Combined Usage of MDK Inhibitor Augments Interferon-γ Anti-Tumor Activity in the SKOV3 Human Ovarian Cancer Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Antibodies and Reagents
2.3. MDK Stable Overexpression
2.4. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.5. Western Blotting Assay
2.6. Cell Cytotoxicity Assay
2.7. Real Time Cell Analysis (RTCA)
2.8. Cell Migration and Invasion Assay
2.9. Statistical Analysis
3. Results
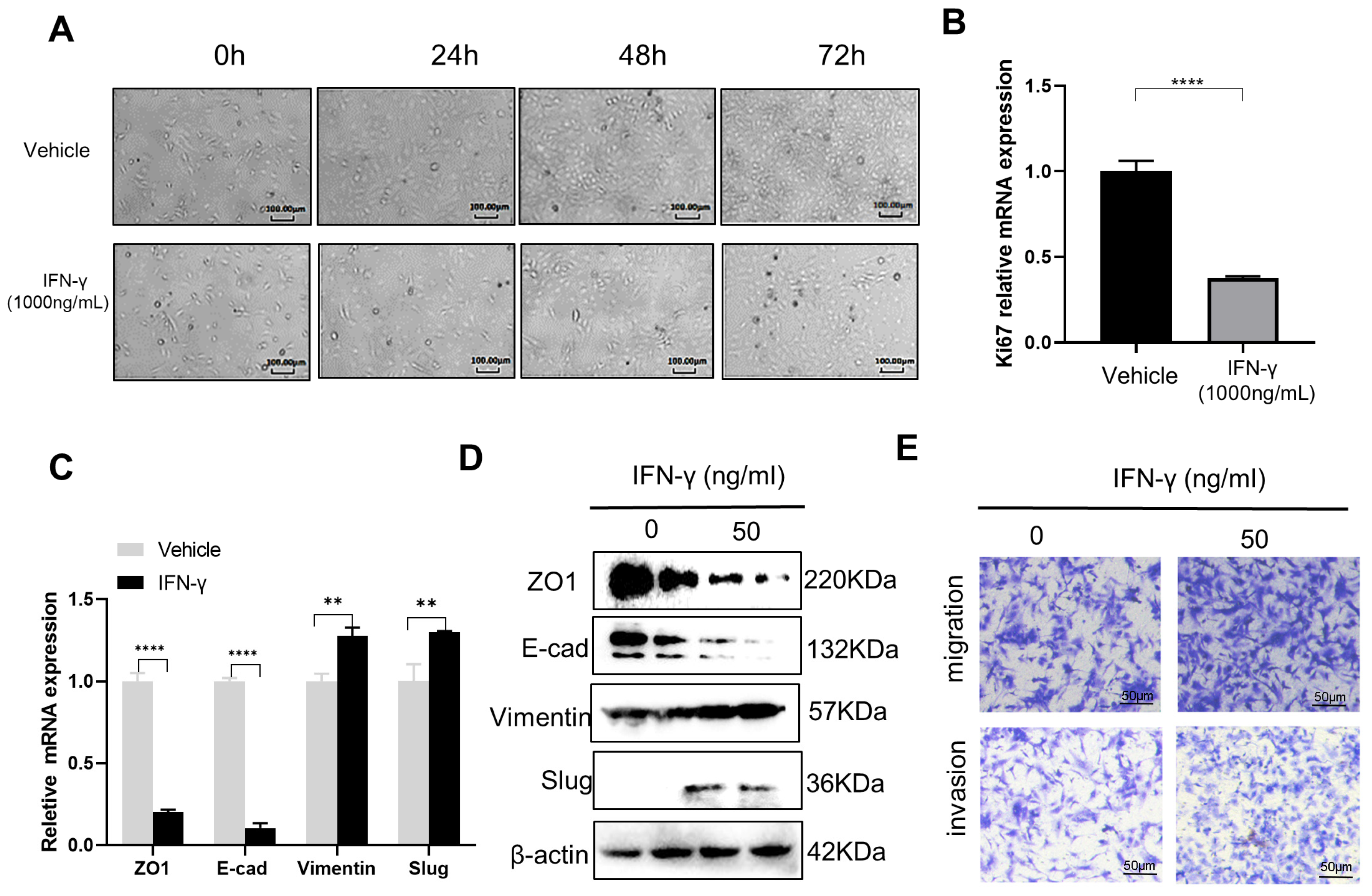
3.1. High-Dose IFN-γ Inhibits Cancer Growth while Low-Dose IFN-γ Promotes Aggressiveness in SKOV3 Cells
3.2. IFN-γ Activates MDK via STAT1 in SKOV3 Cells
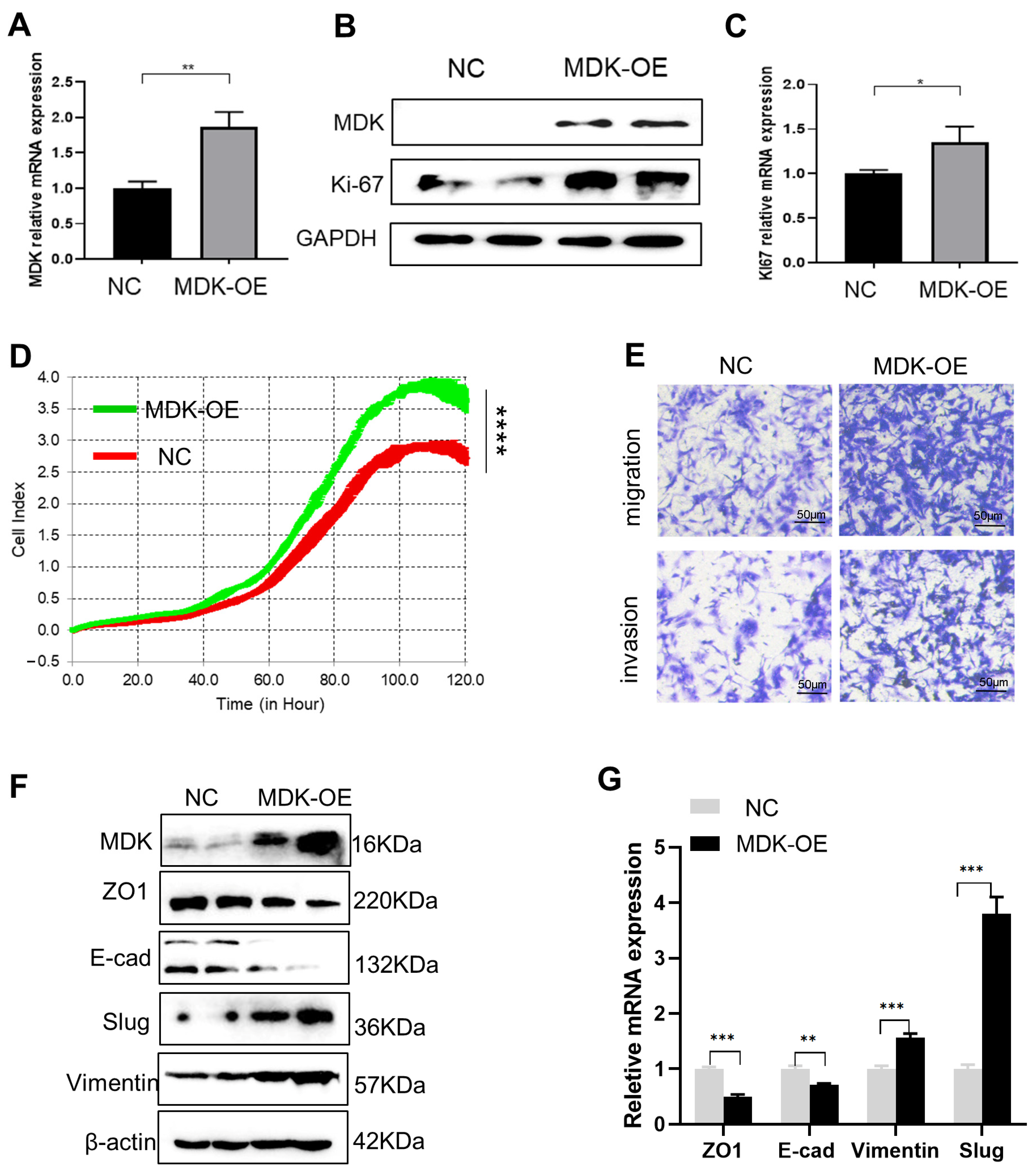
3.3. MDK Promotes Cell Proliferation, Migration, and Invasion in SKOV3 Cells
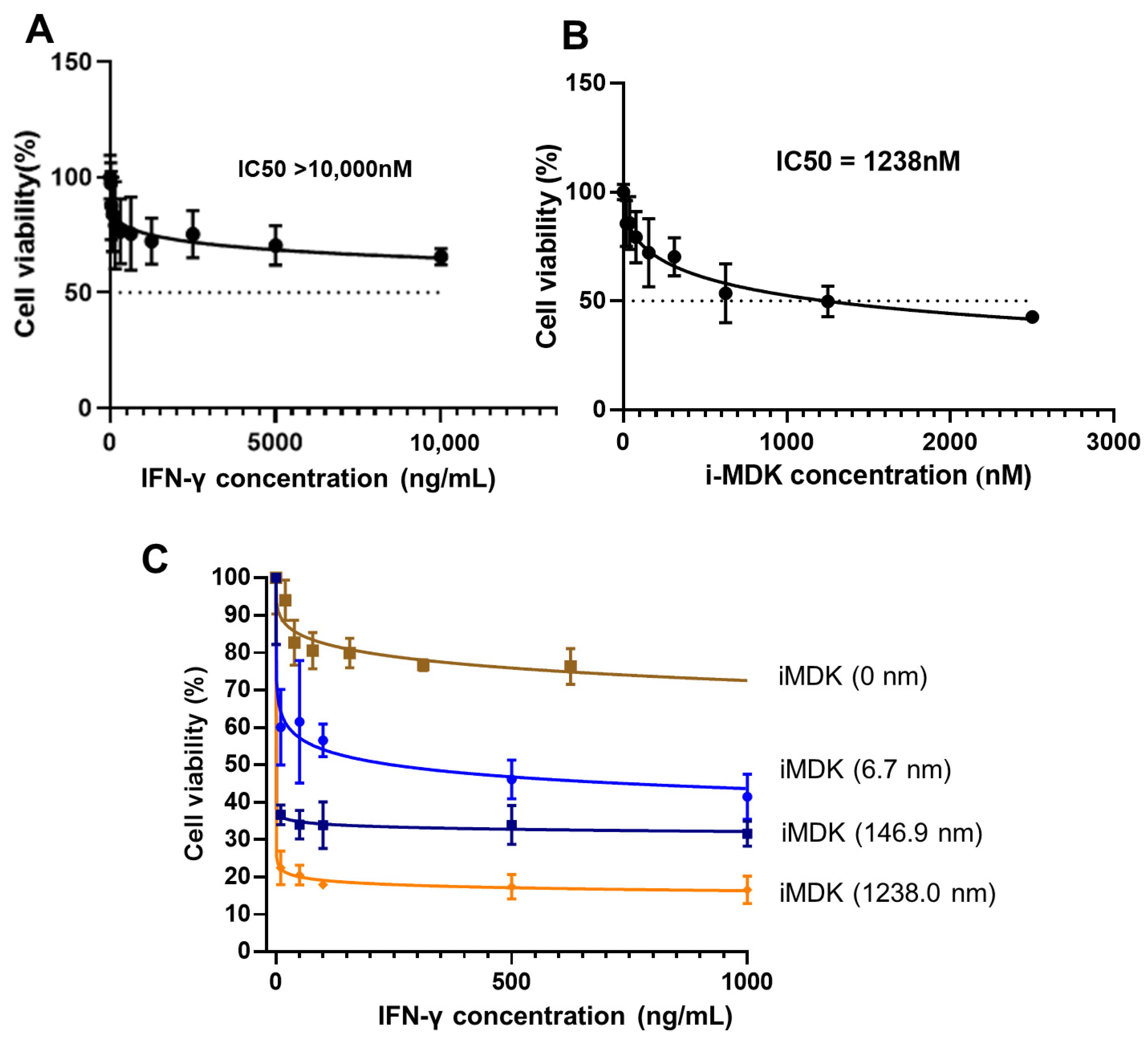
3.4. MDK Inhibition Potentiates the Tumoricidal Effect of IFN-γ in SKOV3 Cells
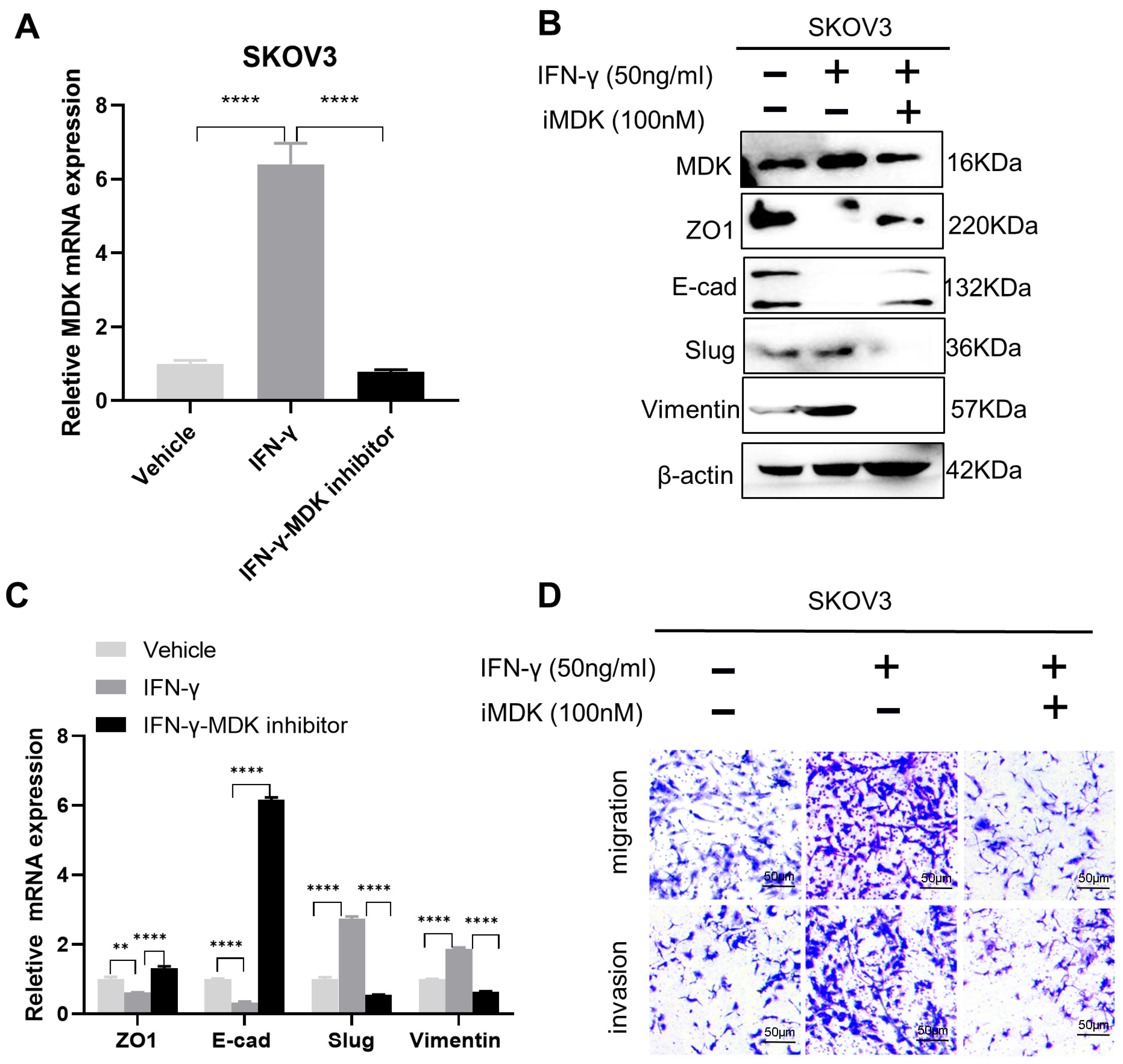
3.5. MDK Inhibition Attenuates the Pro-Metastatic Adverse Effect of IFN-γ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pastor, R.; Goedegebuure, P.S.; Curiel, D.T. Understanding and addressing barriers to successful adenovirus-based virotherapy for ovarian cancer. Cancer Gene Ther. 2021, 28, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Savage, S.R.; Calinawan, A.P.; Lin, C.; Zhang, B.; Wang, P.; Starr, T.K.; Birrer, M.J.; Paulovich, A.G. A highly annotated database of genes associated with platinum resistance in cancer. Oncogene 2021, 40, 6395–6405. