Current Evidence on Vasa Previa without Velamentous Cord Insertion or Placental Morphological Anomalies (Type III Vasa Previa): Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Approach for Systematic Review
2.2. The Definition of Type I, II, and III Vasa Previa
2.3. Search Strategy
2.4. Analysis of Outcome Measures
2.5. Selection of Previous Studies
2.6. Data Extraction
2.7. Assessment of Bias Risk
2.8. Meta-Analysis Plan
2.9. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
Risk of Bias of included Studies
3.3. Primary Outcome: Obstetric and Neonatal Outcomes of Women with Type III Vasa Previa
3.4. Co-Primary Outcome: The Obstetric Outcomes (Type III Versus Other Types)
3.5. Co-Secondary Outcome: The Estimated Prevalence of Type III Vasa Previa
4. Discussion
4.1. Key Findings
4.2. Strengths and Limitations
4.3. Comparison with Existing Literature
4.3.1. Characteristics, and Obstetric and Neonatal Outcomes of Women with Type III VP
4.3.2. The Estimated Prevalence of Type III Vasa Previa and Diagnosis Pitfall
4.3.3. The Mechanism of Developing Type III Vasa Previa
4.3.4. The Validity of Classifying Vasa Previa into Three Categories
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Clinical Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, S.J.; Ngo, G.; Maurel, K.A.; Hasegawa, J.; Arakaki, T.; Melcer, Y.; Maymon, R.; Vendittelli, F.; Shamshirsaz, A.A.; Erfani, H.; et al. Timing of birth and adverse pregnancy outcomes in cases of prenatally diagnosed vasa previa: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022, 227, 173–181.e24. [Google Scholar] [CrossRef]
- Pavalagantharajah, S.; Villani, L.A.; D’Souza, R. Vasa previa and associated risk factors: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100117. [Google Scholar] [CrossRef]
- Melcer, Y.; Maymon, R.; Jauniaux, E. Vasa previa: Prenatal diagnosis and management. Curr. Opin. Obstet. Gynecol. 2018, 30, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Melcer, Y.; Maymon, R. Prenatal diagnosis and management of vasa previa in twin pregnancies: A case series and systematic review. Am. J. Obstet. Gynecol. 2017, 216, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Endo, M.; Kimura, T. Vasa previa with an intact amniotic membrane. Am. J. Obstet. Gynecol. 2017, 216, 616.e611–616.e612. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Kimura, T. Vasa Previa. N. Engl. J. Med. 2019, 380, 274. [Google Scholar] [CrossRef] [PubMed]
- Oyelese, Y. Vasa previa: Time to make a difference. Am. J. Obstet. Gynecol. 2019, 221, 539–541. [Google Scholar] [CrossRef]
- Bronsteen, R.; Whitten, A.; Balasubramanian, M.; Lee, W.; Lorenz, R.; Redman, M.; Goncalves, L.; Seubert, D.; Bauer, S.; Comstock, C. Vasa previa: Clinical presentations, outcomes, and implications for management. Obstet. Gynecol. 2013, 122, 352–357. [Google Scholar] [CrossRef]
- Kulkarni, A.; Powel, J.; Aziz, M.; Shah, L.; Lashley, S.; Benito, C.; Oyelese, Y. Vasa Previa: Prenatal Diagnosis and Outcomes: Thirty-five Cases From a Single Maternal-Fetal Medicine Practice. J. Ultrasound Med. 2018, 37, 1017–1024. [Google Scholar] [CrossRef]
- Swank, M.L.; Garite, T.J.; Maurel, K.; Das, A.; Perlow, J.H.; Combs, C.A.; Fishman, S.; Vanderhoeven, J.; Nageotte, M.; Bush, M.; et al. Vasa previa: Diagnosis and management. Am. J. Obstet. Gynecol. 2016, 215, 223.e221–223.e226. [Google Scholar] [CrossRef]
- Klahr, R.; Fox, N.S.; Zafman, K.; Hill, M.B.; Connolly, C.T.; Rebarber, A. Frequency of spontaneous resolution of vasa previa with advancing gestational age. Am. J. Obstet. Gynecol. 2019, 221, 646.e641–646.e647. [Google Scholar] [CrossRef]
- Sinkey, R.G.; Odibo, A.O. Vasa previa screening strategies: Decision and cost-effectiveness analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 52, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Chiu, S.; Manor, E.; Smith, L.; Oyelese, Y. The association of gestational age at delivery with neonatal outcomes in prenatally diagnosed vasa previa. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2022, 35, 10162–10167. [Google Scholar] [CrossRef]
- Sutera, M.; Garofalo, A.; Pilloni, E.; Parisi, S.; Alemanno, M.G.; Menato, G.; Sciarrone, A.; Viora, E. Vasa previa: When antenatal diagnosis can change fetal prognosis. J. Perinat. Med. 2021, 49, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions; Version 6.1; The Cochrane Collaboration, 2020; Chapter 10: Analysing Data and Undertaking Meta-Analyses. Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 20 December 2022).
