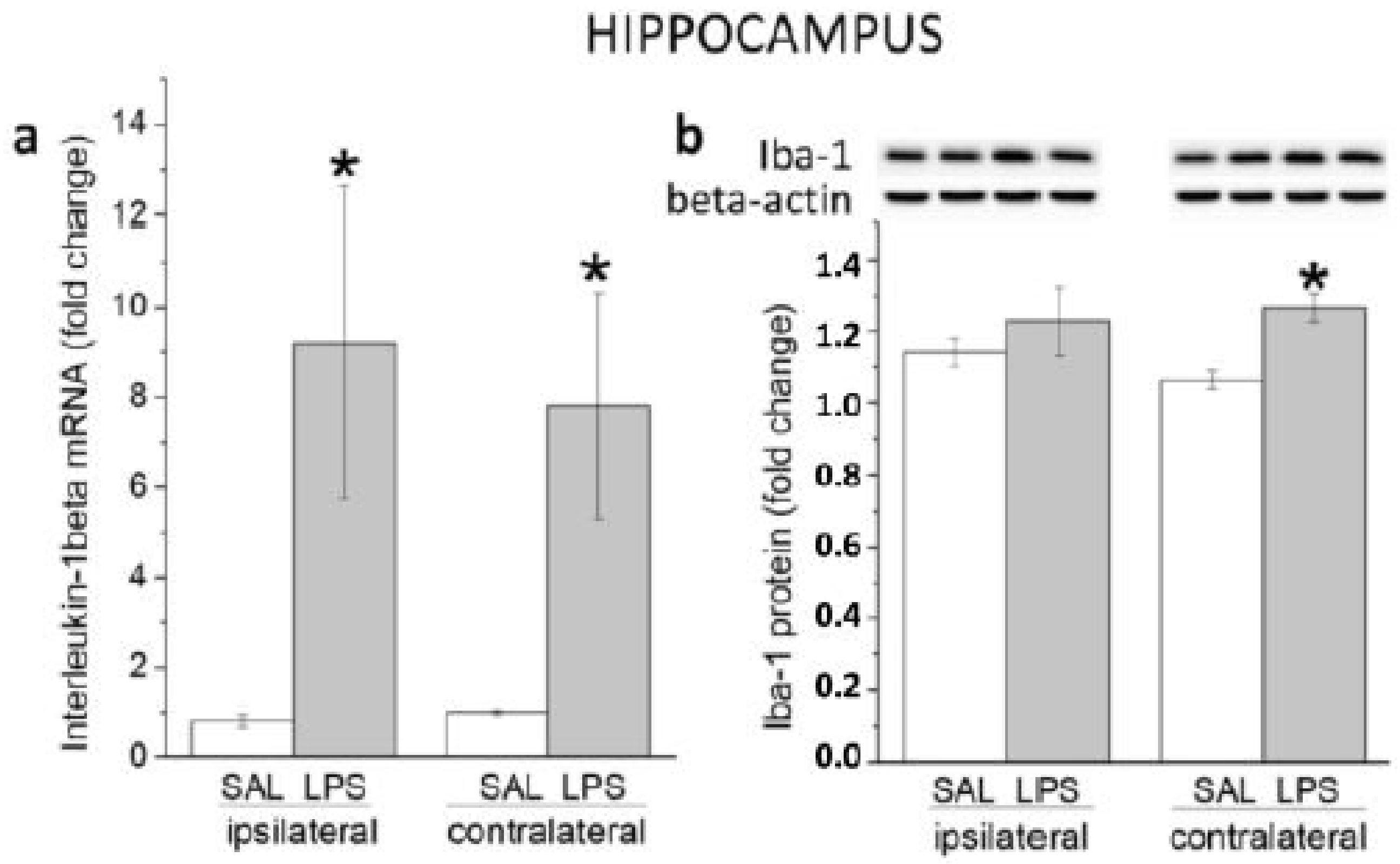
3.1.2. Hippocampus
3.2. Behavioral Effects of Intra-Striatal LPS and Pretreatment with DEX at 24 h and 3 Months
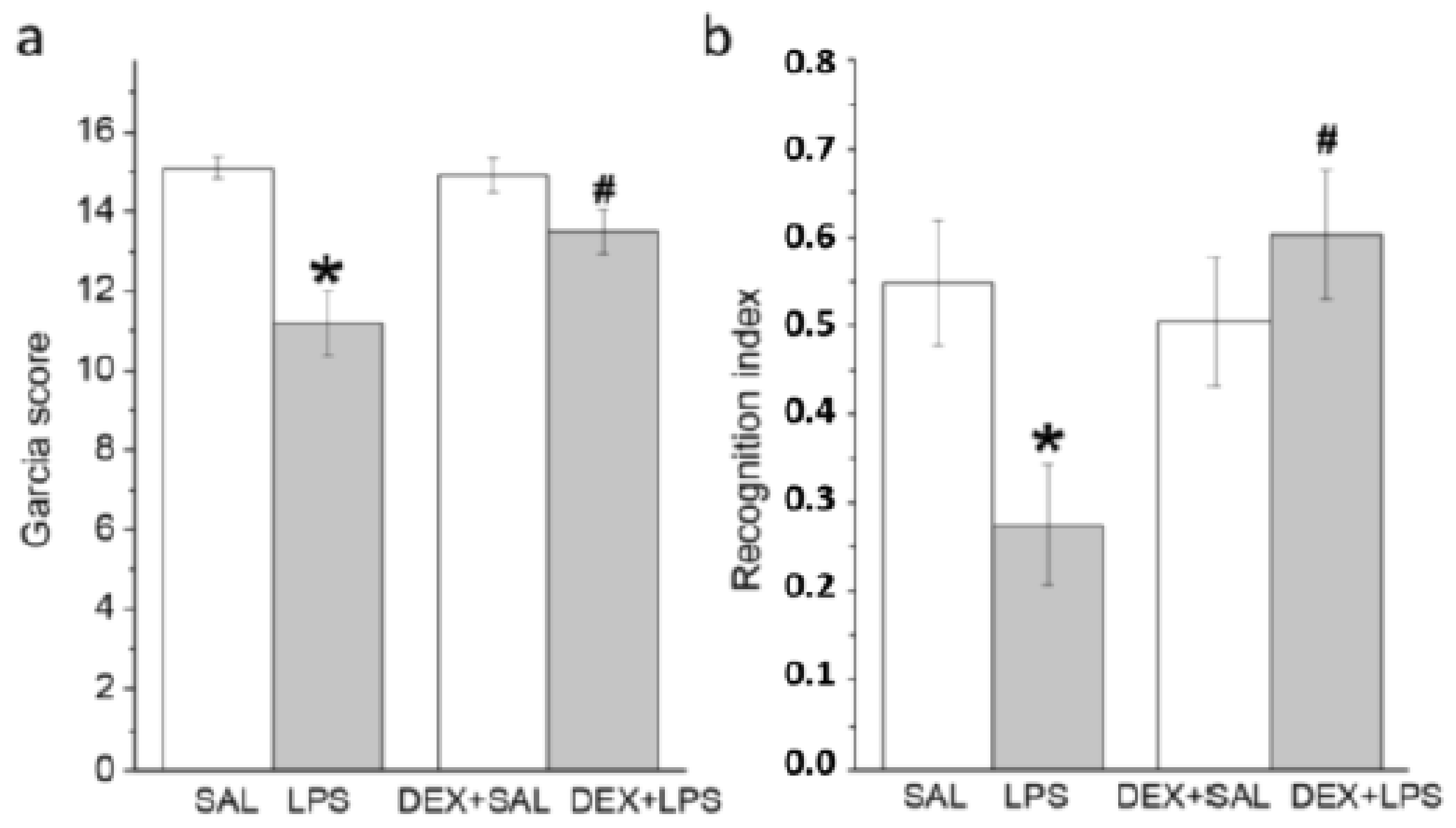
3.2.1. A Single Pretreatment with DEX Attenuated the Acute LPS-Induced Neurological Deficit
3.2.2. A Single Pretreatment with DEX Prevented LPS-Induced Long-Lasting Memory Impairment
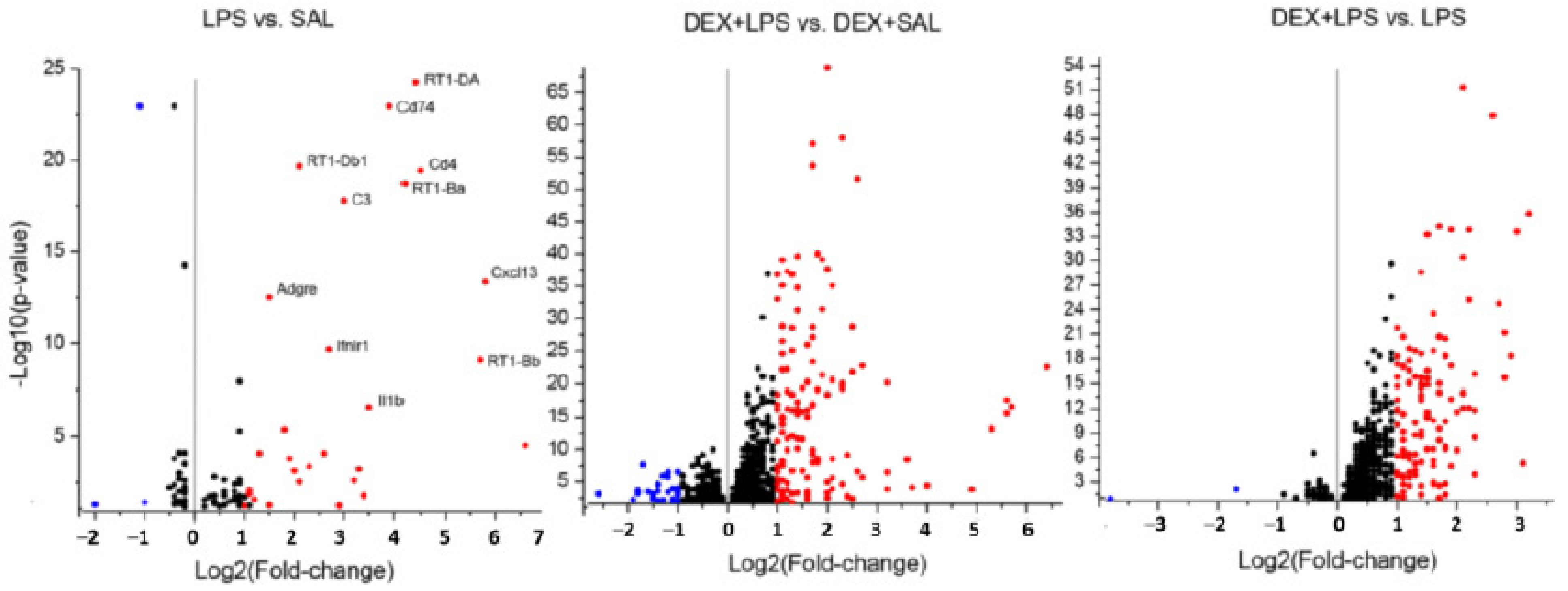
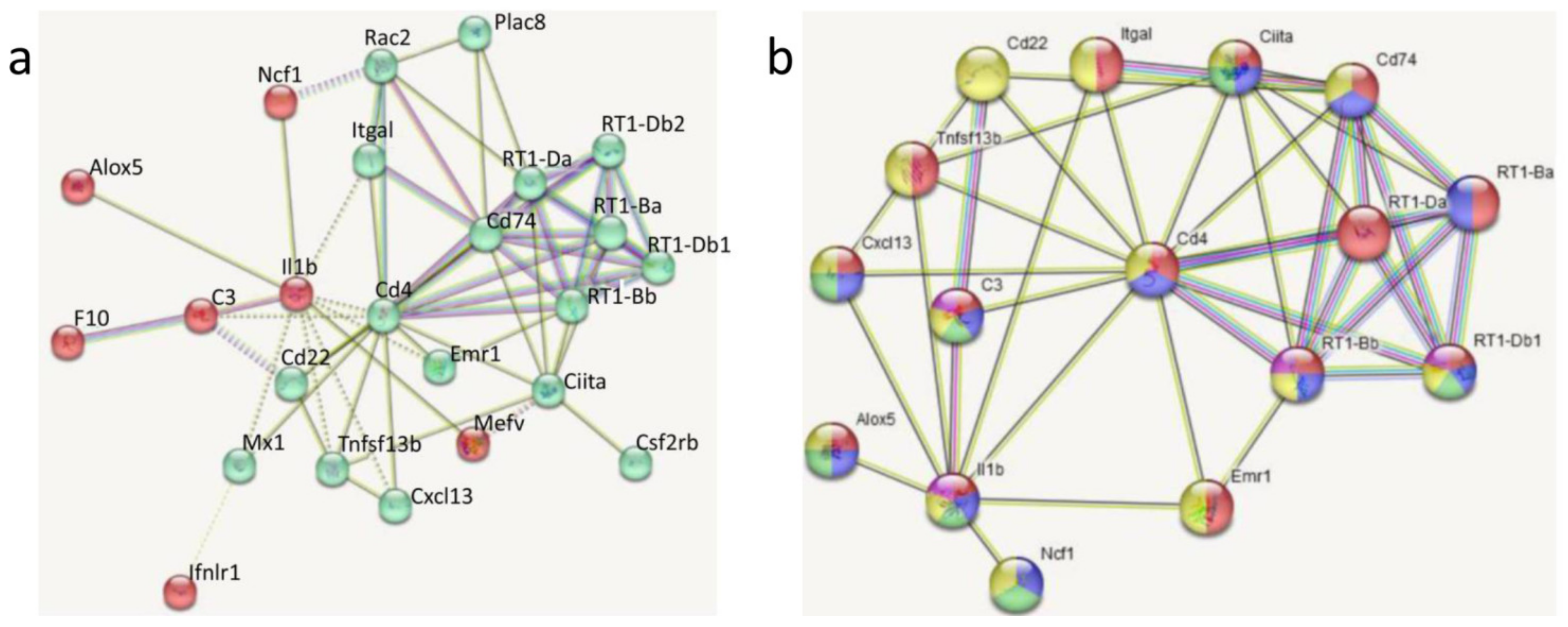
3.3. Transcriptomic Analyses
4. Discussion
4.1. Modeling of Inflammation
4.2. Acute and Delayed Behavioral Effects of LPS: Influence of DEX Pretreatment
4.3. Influence of DEX Pretreatment on Transcriptomic LPS Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öberg, M.; Fabrik, I.; Fabrikova, D.; Zehetner, N.; Härtlova, A. The role of innate immunity and inflammation in Parkinson’s disease. Scand. J. Immunol. 2021, 93, e13022. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Deng, M.; Hu, G.; Li, N.; Yuan, H.; Zhou, Y. New Insights into Microglial Mechanisms of Memory Impairment in Alzheimer’s Disease. Biomolecules 2022, 12, 1722. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, K.; François, M.; Liu, J.W.; Doecke, J.D.; Hecker, J.; Faunt, J.; Maddison, J.; Johns, S.; Pukala, T.L.; Rush, R.A.; et al. Salivary inflammatory biomarkers are predictive of mild cognitive impairment and Alzheimer’s disease in a feasibility study. Front. Aging Neurosci. 2022, 14, 1019296. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.Z.; Zou, C.J.; Mei, X.; Li, X.F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar] [PubMed]
- Schimmel, S.J.; Acosta, S.; Lozano, D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135–142. [Google Scholar] [PubMed]