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Sonego, M.; Pucci, B.; Addi, L.; Iannelli, F.; Capone, F.; Alfano, L.; Roca, M.S.; Milone, M.R.; Moccia, T.; et al. HSP90 identified by a proteomic approach as druggable target to reverse platinum resistance in ovarian cancer. Mol. Oncol. 2021, 15, 1005–1023. [Google Scholar] [CrossRef]
- Li, S.S.; Ma, J.; Wong, A. Chemoresistance in ovarian cancer: Exploiting cancer stem cell metabolism. J. Gynecol. Oncol. 2018, 29, e32. [Google Scholar] [CrossRef]
- Bartoletti, M.; Musacchio, L.; Giannone, G.; Tuninetti, V.; Bergamini, A.; Scambia, G.; Pignata, S. Emerging molecular alterations leading to histology-specific targeted therapies in ovarian cancer beyond PARP inhibitors. Cancer Treat. Rev. 2021, 101, 102298. [Google Scholar] [CrossRef]
- Pietilä, E.A.; Gonzalez-Molina, J.; Moyano-Galceran, L.; Jamalzadeh, S.; Zhang, K.; Lehtinen, L.; Lehti, K. Co-evolution of matrisome and adaptive adhesion dynamics drives ovarian cancer chemoresistance. Nat. Commun. 2021, 12, 3904. [Google Scholar] [CrossRef]
- Miller, C.H.; Maher, S.G.; Young, H.A. Clinical Use of Interferon-gamma. Ann. N. Y. Acad. Sci. 2009, 1182, 69–79. [Google Scholar] [CrossRef]
- Zaidi, M.R. The Interferon-Gamma Paradox in Cancer. J. Interferon Cytokine Res. 2019, 39, 30–38. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Wall, L.; Burke, F.; Barton, C.; Smyth, J.; Balkwill, F. IFN-gamma induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin. Cancer Res. 2003, 9, 2487–2496. [Google Scholar]