- Matsuzaki, S.; Ueda, Y.; Matsuzaki, S.; Nagase, Y.; Kakuda, M.; Lee, M.; Maeda, M.; Kurahashi, H.; Hayashida, H.; Hisa, T.; et al. Assisted Reproductive Technique and Abnormal Cord Insertion: A Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Society for Maternal-Fetal Medicine; Ghidini, A.; Gandhi, M.; McCoy, J.; Kuller, J.A.; Publications, C. Society for Maternal-Fetal Medicine Consult Series #60: Management of pregnancies resulting from in vitro fertilization. Am. J. Obstet. Gynecol. 2022, 226, B2–B12. [Google Scholar] [CrossRef]
- Gagnon, R. No. 231-Guidelines for the Management of Vasa Previa. J. Obstet. Gynaecol. Can. JOGC 2017, 39, e415–e421. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, I.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Diagnosis and Management of Vasa Previa: A Comparison of 4 National Guidelines. Obstet. Gynecol. Surv. 2019, 74, 436–442. [Google Scholar] [CrossRef]
- Skjeldestad, F.E.; Oian, P. Blood loss after cesarean delivery: A registry-based study in Norway, 1999–2008. Am. J. Obstet. Gynecol. 2012, 206, 76.e71–76.e77. [Google Scholar] [CrossRef]
- Marotta, C.; Pisani, L.; Di Gennaro, F.; Cavallin, F.; Bah, S.; Pisani, V.; Haniffa, R.; Beane, A.; Trevisanuto, D.; Hanciles, E.; et al. Epidemiology, Outcomes, and Risk Factors for Mortality in Critically Ill Women Admitted to an Obstetric High-Dependency Unit in Sierra Leone. Am. J. Trop. Med. Hyg. 2020, 103, 2142–2148. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Marotta, C.; Pisani, L.; Veronese, N.; Pisani, V.; Lippolis, V.; Pellizer, G.; Pizzol, D.; Tognon, F.; Bavaro, D.F.; et al. Maternal caesarean section infection (MACSI) in Sierra Leone: A case-control study. Epidemiol. Infect. 2020, 148, e40. [Google Scholar] [CrossRef] [PubMed]
- King, L.J.; Dhanya Mackeen, A.; Nordberg, C.; Paglia, M.J. Maternal risk factors associated with persistent placenta previa. Placenta 2020, 99, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.L.; Hinkle, S.N.; Sjaarda, L.A.; Albert, P.S.; Grantz, K.L. Previous prelabor or intrapartum cesarean delivery and risk of placenta previa. Am. J. Obstet. Gynecol. 2015, 212, 669.e661–669.e666. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Xia, Q.; Liu, L.; Wu, S.; Tian, G.; Wang, W.; Wu, S.; Guo, X.; Liu, Z. The Incidence of Postpartum Hemorrhage in Pregnant Women with Placenta Previa: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0170194. [Google Scholar] [CrossRef]
- Deng, L.; Chang, Q.; Wang, Y.; Wang, L.; Li, Y.; Hu, Q. Tourniquet device for hemorrhage control during cesarean section of complete placenta previa pregnancies. J. Obstet. Gynaecol. Res. 2014, 40, 399–404. [Google Scholar] [CrossRef]
- Catanzarite, V.; Maida, C.; Thomas, W.; Mendoza, A.; Stanco, L.; Piacquadio, K.M. Prenatal sonographic diagnosis of vasa previa: Ultrasound findings and obstetric outcome in ten cases. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2001, 18, 109–115. [Google Scholar] [CrossRef]
- Jauniaux, E.; Alfirevic, Z.; Bhide, A.G.; Burton, G.J.; Collins, S.L.; Silver, R. Vasa Praevia: Diagnosis and Management: Green-top Guideline No. 27b. BJOG Int. J. Obstet. Gynaecol. 2019, 126, e49–e61. [Google Scholar] [CrossRef]