- Thiel, A.; Cechetto, D.F.; Heiss, W.D.; Hachinski, V.; Whitehead, S.N. Amyloid burden, neuroinflammation, and links to cognitive decline after ischemic stroke. Stroke 2014, 45, 2825–2829. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, G.T.; Kalinina, T.S.; Gulyaeva, N.V.; Lanshakov, D.A.; Dygalo, N.N. Changes in Gene Expression and Neuroinflammation in the Hippocampus after Focal Brain Ischemia: Involvement in the Long-Term Cognitive and Mental Disorders. Biochemistry 2021, 86, 657–666. [Google Scholar] [CrossRef]
- Da Ré, C.; Souza, J.M.; Fróes, F.; Taday, J.; Dos Santos, J.P.; Rodrigues, L.; Sesterheim, P.; Gonçalves, C.A.; Leite, M.C. Neuroinflammation induced by lipopolysaccharide leads to memory impairment and alterations in hippocampal leptin signaling. Behav. Brain Res. 2020, 379, 112360. [Google Scholar] [CrossRef]
- Shishkina, G.T.; Kalinina, T.S.; Lanshakov, D.A.; Komysheva, N.P.; Sukhareva, E.V.; Dygalo, N.N. Acute and delayed behavioral effects of lipopolysaccharide with a focus on hippocampal interleukin-1β. Eur. Neuropsychopharmacol. 2021, 53 (Suppl. S1), S93. [Google Scholar] [CrossRef]
- Brown, E.S. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann. N. Y. Acad. Sci. 2009, 1179, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Song, Y.; Liu, Y.; Sun, X.; Ren, W. Impact of Dexamethasone Preconditioning on Prevention of Development of Cognitive Impairment following Acute Inflammation. Contrast Media Mol. Imaging. 2022, 2022, 6064007. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.H.; Aïd, S.; Zhang, Y.; Becker, K.G.; Bosetti, F. The brain expression of genes involved in inflammatory response, the ribosome, and learning and memory is altered by centrally injected lipopolysaccharide in mice. Pharmacogenom. J. 2009, 9, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, G.T.; Gulyaeva, N.V.; Lanshakov, D.A.; Kalinina, T.S.; Onufriev, M.V.; Moiseeva, Y.V.; Sukhareva, E.V.; Babenko, V.N.; Dygalo, N.N. Identifying the Involvement of Pro-Inflammatory Signal in Hippocampal Gene Expression Changes after Experimental Ischemia: Transcriptome-Wide Analysis. Biomedicines 2021, 9, 1840. