Sedentary Behavior and Pain after Physical Activity in Women with Fibromyalgia—The Influence of Pain-Avoidance Goals and Catastrophizing
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
- –
- Occipital insertions of the suboccipital muscles;
- –
- Anterior cervical projection of the C5–C7 intertransverse spaces;
- –
- Midpoint of the upper border of the trapezius;
- –
- Origin of supraspinatus;
- –
- Second chondrosternal junction;
- –
- Two centimeters distally from the epicondyle;
- –
- Upper outer quadrant of the buttock;
- –
- Posterior aspect of the greater trochanter;
- –
- Adipose cushion of the inner aspect of the knee.
2.2. Instruments
2.2.1. Prior to Carrying Out the 6-Minute Walking Test
2.2.2. Six-Minute Walk Test (6MWT)
2.2.3. After Carrying Out the 6-Minute Walking Test
2.3. Statistical Analysis
3. Results
3.1. Descriptive Analyses and Correlations between Study Variables of the Proposed Moderated-Mediation Model
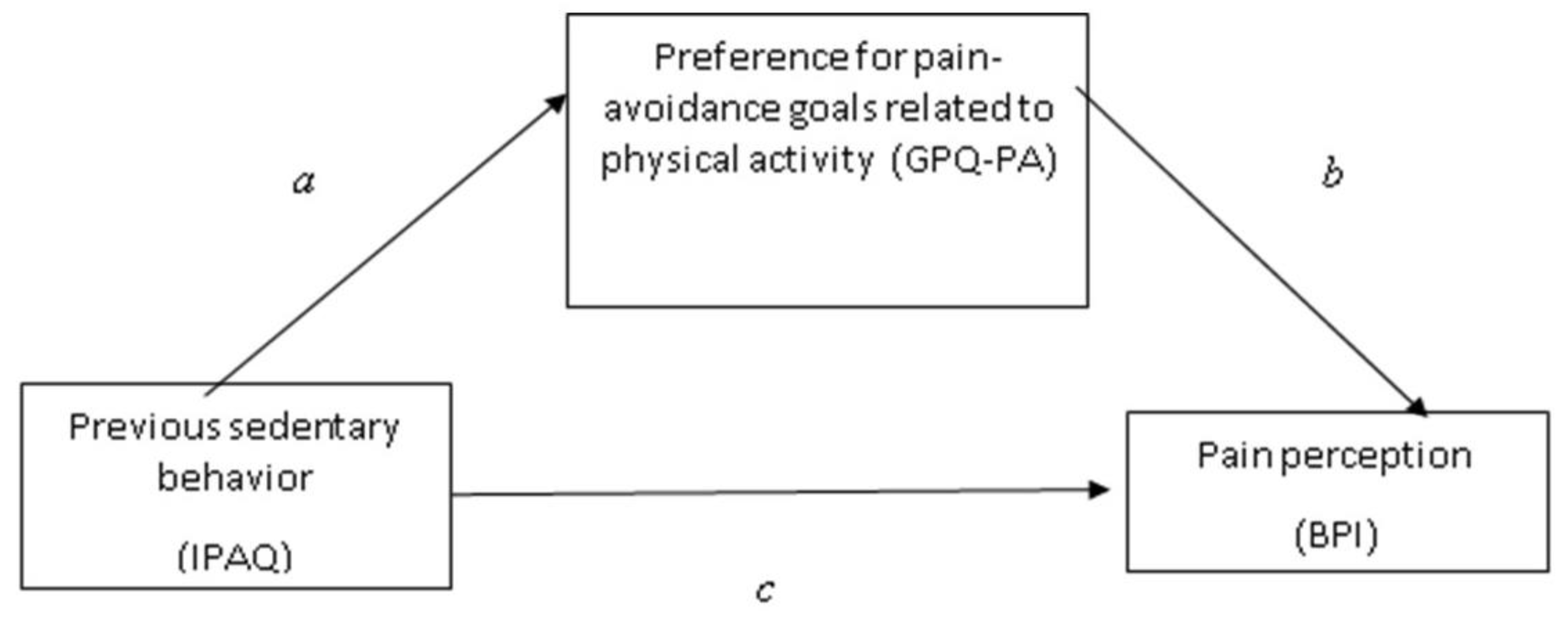
3.2. Testing the Mediation Effect of the Preference for Pain-Avoidance Goals Related to Physical Activity between Previous Sedentary Behavior and Pain Perception
3.3. Testing the Moderated-Mediation Model Based on the Pain Catastrophizing Levels
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, L.M.; Choy, E.; Clauw, D.J.; Goldenberg, D.L.; Harris, R.E.; Helfenstein, M.; Jensen, T.S.; Noguchi, K.; Silverman, S.L.; Ushida, T.; et al. Fibromyalgia and chronic pain syndromes: A white paper detailing current challenges in the field. Clin. J. Pain 2016, 32, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.; Mann, R.; Masters, E.T.; Cappelleri, J.C.; Daniel, S.R.; Zlateva, G.; McElroy, H.J.; Chandran, A.B.; Adams, E.H.; Assaf, A.R.; et al. The Comparative Burden of Chronic Widespread Pain and Fibromyalgia in the United States. Pain. Pract. 2016, 6, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Arnold, L.M.; McCarberag, B.H. Fibro Collaborative. The science of fibromyalgia. Mayo. Clin. Proc. 2011, 86, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Nahin, R.L.; Katz, R.S.; Bergman, M.J.; Wolfe, F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS ONE 2015, 10, e0138024. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, pathogenesis and treatment. Nat. Rev. Rheum. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Sullivan, M.D.; Ballantyne, J.C. Must we reduce pain intensity to treat chronic pain? Pain 2016, 157, 65–69. [Google Scholar] [CrossRef]
- Häuser, W.; Ablin, J.; Fitzcharles, M.A.; Littlejohn, G.; Luciano, J.V.; Usui, C.; Walitt, B. Fibromyalgia. Nat. Rev. 2015, 1, 15022. [Google Scholar] [CrossRef]
- Häuser, W.; Klose, P.; Langhorst, J.; Moradi, B.; Steinbach, M.; Schiltenwolf, M.; Busch, A. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomised controlled trials. Arthr. Research. Ther. 2010, 12, R79. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Exercise improves global well-being in adults with fibromyalgia: Confirmation of previous meta-analytic results using a recently developed and novel varying coefficient model. Clin. Exp. Rheum. 2011, 29, S60–S62. [Google Scholar]
- Cedraschi, C.; Desmeules, J.; Rapiti, E.; Baumgartner, E.; Cohen, P.; Finckh, A.; Allaz, A.F.; Vischert, T.L. Fibromyalgia: A randomised, controlled trial of a treatment programme based on self-management. Ann. Rheum. Dis. 2004, 63, 290–296. [Google Scholar] [CrossRef]