- Wall, L.; Burke, F.; Smyth, J.F.; Balkwill, F. The anti-proliferative activity of interferon-gamma on ovarian cancer: In vitro and in vivo. Gynecol. Oncol. 2003, 88 Pt 2, S149–S151. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Guastalla, J.P.; Colombo, N.; Francois, E.; Fumoleau, P.; Monnier, A.; Brandely, M. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J. Clin. Oncol. 1996, 14, 343–350. [Google Scholar] [CrossRef]
- Windbichler, G.H.; Hausmaninger, H.; Stummvoll, W.; Graf, A.H.; Kainz, C.; Lahodny, J. Interferon-gamma in the first-line therapy of ovarian cancer: A randomized phase III trial. Br. J. Cancer. 2000, 82, 1138–1144. [Google Scholar] [CrossRef]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef]
- Devane, J.G.; Martin, M.L.; Matson, M.A. A short 2 week dose titration regimen reduces the severity of flu-like symptoms with initial interferon gamma-1b treatment. Curr. Med. Res. Opin. 2014, 30, 1179–1187. [Google Scholar] [CrossRef]
- Song, M.; Ping, Y.; Zhang, K.; Yang, L.; Li, F.; Zhang, C.; Zhang, Y. Low-Dose IFNγ Induces Tumor Cell Stemness in Tumor Microenvironment of Non-Small Cell Lung Cancer. Cancer Res. 2019, 79, 3737–3748. [Google Scholar] [CrossRef]
- Lo, U.G.; Bao, J.; Cen, J.; Yeh, H.C.; Luo, J.; Tan, W.; Hsieh, J.T. Interferon-induced IFIT5 promotes epithelial-to-mesenchymal transition leading to renal cancer invasion. Am. J. Clin. Exp. Urol. 2019, 7, 31–45. [Google Scholar]