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.; Planas, A.; Dresselaers, T.; Gsell, W.; Postnov, A.; Celen, S.; Casteels, C.; Himmelreich, U.; Debyser, Z.; Van Laere, K.; et al. PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl. Med. Biol. 2015, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-de Sauvage, M.A.; Maatouk, L.; Arnoux, I.; Pasco, M.; Sanz Diez, A.; Delahaye, M.; Herrero, M.T.; Newman, T.A.; Calvo, C.F.; Audinat, E.; et al. Potent and multiple regulatory actions of microglial glucocorticoid receptors during CNS inflammation. Cell Death Differ. 2013, 20, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.H.; Wagner, S.; Liu, K.F.; Hu, X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995, 26, 627–634; discussion 635. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Gingeras, T.R. Mapping RNA-seq Reads with STAR. Curr. Protoc. Bioinform. 2015, 51, 11.14.1–11.14.19. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, G.T.; Bannova, A.V.; Komysheva, N.P.; Dygalo, N.N. Anxiogenic-like effect of chronic lipopolysaccharide is associated with increased expression of matrix metalloproteinase 9 in the rat amygdala. Stress 2020, 23, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; De Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.; Ver Donck, L.; et al. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediat. Inflamm. 2013, 2013, 271359. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Langenbach, R.; Bosetti, F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008, 22, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Li, T.; Kury, L.T.A.; Zeb, A.; Khatoon, S.; Liu, G.; Yang, X.; Liu, F.; Yao, H.; Khan, A.U.; et al. Pathological Comparisons of the Hippocampal Changes in the Transient and Permanent Middle Cerebral Artery Occlusion Rat Models. Front. Neurol. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Uchida, H.; Fujita, Y.; Matsueda, M.; Umeda, M.; Matsuda, S.; Kato, H.; Kasahara, J.; Araki, T. Damage to neurons and oligodendrocytes in the hippocampal CA1 sector after transient focal ischemia in rats. Cell. Mol. Neurobiol. 2010, 30, 1125–1134. [Google Scholar] [CrossRef]