- Kroese, M.; Schulpen, G.; Bessems, M.; Nijhuis, F.; Severens, J.; Landewé, R. The feasibility and efficacy of a multidisciplinary intervention with aftercare meetings for fibromyalgia. Clin. Rheumatol. 2009, 28, 923–929. [Google Scholar] [CrossRef]
- Burckhardt, C. Multidisciplinary approaches for management of fibromyalgia. Curr. Pharm. Des. 2006, 12, 59–66. [Google Scholar] [CrossRef]
- Häuser, W.; Bernardy, K.; Arnold, B.; Offenbächer, M.; Schiltenwolf, M. Efficacy of multicomponent treatment in fibromyalgia syndrome: A meta-analysis of randomized controlled clinical trials. Arthritis. Rheum. 2009, 61, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Torre, F.; Aguirre, U.; González, N.; Padierna, A.; Matellanes, B.; Quintana, J.M. Evaluation of the interdisciplinary PSYMEPHY treatment on patients with fibromyalgia: A randomized control trial. Pain. Med. 2014, 15, 682–691. [Google Scholar] [CrossRef]
- van Koulil, S.; Effting, M.; Kraaimaat, F.; Van Lankveld, W.; Van Helmond, T.; Cats, H.; Van Riel, P.L.C.M.; De Jong, A.J.L.; Haverman, J.F.; Evers, A.W.M. Cognitive-behavioural therapies and exercise programmes for patients with fibromyalgia: State of the art and future directions. Ann. Rheum. Dis. 2007, 66, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Korszun, A.; Young, E.A.; Engleberg, N.C.; Brucksch, C.B.; Greden, J.F.; Crofford, L.A. Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. J. Psychosom. Res. 2002, 52, 439–443. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, M.J.; Colbert, L.H.; Stegner, A.J.; Cook, D.B. Are women with fibromyalgia less physically active than healthy women? Med. Sci. Sport. Exerc. 2011, 43, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Kop, W.J.; Lyden, A.; Berlin, A.A.; Ambrose, K.; Olsen, C.; Gracely, R.H.; Williams, D.A.; Clauw, D.J. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis. Rheum. 2005, 52, 296–303. [Google Scholar] [CrossRef]
- O’Connor, S.R.; Tully, M.A.; Ryan, B.; Bleakley, C.M.; Baxter, G.D.; Bradley, J.M.; McDonough, S.M. Walking exercise for chronic musculoskeletal pain: Systematic review and meta-analysis. Arch. Phys. Med. Rehab. 2015, 96, 724–734. [Google Scholar] [CrossRef]
- Pastor, M.A.; López-Roig, S.; Sanz, Y.; Peñacoba, C.; Cigarán, M.; Lledó, A.; Écija, C. Andar como forma de ejercicio físico en la Fibromialgia: Un estudio de identificación de creencias desde la Teoría de la Acción Planeada. Anal. Psicol. 2015, 31, 433. [Google Scholar] [CrossRef]
- Peñacoba, C.; Pastor, M.Á.; López-Roig, S.; Sanz, Y.; Velasco, L. Healthcare Provider Advice to Engage in Walking Regimens and Adherence in Women With Fibromyalgia. Rehabil. Nurs. 2019, 44, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Campos, E.; Párraga-Montilla, J.A.; Aragón-Vela, J.; Latorre-Román, P.A. Effects of a functional training program in patients with fibromyalgia: A 9-year prospective longitudinal cohort study. Scand. J. Med. Sci. Sports 2020, 30, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.; Weiss, J.E. Pain, functional disability, and their Association in Juvenile Fibromyalgia Compared to other pediatric rheumatic diseases. Pediatr. Rheumatol. 2019, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Crombez, G.; Van Damme, S.; Eccleston, C. Hypervigilance to pain: An experimental and clinical analysis. Pain 2005, 116, 4–7. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Vase, L.; Egsgaard, L.L.; Nikolajsen, L.; Svensson, P.; Jensen, T.S.; Arendt-Nielsen, L. Pain catastrophizing and cortical responses in amputees with varying levels of phantom limb pain: A high-density EEG brain-mapping study. Exp. Brain. Res. 2012, 218, 407–417. [Google Scholar] [CrossRef]