- Gross, A.; Markota Ajd, B.; Specht, C.; Scheier, M. Systematic screening for vasa previa at the 20-week anomaly scan. Acta Obstet. Et Gynecol. Scand. 2021, 100, 1694–1699. [Google Scholar] [CrossRef]
- Westcott, J.M.; Simpson, S.; Chasen, S.; Vieira, L.; Stone, J.; Doulaveris, G.; Dar, P.; Bernstein, P.S.; Atallah, F.; Dolin, C.D.; et al. Prenatally diagnosed vasa previa: Association with adverse obstetrical and neonatal outcomes. Am. J. Obstet. Gynecol. MFM 2020, 2, 100206. [Google Scholar] [CrossRef]
- Hasegawa, J.; Farina, A.; Nakamura, M.; Matsuoka, R.; Ichizuka, K.; Sekizawa, A.; Okai, T. Analysis of the ultrasonographic findings predictive of vasa previa. Prenat. Diagn. 2010, 30, 1121–1125. [Google Scholar] [CrossRef]
- Melcer, Y.; Maymon, R.; Pekar-Zlotin, M.; Levinsohn-Tavor, O.; Tovbin, J.; Jauniaux, E. Evaluation of the impact of vasa previa on feto-placental hormonal synthesis and fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, Y.; Steetskamp, J.; Macchiella, D.; Hasenburg, A.; Hasenburg, A. Vasa previa: A rare obstetric complication—A case series and a literature review. Clin. Case Rep. 2022, 10, e05608. [Google Scholar] [CrossRef] [PubMed]
- Chmait, R.H.; Chavira, E.; Kontopoulos, E.V.; Quintero, R.A. Third trimester fetoscopic laser ablation of type II vasa previa. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2010, 23, 459–462. [Google Scholar] [CrossRef]
- Ruiter, L.; Kok, N.; Limpens, J.; Derks, J.B.; de Graaf, I.M.; Mol, B.W.; Pajkrt, E. Systematic review of accuracy of ultrasound in the diagnosis of vasa previa. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2015, 45, 516–522. [Google Scholar] [CrossRef]
- Ruiter, L.; Kok, N.; Limpens, J.; Derks, J.B.; de Graaf, I.M.; Mol, B.; Pajkrt, E. Incidence of and risk indicators for vasa praevia: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1278–1287. [Google Scholar] [CrossRef]
- Chmait, R.H.; Catanzarite, V.; Chon, A.H.; Korst, L.M.; Llanes, A.; Ouzounian, J.G. Fetoscopic Laser Ablation Therapy for Type II Vasa Previa. Fetal Diagn. Ther. 2020, 47, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Catanzarite, V.; Cousins, L.; Daneshmand, S.; Schwendemann, W.; Casele, H.; Adamczak, J.; Tith, T.; Patel, A. Prenatally Diagnosed Vasa Previa: A Single-Institution Series of 96 Cases. Obstet. Gynecol. 2016, 128, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Matsuzaki, S.; Kakigano, A.; Mimura, K.; Takiuchi, T.; Kimura, T. A rare type of vasa previa. Clin. Case Rep. 2019, 7, 2263–2264. [Google Scholar] [CrossRef] [PubMed]
- Suekane, T.; Tachibana, D.; Pooh, R.K.; Misugi, T.; Koyama, M. Type-3 vasa previa: Normal umbilical cord insertion cannot exclude vasa previa in cases with abnormal placental location. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2020, 55, 556–557. [Google Scholar] [CrossRef]
- Lo, A.; Berman, S.; Chaiworapongsa, T.; Asaad, R.; Gonik, B. Vasa previa with pulsed wave Doppler depicting maternal heart rate. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2020, 56, 460–461. [Google Scholar] [CrossRef]
- Hata, T.; Takayoshi, R.; Koyanagi, A.; Miyake, T. Antenatal Diagnosis of Type-3 Vasa Previa. Donald Sch. J Ultrasound Obs. Gynecol. 2021, 15, 1–3. [Google Scholar]