- Boyle, A.J.; Murrell, E.; Tong, J.; Schifani, C.; Narvaez, A.; Wuest, M.; West, F.; Wuest, F.; Vasdev, N. PET Imaging of Fructose Metabolism in a Rodent Model of Neuroinflammation with 6-[18F]fluoro-6-deoxy-D-fructose. Molecules 2022, 27, 8529. [Google Scholar] [CrossRef]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef]
- Burguillos, M.A.; Deierborg, T.; Kavanagh, E.; Persson, A.; Hajji, N.; Garcia-Quintanilla, A.; Cano, J.; Brundin, P.; Englund, E.; Venero, J.L.; et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 2011, 472, 319–324. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.W.; Wang, Y.; Adam, L.; Oudin, G.; Louis, J.S.; Tang, J.; Zhang, J.H. Correlation Between Subacute Sensorimotor Deficits and Brain Edema in Rats after Surgical Brain Injury. Acta Neurochir. Suppl. 2016, 121, 317–321. [Google Scholar] [PubMed]
- Jacovides, C.L.; Ahmed, S.; Suto, Y.; Paris, A.J.; Leone, R.; McCarry, J.; Christofidou-Solomidou, M.; Kaplan, L.J.; Smith, D.H.; Holena, D.N.; et al. An inflammatory pulmonary insult post-traumatic brain injury worsens subsequent spatial learning and neurological outcomes. J. Trauma Acute Care Surg. 2019, 87, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, I.R.; Cloutier, C.J.; Tyson, C.D.; Matic, V.M.; Kavaliers, M.; Ossenkopp, K.P. Infection, learning, and memory: Focus on immune activation and aversive conditioning. Neurosci. Biobehav. Rev. 2022, 142, 104898. [Google Scholar] [CrossRef] [PubMed]
- Hennigan, A.; Trotter, C.; Kelly, A.M. Lipopolysaccharide impairs long-term potentiation and recognition memory and increases p75NTR expression in the rat dentate gyrus. Brain Res. 2007, 1130, 158–166. [Google Scholar] [CrossRef]