- Fashler, S.; Katz, J. More than meets the eye: Visual attention biases in individuals reporting chronic pain. J. Pain. Res. 2014, 7, 557–570. [Google Scholar] [CrossRef]
- Van Damme, S.; Crombez, G.; Eccleston, C. The anticipation of pain modulates spatial attention: Evidence for pain-specificity in high-pain catastrophizers. Pain 2004, 111, 392–399. [Google Scholar] [CrossRef]
- Galambos, A.; Szabó, E.; Nagy, Z.; Édes, A.E.; Kocsel, N.; Juhász, G.; Kökönyei, G. A systematic review of structural and functional MRI studies on pain catastrophizing. J. Pain. Res. 2019, 11, 1155–1178. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005, 9, 463. [Google Scholar] [CrossRef]
- Tracey, I.; Mantyh, P.W. The cerebral signature for pain perception and its modulation. Neuron. 2007, 55, 377–391. [Google Scholar] [CrossRef]
- Crombez, G.; Eccleston, C.; Van Damme, S.; Vlaeyen, J.W.S.; Karoly, P. Fear-Avoidance Model of Chronic Pain: The next generation. Clin. J. Pain 2012, 28, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Écija, C.; Luque-Reca, O.; Suso-Ribera, C.; Catalá, P.; Peñacoba, C. Associations of cognitive fusion and pain catastrophizing with fibromyalgia impact through fatigue, pain severity, and depression: An exploratory study using structural equation modeling. J. Clin. Med. 2020, 9, 1763. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Mira, M.A.; López-Roig, S.; Martínez-Zaragoza, F.; León, E.; Abad, E.; Lledó, A.; Peñacoba, C. Goal Preferences, Affect, Activity Patterns and Health Outcomes in Women With Fibromyalgia. Front. Psychol. 2019, 10, 1912. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Sheon, R.P.; et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis. Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthr. Care. Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Carrillo-de-la-Peña, M.T.; Triñanes, Y.; González-Villar, A.; Romero-Yuste, S.; Gómez-Perretta, C.; Arias, M.; Wolfe, F. Convergence between the 1990 and 2010 ACR diagnostic criteria and validation of the Spanish version of the Fibromyalgia Survey Questionnaire (FSQ). Rheumatol. Int. 2015, 35, 141–151. [Google Scholar] [CrossRef]
- Pankoff, B.; Overend, T.; Lucy, D.; White, K. Validity and responsiveness of the 6 minute walk test for people with fibromyalgia. J. Rheum. 2000, 27, 2666–2670. [Google Scholar]
- Rivera, J.; Alegre, C.; Ballina, F.J.; Carbonell, J.; Carmona, L.; Castel, B.; Collado, A.; Esteve, J.J.; Vidal, J.; Tornero, J.; et al. Documento de consenso de la Sociedad Española de Reumatología sobre la fibromialgia. Reumat. Clín. 2006, 2, S55–S66. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Martínez, F.G.; Leon, A.S. 2011 compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports. Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- García-Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de la versión española de la escala de la catastrofización ante el dolor (Pain Catastrophizing Scale) en la fibromialgia. Med. Clín. 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Osman, A.; Barrios, F.X.; Kopper, B.A.; Hauptmann, W.; Jones, J.; O’Neill, E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J. Behav. Med. 1997, 20, 589–605. [Google Scholar] [CrossRef]