- Gong, W.; Zhang, G.M.; Liu, Y.; Lei, Z.; Li, D.; Yuan, Y.; Feng, Z.H. IFN-gamma withdrawal after immunotherapy potentiates B16 melanoma invasion and metastasis by intensifying tumor integrin alphavbeta3 signaling. Int. J. Cancer 2008, 123, 702–708. [Google Scholar] [CrossRef]
- Kelly, S.A.; Gschmeissner, S.; East, N.; Balkwill, F.R. Enhancement of metastatic potential by gamma-interferon. Cancer Res. 1991, 51, 4020–4027. [Google Scholar] [PubMed]
- Zheng, L.; Liu, Q.; Li, R.; Chen, S.; Tan, J.; Li, L.; Liu, J. Targeting MDK Abrogates IFN-γ-Elicited Metastasis inCancers of Various Origins. Front. Oncol. 2022, 12, 885656. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Marth, C.; Alvarez, R.D.; Johnson, G.; Bidzinski, M.; Kardatzke, D.R. Randomized phase 3 trial of interferon gamma-1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: Results from a prospectively designed analysis of progression-free survival. Gynecol. Oncol. 2008, 109, 174–181. [Google Scholar] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, H.; Wu, J.; Guan, Y. Effect of plasmid-mediated stable interferon-γ expression on proliferation and cell death in the SKOV-3 human ovarian cancer cell line. Immunopharmacol. Immunotoxicol. 2011, 33, 498–503. [Google Scholar] [CrossRef]
- Lo, U.G.; Pong, R.C.; Yang, D.; Gandee, L.; Hernandez, E.; Dang, A.; Hsieh, J.T. IFNγ-Induced IFIT5 Promotes Epithelial-to-Mesenchymal Transition in Prostate Cancer via miRNA Processing. Cancer Res. 2019, 79, 1098–1112. [Google Scholar] [CrossRef]
- Chow, K.T.; Gale, M., Jr. SnapShot: Interferon Signaling. Cell 2015, 163, 1808.e1. [Google Scholar] [CrossRef]