- Czerniawski, J.; Miyashita, T.; Lewandowski, G.; Guzowski, J.F. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: Evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav. Immun. 2015, 44, 159–166. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Harte, M.K.; Stenson, G.; Reynolds, G.P. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav. Brain Res. 2009, 205, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Barter, J.; Kumar, A.; Rani, A.; Colon-Perez, L.M.; Febo, M.; Foster, T.C. Differential Effect of Repeated Lipopolysaccharide Treatment and Aging on Hippocampal Function and Biomarkers of Hippocampal Senescence. Mol. Neurobiol. 2020, 57, 4045–4059. [Google Scholar] [CrossRef]
- Tchessalova, D.; Tronson, N.C. Memory deficits in males and females long after subchronic immune challenge. Neurobiol. Learn. Mem. 2019, 158, 60–72. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Bossu, P.; Cutuli, D.; Palladino, I.; Caporali, P.; Angelucci, F.; Laricchiuta, D.; Gelfo, F.; De Bartolo, P.; Caltagirone, C.; Petrosini, L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J. Neuroinflamm. 2012, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Tchessalova, D.; Tronson, N.C. Enduring and Sex-specific Changes in Hippocampal Gene Expression after a Subchronic Immune Challenge. Neuroscience 2020, 428, 76–89. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Bixler, G.V.; Brucklacher, R.M.; Farley, J.A.; Yan, H.; Warrington, J.P.; Sonntag, W.E.; Freeman, W.M. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J. Neuroinflamm. 2011, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Morikawa, A.; Imori, S.; Waki, S.; Tamura, T.; Yamanaka, D.; Yamazaki, F.; Yokoyama, M. Preventive effects of multisensory rehabilitation on development of cognitive dysfunction following systemic inflammation in aged rats. J. Anesth. 2014, 28, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, C.; Yu, H.; Cai, X.; Shen, X.; Sun, X.; Wang, J.; Zhang, Y.; Wang, C. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J. Neuroinflamm. 