- Pastor-Mira, M.A.; López-Roig, S.; Martínez-Zaragoza, F.; Toribio, E.; Nardi-Rodríguez, A.; Peñacoba, C. Motivational determinants of objective physical activity in women with fibromyalgia who attended rehabilitation settings. J. Clin. Med. 2021, 10, 5547. [Google Scholar] [CrossRef] [PubMed]
- Karsdorp, P.A.; Vlaeyen, J.W.S. Goals matter: Both achievement and pain-avoidance goals are associated with pain severity and disability in patients with low back and upper extremity pain. Pain 2011, 152, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Mannerkorpi, K.; Svantesson, U.; Carlsson, J.; Ekdahl, C. Tests of functional limitations in fibromyalgia syndrome: A reliability study. Arthritis. Care. Res. 1999, 12, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Homann, D.; Stefanella, J.M.; Goes, S.M.; Leite, N. Impaired functional capacity and exacerbation of pain and exertion during the 6-minute walk test in women with fibromyalgia. Rev. Bras. Fisioter 2011, 15, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Peppin, J.F.; Marcum, S.; Kirsh, K.L. The chronic pain patient and functional assessment: Use of the 6-minute walk test in a multidisciplinary pain clinic. Curr. Med. 2014, 30, 361–365. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. 1994, 23, 129–138. [Google Scholar]
- Anderson, K.O. Role of cutpoints: Why grade pain intensity? Pain 2005, 113, 5–6. [Google Scholar] [CrossRef]
- Tan, G.; Jensen, M.P.; Thornby, J.I.; Shanti, B.F. Validation of the brief pain inventory for chronic nonmalignant pain. J. Pain 2004, 5, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.M.; Kenny, D.A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Social. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F.; Preacher, K.J. Statistical mediation analysis with a multicategorical independent variable. British J. Math. Statist. Psychol. 2014, 67, 451–470. [Google Scholar] [CrossRef]
- Hayes, A.F. Mediation, Moderation and Conditional Process Analysis. A Regression-Based Approach, 3rd ed.; Guilford Press: New York, NY, USA, 2017. [Google Scholar]
- Hayes, A.F. Introduction to Mediation: A Regression-Based Approach; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Vervoort, T.; Trost, Z. Examining Affective-Motivational Dynamics and Behavioral Implications Within The Interpersonal Context of Pain. J. Pain 2017, 18, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Karsdorp, P.A.; Ranson, S.; Schrooten, M.G.S.; Vlaeyen, J.W.S. Pain catastrophizing, threat, and the informational value of mood: Task persistence during a painful finger pressing task. Pain 2012, 153, 1410–1417. [Google Scholar] [CrossRef]
- Peñacoba, C.; López-Gómez, I.; Pastor-Mira, M.A.; López- Roig, S.; Ecija, C. Contextualizing goal preferences in fear-avoidance models Looking at fatigue as a disabling symptom in fibromyalgia patients. PLoS ONE 2021, 16, e0254200. [Google Scholar] [CrossRef] [PubMed]
- Racine, M.; Galán, S.; de la Vega, R.; Pires, C.T.; Solé, E.; Nielson, W.R.; Miró, J.; Moulin, D.E.; Jensen, M.P. Pain-related activity management patterns and function in patients with fibromyalgia syndrome. J. Pain 2018, 34, 122–129. [Google Scholar] [CrossRef]