- Liu, C.; Gao, A.C. IFNγ, a Double-Edged Sword in Cancer Immunity and Metastasis. Cancer Res. 2019, 79, 1032–1033. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Xue, H.; Li, C.; Liu, Q.; Zhou, Y.; Wen, T. A TGF-β-MTA1-SOX4-EZH2 signaling axis drives epithelial-mesenchymal transition in tumor metastasis. Oncogene 2020, 39, 2125–2139. [Google Scholar] [CrossRef]
- Menon, U.; Karpinskyj, C.; Gentry-Maharaj, A. Ovarian Cancer Prevention and Screening. Obstet. Gynecol. 2018, 131, 909–927. [Google Scholar] [CrossRef]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef]
- Govindarajan, M.; Wohlmuth, C.; Waas, M.; Bernardini, M.Q.; Kislinger, T. High-throughput approaches for precision medicine in high-grade serous ovarian cancer. J. Hematol. Oncol. 2020, 13, 134. [Google Scholar] [CrossRef]
- Malik, S.T.; Knowles, R.G.; East, N.; Lando, D.; Stamp, G.; Balkwill, F.R. Antitumor activity of gamma-interferon in ascitic and solid tumor models of human ovarian cancer. Cancer Res. 1991, 51, 6643–6649. [Google Scholar]
- Razaghi, A.; Villacrés, C.; Jung, V.; Mashkour, N.; Butler, M.; Owens, L.; Heimann, K. Improved therapeutic efficacy of mammalian expressed-recombinant interferon gamma against ovarian cancer cells. Exp. Cell Res. 2017, 359, 20–29. [Google Scholar] [CrossRef]
- Burke, F.; East, N.; Upton, C.; Patel, K.; Balkwill, F.R. Interferon gamma induces cell cycle arrest and apoptosis in a model of ovarian cancer: Enhancement of effect by batimastat. Eur. J. Cancer 1997, 33, 1114–1121. [Google Scholar] [CrossRef]