2017, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeon, S.G.; Jeong, H.R.; Park, H.; Kim, J.I.; Hoe, H.S. L-Type Ca2+ Channel Inhibition Rescues the LPS-Induced Neuroinflammatory Response and Impairments in Spatial Memory and Dendritic Spine Formation. Int. J. Mol. Sci. 2022, 23, 13606. [Google Scholar] [CrossRef] [PubMed]
- Pregnolato, S.; Chakkarapani, E.; Isles, A.R.; Luyt, K. Glutamate Transport and Preterm Brain Injury. Front. Physiol. 2019, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Dygalo, N.N.; Kalinina, T.S.; Shishkina, G.T. Stress-induced expression pattern of glutamate signaling genes associated with anhedonia. Stress 2020, 23, 700–707. [Google Scholar] [CrossRef]
- Kalinina, T.S.; Shishkina, G.T.; Lanshakov, D.A.; Sukhareva, E.V.; Onufriev, M.V.; Moiseeva, Y.V.; Gulyaeva, N.V.; Dygalo, N.N. Comparative Investigation of Expression of Glutamatergic and GABAergic Genes in the Rat Hippocampus after Focal Brain Ischemia and Central LPS Administration. Biochemistry 2023, 88, 539–550. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, X.; Zhang, L.; Hu, J.; Chen, Y.; Zhan, L.; Gao, Z. The Functional and Molecular Properties, Physiological Functions, and Pathophysiological Roles of GluN2A in the Central Nervous System. Mol. Neurobiol. 2017, 54, 1008–1021. [Google Scholar] [CrossRef]
- Rossato, J.I.; Radiske, A.; Gonzalez, M.C.; Apolinário, G.; de Araújo, R.L.S.; Bevilaqua, L.R.M.; Cammarota, M. NMDARs control object recognition memory destabilization and reconsolidation. Brain Res. Bull. 2023, 197, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Brigman, J.L.; Feyder, M.; Saksida, L.M.; Bussey, T.J.; Mishina, M.; Holmes, A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn. Mem. 2008, 15, 50–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abd-Elrahman, K.S.; Ferguson, S.S.G. Noncanonical Metabotropic Glutamate Receptor 5 Signaling in Alzheimer’s Disease. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Cortese, G.P.; Olin, A.; O’Riordan, K.; Hullinger, R.; Burger, C. Environmental enrichment improves hippocampal function in aged rats by enhancing learning and memory, LTP, and mGluR5-Homer1c activity. Neurobiol. Aging 2018, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mecca, A.P.; McDonald, J.W.; Michalak, H.R.; Godek, T.A.; Harris, J.E.; Pugh, E.A.; Kemp, E.C.; Chen, M.K.; Salardini, A.; Nabulsi, N.B.; et al. PET imaging of mGluR5 in Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Brothers, H.M.; Bardou, I.; Hopp, S.C.; Marchalant, Y.; Kaercher, R.M.; Turner, S.M.; Mitchem, M.R.; Kigerl, K.; Wenk, G.L. Time-Dependent Compensatory Responses to Chronic Neuroinflammation in Hippocampus and Brainstem: The Potential Role of Glutamate Neurotransmission. J. Alzheimers Dis. Park. 2013, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, I.J.; Yue, C.; Takano, H.; Terzic, B.; Pance, K.; Lee, J.Y.; Cui, Y.; Coulter, D.A.; Zhou, Z. Loss of CDKL5 in Glutamatergic Neurons Disrupts Hippocampal Microcircuitry and Leads to Memory Impairment in Mice. J. Neurosci. 2017, 37, 7420–7437. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Wu, X.M.; Zhou, Z.Q.; Liu, P.M.; Yang, J.J.; Ji, M.H. Dysfunction of NRG1/ErbB4 Signaling in the Hippocampus Might Mediate Long-term Memory Decline After Systemic Inflammation. Mol. Neurobiol. 2023, 60, 3210–3226. [Google Scholar] [CrossRef]
- De Kloet, E.R. Why Dexamethasone Poorly Penetrates in Brain. Stress 1997, 2, 13–20. [Google Scholar] [CrossRef]
- Wang, K.; Shi, Y.; Liu, W.; Liu, S.; Sun, M.Z. Taurine improves neuron injuries and cognitive impairment in a mouse Parkinson’s disease model through inhibition of microglial activation. Neurotoxicology 2021, 83, 129–136. [Google Scholar] [CrossRef]
- Cuong, T.T.; Yang, C.S.; Yuk, J.M.; Lee, H.M.; Ko, S.R.; Cho, B.G.; Jo, E.K. Glucocorticoid receptor agonist compound K regulates Dectin-1-dependent inflammatory signaling through inhibition of reactive oxygen species. Life Sci. 2009, 85, 625–633. [Google Scholar] [CrossRef]