- Ochiai, D.; Endo, T.; Oishi, M.; Kasuga, Y.; Ikenoue, S.; Tanaka, M. Vasa previa with fetal vessels running transversely across the cervix: A diagnostic pitfall. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 58, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Ueda, Y.; Matsuzaki, S.; Kakuda, M.; Lee, M.; Takemoto, Y.; Hayashida, H.; Maeda, M.; Kakubari, R.; Hisa, T.; et al. The Characteristics and Obstetric Outcomes of Type II Vasa Previa: Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 3263. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Lee, M.; Nagase, Y.; Jitsumori, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Ueda, Y.; et al. A systematic review and meta-analysis of obstetric and maternal outcomes after prior uterine artery embolization. Sci. Rep. 2021, 11, 16914. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Endo, M.; Ueda, Y.; Mimura, K.; Kakigano, A.; Egawa-Takata, T.; Kumasawa, K.; Yoshino, K.; Kimura, T. A case of acute Sheehan’s syndrome and literature review: A rare but life-threatening complication of postpartum hemorrhage. BMC Pregnancy Childbirth 2017, 17, 188. [Google Scholar] [CrossRef]
- Kamijo, K.; Miyamoto, T.; Ando, H.; Tanaka, Y.; Kikuchi, N.; Shinagawa, M.; Yamada, S.; Asaka, R.; Fuseya, C.; Ohira, S.; et al. Clinical characteristics of a novel “Type 3” vasa previa: Case series at a single center. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2022, 35, 7730–7736. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, D.; Misugi, T.; Pooh, R.K.; Kitada, K.; Kurihara, Y.; Tahara, M.; Hamuro, A.; Nakano, A.; Koyama, M. Placental Types and Effective Perinatal Management of Vasa Previa: Lessons from 55 Cases in a Single Institution. Diagnostics 2021, 11, 1369. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Nagase, Y.; Ueda, Y.; Lee, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Endo, M.; et al. The association of endometriosis with placenta previa and postpartum hemorrhage: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100417. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Danna, S.M.; Graham, E.; Burns, R.J.; Deschenes, S.S.; Schmitz, N. Association between Depressive Symptoms and Cognitive Function in Persons with Diabetes Mellitus: A Systematic Review. PLoS ONE 2016, 11, e0160809. [Google Scholar] [CrossRef] [PubMed]
- ROBINS-I Detailed Guidance. 2016. Available online: https://www.riskofbias.info/welcome/home/current-version-of-robins-i/robins-i-detailed-guidance-2016 (accessed on 20 December 2022).
- Liu, N.; Hu, Q.; Liao, H.; Wang, X.; Yu, H. Vasa previa: Perinatal outcomes in singleton and multiple pregnancies. Biosci. Trends 2021, 15, 118–125. [Google Scholar] [CrossRef]
- Kanda, E.; Matsuda, Y.; Kamitomo, M.; Maeda, T.; Mihara, K.; Hatae, M. Prenatal diagnosis and management of vasa previa: A 6-year review. J. Obstet. Gynaecol. Res. 2011, 37, 1391–1396. [Google Scholar] [CrossRef]
- Oyelese, K.O.; Turner, M.; Lees, C.; Campbell, S. Vasa previa: An avoidable obstetric tragedy. Obstet. Gynecol. Surv. 1999, 54, 138–145. [Google Scholar] [CrossRef]
- Schachter, M.; Tovbin, Y.; Arieli, S.; Friedler, S.; Ron-El, R.; Sherman, D. In vitro fertilization is a risk factor for vasa previa. Fertil. Steril. 2002, 78, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Pirtea, L.C.; Grigoraş, D.; Sas, I.; Ilie, A.C.; Stana, L.G.; Motoc, A.G.; Jianu, A.M.; Mazilu, O. In vitro fertilization represents a risk factor for vasa praevia. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2016, 57, 627–632. [Google Scholar]
