Therapy Defining at Initial Diagnosis of Primary Brain Tumor—The Role of 18F-FET PET/CT and MRI
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. PET/CT
3.2. CE and rCBV MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A2;3;4 | astrocytoma grade 2;3;4 |
| ADC | apparent diffusion coefficient |
| BBB | blood–brain barrier |
| BTV | biological tumor volume |
| CNS | central nervous system |
| CT | computer tomography |
| DWI | Diffusion-weighted imaging |
| E3 | Ependymoma grade 3 |
| EANO | European Association of Neuro-oncology |
| ETMR | Embryonal tumor with multilayer rosettes |
| FDG | fluoro-deoxy-glucose |
| FDOPA | 6-[18F]-L-fluoro-L-3, 4-dihydroxyphenylalanine |
| FET | O-(2-[18F]fluoroethyl)-L-tyrosine |
| FLAIR | fluid-attenuated inversion recovery |
| GBM | glioblastoma |
| MET | L-[11C]methyl-methionine |
| MRI | magnetic resonance imaging |
| MRS | magnetic resonance spectroscopy |
| NCCN | National Comprehensive Cancer Network |
| O2;3 | Oligodendroglioma grade 2;3 |
| OSEM | ordered subset expectation maximization |
| PBL | Bayesian penalized likelihood |
| PET | positron emission tomography |
| PWI | perfusion-weighted imaging |
| rCBV | relative cerebral blood volume |
| TBR | tumor brain ratio |
| TOF | time of flight |
References
- Globocan. Cancer Today. 2020. Available online: https://gco.iarc.fr (accessed on 15 September 2022).
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Richardson, T.E.; Kumar, A.; Xing, C.; Hatanpaa, K.J.; Walker, J.M. Overcoming the Odds: Toward a Molecular Profile of Long-Term Survival in Glioblastoma. J. Neuropathol. Exp. Neurol. 2020, 79, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020, 18, 170–186. [Google Scholar] [CrossRef]
- Bent, M.J.V.D.; Tesileanu, C.M.S.; Wick, W.; Sanson, M.; Brandes, A.A.; Clement, P.M.; Erridge, S.; Vogelbaum, M.A.; Nowak, A.K.; Baurain, J.F.; et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): Second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 813–823. [Google Scholar] [CrossRef]
- Andronesi, O.C.; Arrillaga-Romany, I.C.; Ly, K.I.; Bogner, W.; Ratai, E.M.; Reitz, K.; Iafrate, A.J.; Dietrich, J.; Gerstner, E.R.; Chi, A.S.; et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat. Commun. 2018, 9, 1474. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, L.E.; Fuller, G.N.; Hashmi, M.; Leeds, N.E.; Schomer, D.F. The Significance of Lack of MR Contrast Enhancement of Supratentorial Brain Tumors in Adults: Histopathological Evaluation of a Series. Surg. Neurol. 1998, 49, 436–440. [Google Scholar] [CrossRef]
- Scott, J.N.; Brasher, P.M.; Sevick, R.J.; Rewcastle, N.B.; Forsyth, P.A. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002, 59, 947–949. [Google Scholar] [CrossRef]
- Borbély, K.; Nyáry, I.; Tóth, M.; Ericson, K.; Gulyás, B. Optimization of semi-quantification in metabolic PET studies with 18F-fluorodeoxyglucose and 11C-methionine in the determination of malignancy of gliomas. J. Neurol. Sci. 2006, 246, 85–94. [Google Scholar] [CrossRef]
- Borbély, K.; Wintermark, M.; Martos, J.; Fedorcsák, I.; Bognar, L.; Kásler, M. The pre-requisite of a second-generation glioma PET biomarker. J. Neurol. Sci. 2010, 298, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, B.; Nyary, I.; Borbély, K. FDG, MET or CHO? The quest for the optimal PET tracer for glioma imaging continues. Nat. Clin. Pract. Cardiovasc. Med. 2008, 4, 470–471. [Google Scholar] [CrossRef]
- Heiss, P.; Mayer, S.; Herz, M.; Wester, H.J.; Schwaiger, M.; Senekowitsch-Schmidtke, R. Investigation of transport mechanism and uptake kinetics of O-(2-[18F]fluoroethyl)-L-tyrosine in vitro and in vivo. J. Nucl. Med. 1999, 40, 1367–1373. [Google Scholar] [PubMed]
- Dunet, V.; Rossier, C.; Buck, A.; Stupp, R.; Prior, J.O. Performance of 18F-Fluoro-Ethyl-Tyrosine (18F-FET) PET for the Differential Diagnosis of Primary Brain Tumor: A Systematic Review and Metaanalysis. J. Nucl. Med. 2012, 53, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegmayr, C.; Willuweit, A.; Lohmann, P.; Langen, K.-J. O-(2-[18F]-Fluoroethyl)-L-Tyrosine (FET) in Neurooncology: A Review of Experimental Results. Curr. Radiopharm. 2019, 12, 201–210. [Google Scholar] [CrossRef]