- Marth, C.; Windbichler, G.H.; Hausmaninger, H.; Petru, E.; Estermann, K.; Pelzer, A.; Mueller-Holzner, E. Interferon-gamma in combination with carboplatin and paclitaxel as a safe and effective first-line treatment option for advanced ovarian cancer: Results of a phase I/II study. Int. J. Gynecol. Cancer 2006, 16, 1522–1528. [Google Scholar] [CrossRef]
- Filippou, P.S.; Karagiannis, G.S.; Constantinidou, A. Midkine (MDK) growth factor: A key player in cancer progression and a promising therapeutic target. Oncogene 2020, 39, 2040–2054. [Google Scholar] [CrossRef]
- Zhang, X.; Karim, M.; Hasan, M.M.; Hooper, J.; Wahab, R.; Roy, S.; Al-Hilal, T.A. Cancer-on-a-Chip: Models for Studying Metastasis. Cancers 2022, 14, 648. [Google Scholar] [CrossRef]
- Kavand, H.; Nasiri, R.; Herland, A. Advanced Materials and Sensors for Microphysiological Systems: Focus on Electronic and Electrooptical Interfaces. Adv Mater. 2022, 34, e2107876. [Google Scholar] [CrossRef]
- Cerezo-Wallis, D.; Contreras-Alcalde, M.; Troulé, K.; Catena, X.; Mucientes, C.; Calvo, T.G.; Soengas, M.S. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat. Med. 2020, 26, 1865–1877. [Google Scholar] [CrossRef]

| NO. | IFN-γ (ng/mL) | iMDK (μM) | FA | CI |
|---|---|---|---|---|
| 1 | 10 | 6.662 | 0.3996 | 0.01426 |
| 2 | 50 | 6.662 | 0.3851 | 0.02502 |
| 3 | 100 | 6.662 | 0.4344 | 0.02188 |
| 4 | 500 | 6.662 | 0.5388 | 0.02493 |
| 5 | 1000 | 6.662 | 0.5852 | 0.02835 |
| 1 | 10 | 146.896 | 0.6339 | 0.03549 |
| 2 | 50 | 146.896 | 0.6599 | 0.02825 |