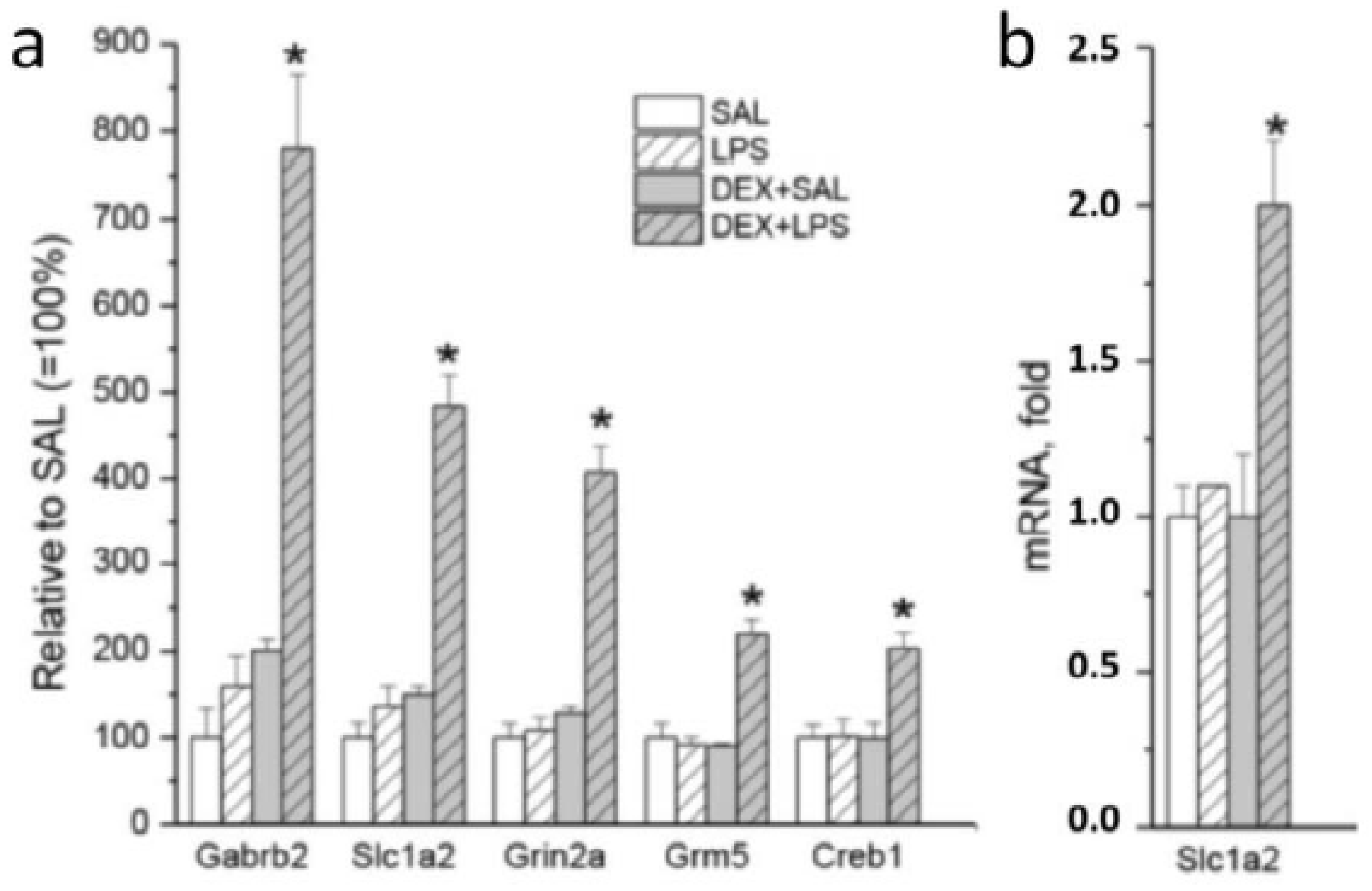
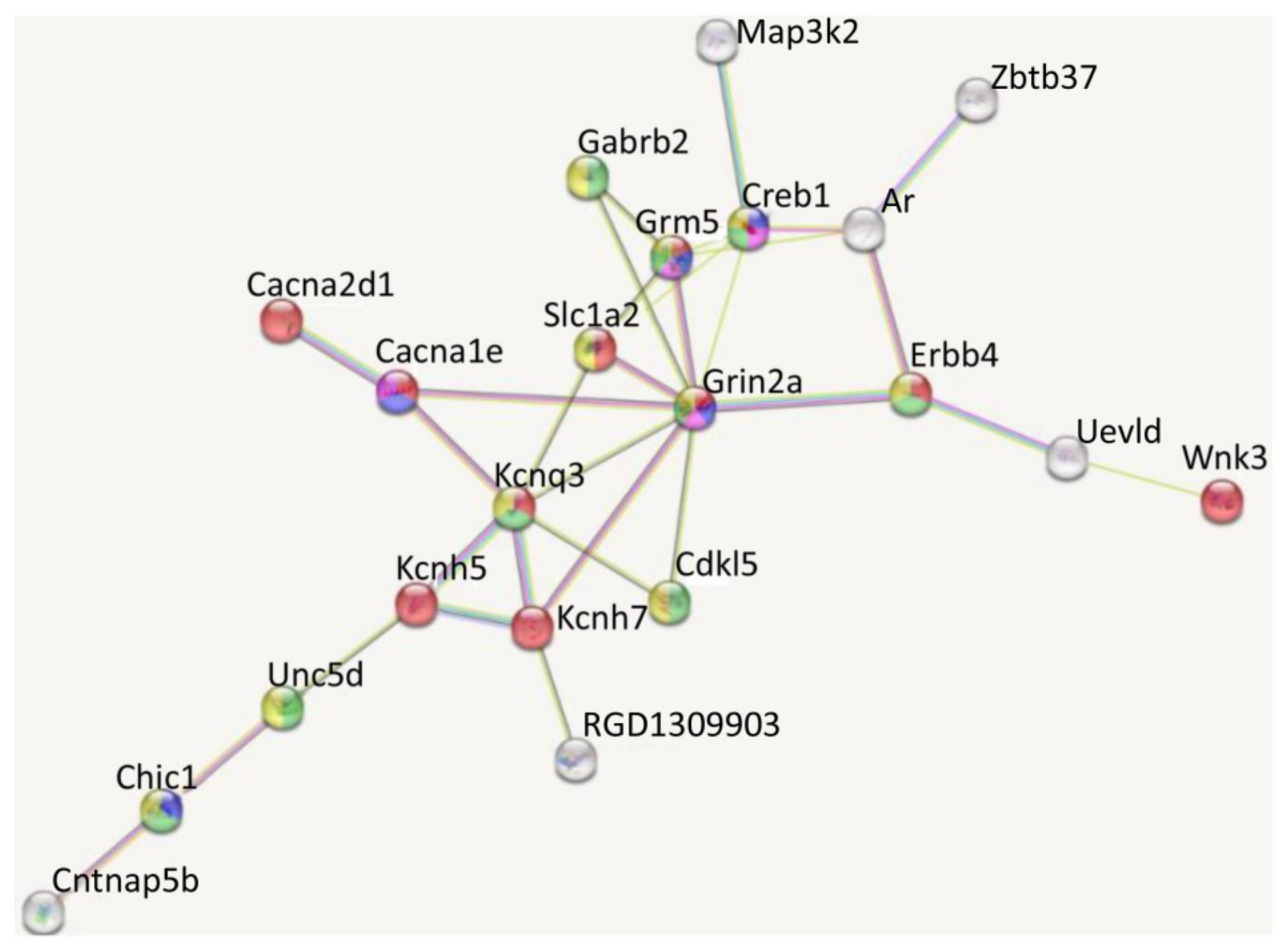
| Gene | LPS vs. SAL (33) | DEX + LPS vs. DEX + SAL (161) | Common DEGs (22) | Specific DEGs (84) |
|---|---|---|---|---|
| Cd4 | 16 | 21 | 13 | - |
| Cd74 | 11 | 14 | 8 | - |
| Ciita | 8 | 11 | 6 | - |
| Creb1 | - | 11 | - | 7 |
| Il1b | 10 | 15 | 8 | - |
| RT1-Bb | 8 | 11 | 7 | - |
| RT1-Da | 9 | 9 | 6 | - |
| Grin2a | - | 9 | - | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkina, G.T.; Kalinina, T.S.; Lanshakov, D.A.; Bulygina, V.V.; Komysheva, N.P.; Bannova, A.V.; Drozd, U.S.; Dygalo, N.N. Genes Involved by Dexamethasone in Prevention of Long-Term Memory Impairment Caused by Lipopolysaccharide-Induced Neuroinflammation. Biomedicines 2023, 11, 2595. https://doi.org/10.3390/biomedicines11102595
Shishkina GT, Kalinina TS, Lanshakov DA, Bulygina VV, Komysheva NP, Bannova AV, Drozd US, Dygalo NN. Genes Involved by Dexamethasone in Prevention of Long-Term Memory Impairment Caused by Lipopolysaccharide-Induced Neuroinflammation. Biomedicines. 2023; 11(10):2595. https://doi.org/10.3390/biomedicines11102595
Chicago/Turabian StyleShishkina, Galina T., Tatyana S. Kalinina, Dmitriy A. Lanshakov, Veta V. Bulygina, Natalya P. Komysheva, Anita V. Bannova, Ulyana S. Drozd, and Nikolay N. Dygalo. 2023. "Genes Involved by Dexamethasone in Prevention of Long-Term Memory Impairment Caused by Lipopolysaccharide-Induced Neuroinflammation" Biomedicines 11, no. 10: 2595. https://doi.org/10.3390/biomedicines11102595
APA StyleShishkina, G. T., Kalinina, T. S., Lanshakov, D. A., Bulygina, V. V., Komysheva, N. P., Bannova, A. V., Drozd, U. S., & Dygalo, N. N. (2023). Genes Involved by Dexamethasone in Prevention of Long-Term Memory Impairment Caused by Lipopolysaccharide-Induced Neuroinflammation. Biomedicines, 11(10), 2595. https://doi.org/10.3390/biomedicines11102595