- Zhang, W.; Geris, S.; Al-Emara, N.; Ramadan, G.; Sotiriadis, A.; Akolekar, R. Perinatal outcome of pregnancies with prenatal diagnosis of vasa previa: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 57, 710–719. [Google Scholar] [CrossRef]
- Baulies, S.; Maiz, N.; Muñoz, A.; Torrents, M.; Echevarría, M.; Serra, B. Prenatal ultrasound diagnosis of vasa praevia and analysis of risk factors. Prenat. Diagn. 2007, 27, 595–599. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, Y.; Li, M.; Li, W.; Li, X.; Zhang, X.; Wang, R.; Zhang, J.; Zhou, F.; Yang, Q.; et al. Clinical features of velamentous umbilical cord insertion and vasa previa: A retrospective analysis based on 501 cases. Medicine 2020, 99, e23166. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, E.; Pando, C.; Kirschen, G.W.; Soucier, D.; Fuchs, A.; Garry, D.J. Assisted reproductive technologies (ART) and placental abnormalities. J. Perinat. Med. 2020, 48, 825–828. [Google Scholar] [CrossRef]
- O’Quinn, C.; Cooper, S.; Tang, S.; Wood, S. Antenatal Diagnosis of Marginal and Velamentous Placental Cord Insertion and Pregnancy Outcomes. Obstet. Gynecol. 2020, 135, 953–959. [Google Scholar] [CrossRef]
- Yanaihara, A.; Hatakeyama, S.; Ohgi, S.; Motomura, K.; Taniguchi, R.; Hirano, A.; Takenaka, S.; Yanaihara, T. Difference in the size of the placenta and umbilical cord between women with natural pregnancy and those with IVF pregnancy. J. Assist. Reprod. Genet. 2018, 35, 431–434. [Google Scholar] [CrossRef]
- Kalafat, E.; Thilaganathan, B.; Papageorghiou, A.; Bhide, A.; Khalil, A. Significance of placental cord insertion site in twin pregnancy. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 52, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, C.; Johnsen, S.L.; Albrechtsen, S.; Sunde, I.D.; Vekseth, C.; Rasmussen, S. Velamentous or marginal cord insertion and the risk of spontaneous preterm birth, prelabor rupture of the membranes, and anomalous cord length, a population-based study. Acta Obstet. Gynecol. Scand. 2017, 96, 78–85. [Google Scholar] [CrossRef]
- Suzuki, S.; Kato, M. Clinical Significance of Pregnancies Complicated by Velamentous Umbilical Cord Insertion Associated With Other Umbilical Cord/Placental Abnormalities. J. Clin. Med. Res. 2015, 7, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, S.; Georgiadis, L.; Harju, M.; Keski-Nisula, L.; Heinonen, S. Risk factors and adverse pregnancy outcomes among births affected by velamentous umbilical cord insertion: A retrospective population-based register study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 231–234. [Google Scholar] [CrossRef]
- Delbaere, I.; Goetgeluk, S.; Derom, C.; De Bacquer, D.; De Sutter, P.; Temmerman, M. Umbilical cord anomalies are more frequent in twins after assisted reproduction. Hum. Reprod. 2007, 22, 2763–2767. [Google Scholar] [CrossRef]
- Jauniaux, E.; Englert, Y.; Vanesse, M.; Hiden, M.; Wilkin, P. Pathologic features of placentas from singleton pregnancies obtained by in vitro fertilization and embryo transfer. Obstet. Gynecol. 1990, 76, 61–64. [Google Scholar] [PubMed]