| 3 | 100 | 146.896 | 0.6613 | 0.02843 |
| 4 | 500 | 146.896 | 0.6608 | 0.03300 |
| 5 | 1000 | 146.896 | 0.6845 | 0.03017 |
| 1 | 10 | 1238 | 0.6339 | 0.06794 |
| 2 | 50 | 1238 | 0.6599 | 0.05325 |
| 3 | 100 | 1238 | 0.6613 | 0.03741 |
| 4 | 500 | 1238 | 0.6608 | 0.03482 |
| 5 | 1000 | 1238 | 0.6845 | 0.03114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Tan, J.; Zhao, Z.; Li, R.; Zheng, L.; Chen, X.; Li, L.; Dong, X.; Wen, T.; Liu, J. Combined Usage of MDK Inhibitor Augments Interferon-γ Anti-Tumor Activity in the SKOV3 Human Ovarian Cancer Cell Line. Biomedicines 2023, 11, 8. https://doi.org/10.3390/biomedicines11010008
Liu Q, Tan J, Zhao Z, Li R, Zheng L, Chen X, Li L, Dong X, Wen T, Liu J. Combined Usage of MDK Inhibitor Augments Interferon-γ Anti-Tumor Activity in the SKOV3 Human Ovarian Cancer Cell Line. Biomedicines. 2023; 11(1):8. https://doi.org/10.3390/biomedicines11010008
Chicago/Turabian StyleLiu, Qun, Jingyu Tan, Zhenguo Zhao, Ruijun Li, Luyu Zheng, Xiangyu Chen, Lina Li, Xichen Dong, Tao Wen, and Jian Liu. 2023. "Combined Usage of MDK Inhibitor Augments Interferon-γ Anti-Tumor Activity in the SKOV3 Human Ovarian Cancer Cell Line" Biomedicines 11, no. 1: 8. https://doi.org/10.3390/biomedicines11010008
APA StyleLiu, Q., Tan, J., Zhao, Z., Li, R., Zheng, L., Chen, X., Li, L., Dong, X., Wen, T., & Liu, J. (2023). Combined Usage of MDK Inhibitor Augments Interferon-γ Anti-Tumor Activity in the SKOV3 Human Ovarian Cancer Cell Line. Biomedicines, 11(1), 8. https://doi.org/10.3390/biomedicines11010008