- Carro, E.; Trejo, J.L.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 2001, 21, 5678–5684. [Google Scholar] [CrossRef]
- Crombez, G.; Eccleston, C.; Baeyens, F.; Eelen, P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain 1998, 75, 187–198. [Google Scholar] [CrossRef]
- Peters, M.L.; Vlaeyen, J.W.S.; van Drunen, C. Do fibromyalgia patients display hypervigilance for innocuous somatosensory stimuli? Application of a body scanning reaction time paradigm. Pain 2000, 86, 283–292. [Google Scholar] [CrossRef]
- Geisser, M.E.; Robinson, M.E.; Keefe, F.J.; Weiner, M.L. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain 1994, 59, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Gracely, R.H.; Geisser, M.E.; Giesecke, T.; Grant, M.A.B.; Petzke, F.; Williams, D.A.; Clauw, D.J. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004, 127, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Han, C.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Fibromyalgia diagnosis: A review of the past, present and future. Exp. Rev. Neurotherap. 2015, 15, 667–679. [Google Scholar] [CrossRef]
- Karoly, P.; Okun, M.A.; Ruehlman, L.S.; Pugliese, J.A. The Impact of Goal Cognition and Pain Severity on Disability and Depression in Adults with Chronic Pain: An Examination of Direct Effects and Mediated Effects via Pain-Induced Fear. Cognit. Ther. Res. 2008, 32, 418–433. [Google Scholar] [CrossRef]
- López-Roig, S.; Pastor, M.A.; Peñacoba, C.; Lledó, A.; Sanz, Y.; Velasco, L. Prevalence and predictors of unsupervised walking and physical activity in a community population of women with fibromyalgia. Rheumatol. Int. 2016, 36, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Baños, Y.; Pastor, M.A.; Velasco, L.; López-Roig, S.; Peñacoba, C.; Lledo, A.; Rodríguez, C. To walk or not to walk: Insights from a qualitative description study with women suffering from fibromyalgia. Rheum. Internat. 2016, 36, 1135–1143. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Kole-Snijders, A.M.J.; Boeren, R.G.B.; van Eek, H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain 1995, 62, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; López-Gómez, I.; Gutiérrez, L.; Écija, C.; Catalá, P.; Peñacoba, C. Exploring the Preference for Fatigue-avoidance Goals as a Mediator Between Pain Catastrophizing, Functional Impairment, and Walking Behavior in Women With Fibromyalgia. Clin. J. Pain 2021, 38, 182–188. [Google Scholar] [CrossRef] [PubMed]
- McCormick, Z.L.; Gagnon, C.M.; Caldwell, M.; Patel, J.; Kornfeld, M.S.; Atchison, D.J.; Stanos, D.S.; Harden, R.N.; Calisoff, R. Short-term functional, emotional and pain outcomes of patients with complex regional pain syndrome treated in a comprehensive interdisciplinary pain management program. Pain. Med. 2015, 16, 2357–2367. [Google Scholar] [CrossRef]
- Yngve, A.; Nilsson, A.; Sjostrom, M.; Ekelund, U. Effect of monitor placement and of activity setting on the MTI accelerometer output. Med. Sci. Sport. Exerc. 2003, 35, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Porres, J.; Delgado, M.; Ruiz, J.R. Comparison of physical activity estimates using International Physical Activity Questionnaire (IPAQ) and accelerometry in fibromyalgia patients: The Al-Andalus study. J. Sport. Sci. 2013, 3, 1741–1752. [Google Scholar] [CrossRef] [PubMed]