- Suzuki, S.; Igarashi, M. Clinical significance of pregnancies with succenturiate lobes of placenta. Arch. Gynecol. Obstet. 2008, 277, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Krief, D.; Naepels, P.; Chevreau, J. Per labor Vasa Previa discovery: A simple clinical diagnosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 284–285. [Google Scholar] [CrossRef]
- Siargkas, A.; Tsakiridis, I.; Pachi, C.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Impact of velamentous cord insertion on perinatal outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2022, 5, 100812. [Google Scholar] [CrossRef] [PubMed]
- Villani, L.A.; Pavalagantharajah, S.; D’Souza, R. Variations in reported outcomes in studies on vasa previa: A systematic review. Am. J. Obstet. Gynecol. MFM 2020, 2, 100116. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Kaushik, S.; Kuruba, N.; Beweley, S. Vasa praevia: A missed diagnosis. J. Obstet. Gynaecol. 2008, 28, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.; Kleinrouweler, C.E.; Kastelein, A.W.; Ruiter, L.; van Leeuwen, E.; Mol, B.W.; Pajkrt, E. Follow-up ultrasound in second-trimester low-positioned anterior and posterior placentae: Prospective cohort study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2020, 56, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Matsuoka, R.; Ichizuka, K.; Otsuki, K.; Sekizawa, A.; Farina, A.; Okai, T. Cord insertion into the lower third of the uterus in the first trimester is associated with placental and umbilical cord abnormalities. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2006, 28, 183–186. [Google Scholar] [CrossRef]
- Silver, R.M. Abnormal Placentation: Placenta Previa, Vasa Previa, and Placenta Accreta. Obstet. Gynecol. 2015, 126, 654–668. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Nagase, Y.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Lee, M.; Matsuzaki, S.; Ueda, Y.; Tomimatsu, T.; Endo, M.; et al. Antenatal diagnosis of placenta accreta spectrum after in vitro fertilization-embryo transfer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 9205. [Google Scholar] [CrossRef]
- Erfani, H.; Haeri, S.; Shainker, S.A.; Saad, A.F.; Ruano, R.; Dunn, T.N.; Rezaei, A.; Aalipour, S.; Nassr, A.A.; Shamshirsaz, A.A.; et al. Vasa previa: A multicenter retrospective cohort study. Am. J. Obstet. Gynecol. 2019, 221, 644.e641–644.e645. [Google Scholar] [CrossRef]
- Hasegawa, J.; Nakamura, M.; Ichizuka, K.; Matsuoka, R.; Sekizawa, A.; Okai, T. Vasa previa is not infrequent. J. Matern. Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2012, 25, 2795–2796. [Google Scholar] [CrossRef]
- Oyelese, Y.; Smulian, J.C. Placenta Previa, Placenta Accreta, and Vasa Previa. Obstet. Gynecol. 2006, 107, 927–941. [Google Scholar] [CrossRef]
- Society of Maternal-Fetal Publications; Sinkey, R.G.; Odibo, A.O.; Dashe, J.S. #37: Diagnosis and management of vasa previa. Am. J. Obstet. Gynecol. 2015, 213, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Conde-Agudelo, A.; Borovac-Pinheiro, A.; Suarez-Rebling, D.; Eckardt, M.; Theron, G.; Burke, T.F. Uterine balloon tamponade for the treatment of postpartum hemorrhage: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 222, 293.e1–293.e52. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Endo, M.; Tomimatsu, T.; Nakagawa, S.; Matsuzaki, S.; Miyake, T.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Ueda, Y.; et al. New dedicated blunt straight needles and sutures for uterine compression sutures: A retrospective study and literature review. BMC Surg. 2019, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.F.; Bishop, D.G.; Fawcus, S.; Morris, E.; Shakur-Still, H.; Devall, A.J.; Gallos, I.D.; Widmer, M.; Oladapo, O.T.; Coomarasamy, A.; et al. Tranexamic acid at cesarean delivery: Drug-error deaths. Am. J. Obstet. Gynecol. 2023, 228, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Madar, H.; Le Lous, M.; Sénat, M.V.; Winer, N.; Rozenberg, P.; Kayem, G.; Verspyck, E.; Fuchs, F.; Azria, E.; et al. Tranexamic acid for the prevention of blood loss after cesarean among women with twins: A secondary analysis of the TRAnexamic Acid for Preventing Postpartum Hemorrhage Following a Cesarean Delivery randomized clinical trial. Am. J. Obstet. Gynecol. 2022, 227, 889.e1–889.e17. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Pergialiotis, V. Tranexamic acid for the prevention of postpartum hemorrhage in women undergoing cesarean delivery: An updated meta-analysis. Am. J. Obstet. Gynecol. 2022, 226, 510–523.e522. [Google Scholar] [CrossRef]
- Phung, L.C.; Farrington, E.K.; Connolly, M.; Wilson, A.N.; Carvalho, B.; Homer, C.S.E.; Vogel, J.P. Intravenous oxytocin dosing regimens for postpartum hemorrhage prevention following cesarean delivery: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 225, 250.e1–250.e38. [Google Scholar] [CrossRef]
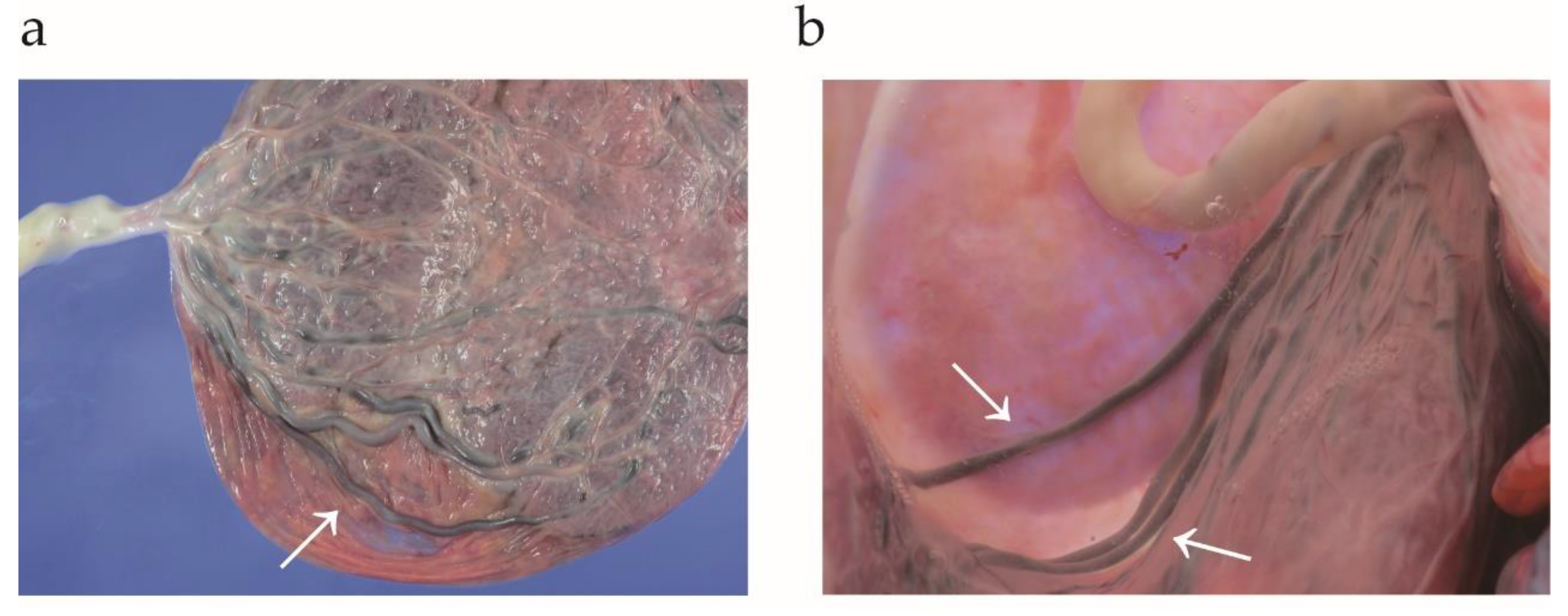
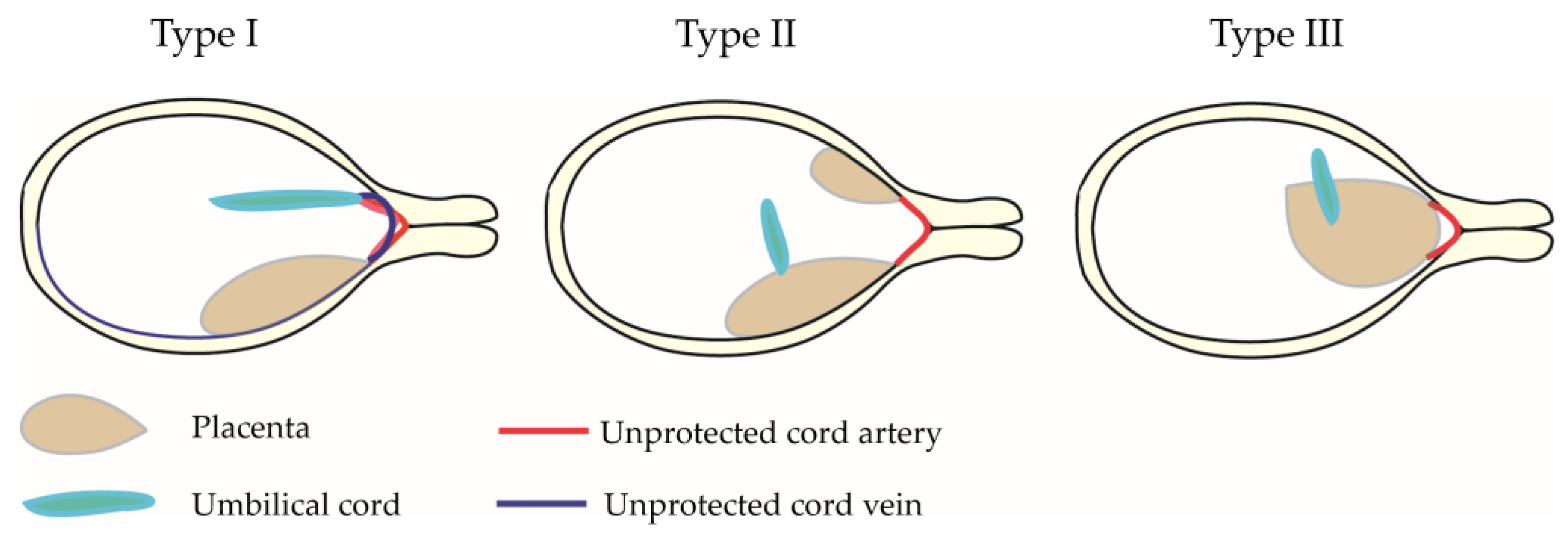
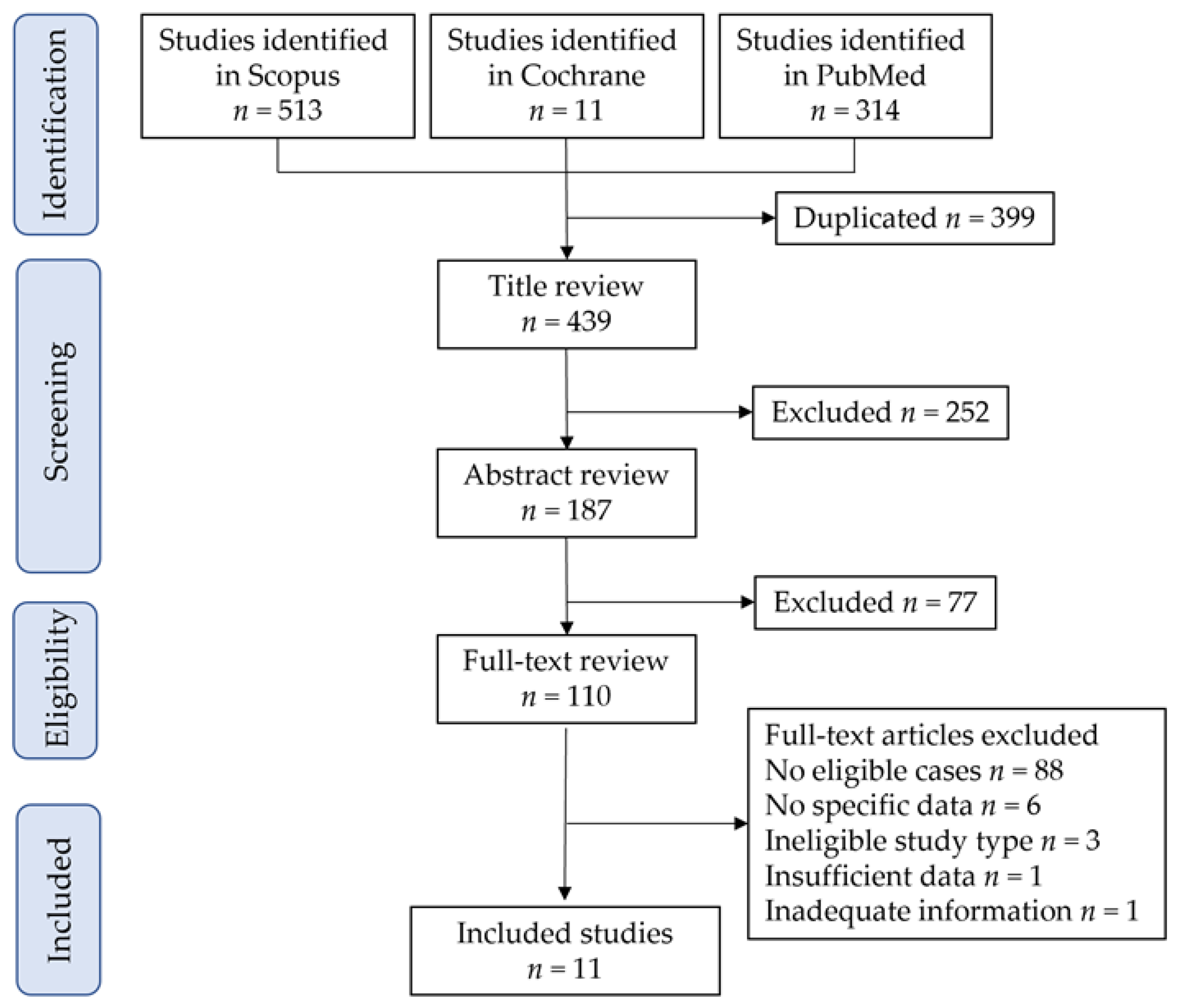
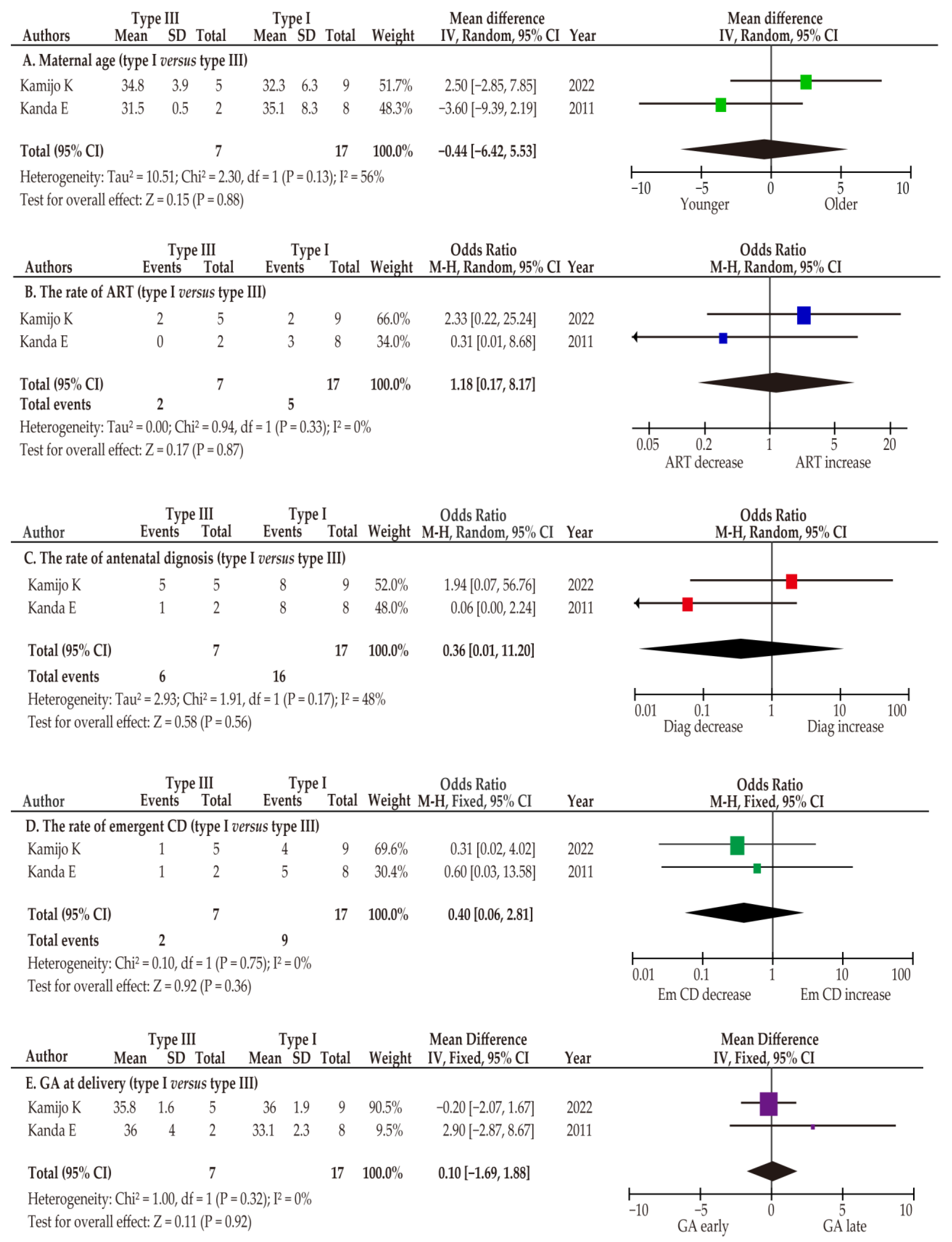
| Author | Year | Location | Total | Cont | VP | Type III |
|---|---|---|---|---|---|---|
| Kamijo K [48] | 2022 | JPN | 8723 | -- | 14 | 5 |
| Tachibana D [49] | 2021 | JPN | -- | -- | 55 | 7 |
| Liu N [54] | 2021 | CHN | 79,647 | 79,486 | 157 | 4 |
| Ochiai D [43] | 2021 | JPN | -- | -- | 1 | 1 |
| Hata T [42] | 2021 | JPN | -- | -- | 1 | 1 |
| Lo A [41] | 2020 | USA | -- | -- | 1 | 1 |
| Suekane T [40] | 2020 | JPN | -- | -- | 1 | 1 |
| Hara T [39] | 2019 | JPN | -- | -- | 1 | 1 |
| Matsuzaki S [5] | 2017 | JPN | -- | -- | 1 | 1 |
| Catanzarite V [38] | 2016 | USA | -- | -- | 96 | 1 |
| Kanda E [55] | 2011 | JPN | 5131 | 5121 | 10 | 2 |
| Author | Year | No. | Age | ART | GA | EmCD | HDP | FGR | PPH | PAS | AD | ND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kamijo K [48] | 2022 | 5 | 32 | Yes | 37 | -- | -- | -- | -- | -- | Yes | -- |
| 39 | -- | 33 | Yes | -- | -- | -- | -- | Yes | -- | |||
| 29 | -- | 37 | -- | -- | -- | -- | -- | Yes | -- | |||
| 39 | Yes | 35 | -- | -- | -- | -- | -- | Yes | -- | |||
| 35 | -- | 37 | -- | -- | -- | -- | -- | Yes | -- | |||
| Ochiai D [43] | 2021 | 1 | 37 | Yes | 34 | -- | -- | -- | -- | -- | Yes | -- |
| Hata T [42] | 2021 | 1 | 38 | Unk | 37 | -- | -- | -- | -- | -- | Yes | -- |
| Lo A [41] | 2020 | 1 | 38 | Unk | 36 | -- | -- | -- | -- | -- | Yes | -- |
| Suekane T [40] | 2020 | 1 | 32 | -- | 35 | -- | -- | -- | -- | -- | Yes | -- |
| Hara T [39] | 2019 | 1 | 36 | Yes | 34 | -- | -- | -- | -- | -- | Yes | -- |
| Matsuzaki S [5] | 2017 | 1 | 31 | Yes | 36 | -- | -- | -- | -- | Yes | -- | -- |
| Kanda E [55] | 2011 | 2 | 32 | -- | 32 | Yes | -- | -- | -- | -- | Yes | -- |