| Variables | Theoric Range | M | SD | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|---|
| 1. Sedentary behavior (minutes) | - | 250.06 | 21.28 | -- | |||
| 2. Pain perception (a.u.) | 0–10 | 6.01 | 2.54 | 0.10 ** | -- | ||
| 3. Pain catastrophizing (a.u.) | 0–52 | 21.26 | 12.05 | 0.10 ** | 0.32 ** | -- | |
| 4. Preference for pain-avoidance goals (a.u.) | 0–30 | 4.71 | 1.05 | 0.84 * | 0.23 ** | 0.27 ** | -- |
| Effect (Path) | Coeff | SE | t | p | LLCI | ULCI |
|---|---|---|---|---|---|---|
| Previous sedentary behavior → Pain-avoidance goals (a) | 0.40 | 0.06 | 2.73 | 0.004 | 0.007 | 0.015 |
| Pain-avoidance goals→ Pain perception (b) | 0.55 | 0.28 | 2.01 | 0.004 | 0.057 | 1.102 |
| Direct effect of previous sedentary behavior→ Pain perception (c’) | 0.90 | 0.013 | 1.72 | 0.047 | 0.017 | 0.035 |
| Total effect of previous sedentary behavior→ Pain perception (c) | 0.120 | 0.013 | 1.87 | 0.038 | 0.015 | 0.038 |
| Bootstrap Results for the Indirect Effect | ||||||
| Effect | Boot SE | Boot LLCI | Boot ULCI | |||
| Indirect effect of previous sedentary behavior→ Pain perception | 0.20 | 0.03 | 0.003 | 0.010 | ||
| Outcome → | Pain Perception | Preferences for Pain-Avoidance Goals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Coeff | SE | t | p | LLCI | ULCI | Coeff | SE | t | p | LLCI | ULCI |
| Previous sedentary behavior (c’) | 0.900 | 0.006 | 0.540 | 0.047 | 0.017 | 0.035 | 0.400 | 0.06 | 0.730 | 0.004 | 0.007 | 0.015 |
| Preference for pain-avoidance goals (b1) | 0.580 | 0.007 | 0.726 | 0.021 | 0.090 | 0.110 | ||||||
| Pain catastrophizing (b2) | 0.570 | 0.024 | 2.371 | 0.021 | 0.090 | 0.107 | ||||||
| Previous sedentary behavior × Pain catastrophizing (b3) | 0.027 | 0.27 | 0.140 | 0.008 | 0.401 | 0.950 | ||||||
| R2 = 0.314 F(2.675) = 4.000, p = 0.038 | R2 = 0.084 F(0.527) = 1.116, p = 0.472 | |||||||||||
| Pain Catastrophizing | Scores | Effect | Boot SE | Boot LLCI | Boot ULCI |
|---|---|---|---|---|---|
| Low | −12.05 | −0.260 | 0.003 | −0.004 | 0.007 |
| Medium | 0.000 | 0.001 | 0.003 | −0.002 | 0.008 |
| High | 12.05 * | 0.570 | 0.021 | 0.09 | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, L.; Écija, C.; Catalá, P.; Peñacoba, C. Sedentary Behavior and Pain after Physical Activity in Women with Fibromyalgia—The Influence of Pain-Avoidance Goals and Catastrophizing. Biomedicines 2023, 11, 154. https://doi.org/10.3390/biomedicines11010154
Gutiérrez L, Écija C, Catalá P, Peñacoba C. Sedentary Behavior and Pain after Physical Activity in Women with Fibromyalgia—The Influence of Pain-Avoidance Goals and Catastrophizing. Biomedicines. 2023; 11(1):154. https://doi.org/10.3390/biomedicines11010154
Chicago/Turabian StyleGutiérrez, Lorena, Carmen Écija, Patricia Catalá, and Cecilia Peñacoba. 2023. "Sedentary Behavior and Pain after Physical Activity in Women with Fibromyalgia—The Influence of Pain-Avoidance Goals and Catastrophizing" Biomedicines 11, no. 1: 154. https://doi.org/10.3390/biomedicines11010154
APA StyleGutiérrez, L., Écija, C., Catalá, P., & Peñacoba, C. (2023). Sedentary Behavior and Pain after Physical Activity in Women with Fibromyalgia—The Influence of Pain-Avoidance Goals and Catastrophizing. Biomedicines, 11(1), 154. https://doi.org/10.3390/biomedicines11010154