| 31 | -- | 40 | -- | -- | Yes | -- | -- | -- | -- |
| Characteristic | (%) |
|---|---|
| No. | n = 13 |
| Age(y) | 35 (31.5–38) |
| ART | 5/11 * (45.5%) |
| Gestational age | 36 (34–37) |
| Antenatal diag | 11 (84.6%) |
| Emergent CD | 2 (15.4%) |
| HDP | 0 |
| FGR | 1 (7.7%) |
| PPH | 0 |
| PAS | 1 (7.7%) |
| Neonatal death | 0 |
| Author | Year | Type I | Type III | OR or MD (95%CI) |
|---|---|---|---|---|
| Age | ||||
| Kamijo K | 2022 | 32.3 ± 6.3 | 34.8 ± 3.9 | 2.50 (−2.85–7.85) |
| Kanda E | 2011 | 35.1 ± 8.3 | 31.5 ± 0.5 | −3.60 (−9.39–2.19) |
| ART | ||||
| Kamijo K | 2022 | 2/9 (22.2%) | 2/5 (40.0%) | 2.33 (0.22–5.24) |
| Kanda E | 2011 | 3/8 (37.5%) | 0/2 (0) | 0.31 (0.01–8.68) |
| Antenatal diagnosis rate | ||||
| Kamijo K | 2022 | 8/9 (88.9%) | 5/5 (100.0%) | 1.94 (0.07–56.76) |
| Kanda E | 2011 | 8/8 (100%) | 1/2 (50.0%) | 0.06 (0.00–2.24) |
| GA at diagnosis | ||||
| Kamijo K | 2022 | 31.8 ± 3.8 | 31.4 ± 3.7 | −0.40 (−4.48–3.68) |
| Kanda E | 2011 | 26.1 ± 3.9 | 30 | -- |
| Emergent CD | ||||
| Kamijo K | 2022 | 4/9 (44.4%) | 1/5 (20.0%) | 0.31 (0.02–4.02) |
| Kanda E | 2011 | 5/8 (62.5%) | 2/2 (100%) | 4.09 (0.15–108.94) |
| GA at delivery | ||||
| Kamijo K | 2022 | 36.0 ± 1.9 | 35.8 ± 1.6 | −0.20 (−2.07–1.67) |
| Kanda E | 2011 | 33.1 ± 2.3 | 36.0 ± 4.0 | 2.90 (−2.87–8.67) |
| ND | ||||
| Kamijo K | 2022 | 0 | 0 | -- |
| Kanda E | 2011 | 0 | 0 | -- |
| Author | Year | Total | Cont | VP | Type III | Prevalence |
|---|---|---|---|---|---|---|
| Kamijo K [48] | 2022 | 8723 | -- | 14 | 5 (35.7%) | 0.06% |
| Tachibana D [49] | 2021 | -- | -- | 55 | 7 (12.7%) | -- |
| Liu N [54] | 2021 | 79,647 | 79,486 | 157 | 4 (2.5%) | 0.005% |
| Catanzarite V [38] | 2016 | -- | -- | 96 | 1 (1.0%) | -- |
| Kanda E [55] | 2011 | 5131 | 5121 | 10 | 2 (20%) | 0.04% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takemoto, Y.; Matsuzaki, S.; Matsuzaki, S.; Kakuda, M.; Lee, M.; Hayashida, H.; Maeda, M.; Kamiura, S. Current Evidence on Vasa Previa without Velamentous Cord Insertion or Placental Morphological Anomalies (Type III Vasa Previa): Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 152. https://doi.org/10.3390/biomedicines11010152
Takemoto Y, Matsuzaki S, Matsuzaki S, Kakuda M, Lee M, Hayashida H, Maeda M, Kamiura S. Current Evidence on Vasa Previa without Velamentous Cord Insertion or Placental Morphological Anomalies (Type III Vasa Previa): Systematic Review and Meta-Analysis. Biomedicines. 2023; 11(1):152. https://doi.org/10.3390/biomedicines11010152
Chicago/Turabian StyleTakemoto, Yuki, Shinya Matsuzaki, Satoko Matsuzaki, Mamoru Kakuda, Misooja Lee, Harue Hayashida, Michihide Maeda, and Shoji Kamiura. 2023. "Current Evidence on Vasa Previa without Velamentous Cord Insertion or Placental Morphological Anomalies (Type III Vasa Previa): Systematic Review and Meta-Analysis" Biomedicines 11, no. 1: 152. https://doi.org/10.3390/biomedicines11010152
APA StyleTakemoto, Y., Matsuzaki, S., Matsuzaki, S., Kakuda, M., Lee, M., Hayashida, H., Maeda, M., & Kamiura, S. (2023). Current Evidence on Vasa Previa without Velamentous Cord Insertion or Placental Morphological Anomalies (Type III Vasa Previa): Systematic Review and Meta-Analysis. Biomedicines, 11(1), 152. https://doi.org/10.3390/biomedicines11010152