- Lohmann, P.; Stavrinou, P.; Lipke, K.; Bauer, E.K.; Ceccon, G.; Werner, J.-M.; Neumaier, B.; Fink, G.R.; Shah, N.J.; Langen, K.-J.; et al. FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur. J. Nucl. Med. 2019, 46, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, O.M.; Larsen, V.A.; Muhic, A.; Hansen, A.E.; Larsson, H.B.W.; Poulsen, H.S.; Law, I. Simultaneous evaluation of brain tumour metabolism, structure and blood volume using [18F]-fluoroethyltyrosine (FET) PET/MRI: Feasibility, agreement and initial experience. Eur. J. Pediatr. 2016, 43, 103–112. [Google Scholar] [CrossRef]
- Kaiser, L.; Holzgreve, A.; Quach, S.; Ingrisch, M.; Unterrainer, M.; Dekorsy, F.J.; Lindner, S.; Ruf, V.; Brosch-Lenz, J.; Delker, A.; et al. Differential Spatial Distribution of TSPO or Amino Acid PET Signal and MRI Contrast Enhancement in Gliomas. Cancers 2021, 14, 53. [Google Scholar] [CrossRef]
- Unterrainer, M.; Fleischmann, D.F.; Diekmann, C.; Vomacka, L.; Lindner, S.; Vettermann, F.; Brendel, M.; Wenter, V.; Ertl-Wagner, B.; Herms, J.; et al. Comparison of 18F-GE-180 and dynamic 18F-FET PET in high grade glioma: A double-tracer pilot study. Eur. J. Nucl. Med. 2018, 46, 580–590. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Guerrini, F.; Roca, E.; Spena, G. Supramarginal Resection for Glioblastoma: It Is Time to Set Boundaries! A Critical Review on a Hot Topic. Brain Sci. 2022, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Certo, F.; Stummer, W.; Farah, J.O.; Freyschlag, C.; Visocchi, M.; Morrone, A.; Altieri, R.; Toccaceli, G.; Peschillo, S.; Thomè, C.; et al. Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J. Neurosurg. Sci. 2020, 63, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Certo, F.; Altieri, R.; Maione, M.; Schonauer, C.; Sortino, G.; Fiumanò, G.; Tirrò, E.; Massimino, M.; Broggi, G.; Vigneri, P.; et al. FLAIRectomy in Supramarginal Resection of Glioblastoma Correlates With Clinical Outcome and Survival Analysis: A Prospective, Single Institution, Case Series. Oper. Neurosurg. 2020, 20, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Ort, J.; Hamou, H.A.; Kernbach, J.M.; Hakvoort, K.; Blume, C.; Lohmann, P.; Galldiks, N.; Heiland, D.H.; Mottaghy, F.M.; Clusmann, H.; et al. 18F-FET-PET-guided gross total resection improves overall survival in patients with WHO grade III/IV glioma: Moving towards a multimodal imaging-guided resection. J. Neuro-Oncology 2021, 155, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Stoffels, G.; Lohmann, P.; Bauer, E.K.; Werner, J.-M.; Wollring, M.; Rapp, M.; Felsberg, J.; Kocher, M.; Fink, G.R.; et al. Prognostic value of pre-irradiation FET PET in patients with not completely resectable IDH-wildtype glioma and minimal or absent contrast enhancement. Sci. Rep. 2021, 11, 20828. [Google Scholar] [CrossRef]
- Ceccon, G.; Lohmann, P.; Werner, J.-M.; Tscherpel, C.; Dunkl, V.; Stoffels, G.; Rosen, J.; Rapp, M.; Sabel, M.; Herrlinger, U.; et al. Early treatment response assessment using 18F-FET PET compared to contrast-enhanced MRI in glioma patients following adjuvant temozolomide chemotherapy. J. Nucl. Med. 2020, 62, 918–925. [Google Scholar] [CrossRef]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients—A report of the PET/RANO group. Neuro-Oncology 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Prather, K.Y.; O’Neal, C.M.; Westrup, A.M.; Tullos, H.J.; Hughes, K.L.; Conner, A.K.; Glenn, C.A.; Battiste, J.D. A systematic review of amino acid PET in assessing treatment response to temozolomide in glioma. Neuro-Oncology Adv. 2022, 4, vdac008. [Google Scholar] [CrossRef]
- Galldiks, N.; Verger, A.; Zaragori, T.; Unterrainer, M.; Suchorska, B.; Lohmann, P.; Tonn, J.C.; Langen, K.-J.; Albert, N.L.; Lohman, P. Comment on “Hypometabolic gliomas on FET-PET—Is there an inverted U-curve for survival?”. Neuro-Oncology 2019, 21, 1612–1613. [Google Scholar] [CrossRef]
- Galldiks, N.; Unterrainer, M.; Judov, N.; Stoffels, G.; Rapp, M.; Lohmann, P.; Vettermann, F.; Dunkl, V.; Suchorska, B.; Tonn, J.C.; et al. Photopenic defects on O-(2-[18F]-fluoroethyl)-L-tyrosine PET: Clinical relevance in glioma patients. Neuro-Oncology 2019, 21, 1331–1338. [Google Scholar] [CrossRef]
- Kamson, D.O. Hypometabolic gliomas on FET-PET—Is there an inverted U-curve for survival? Neuro-Oncology 2019, 21, 1221–1222. [Google Scholar] [CrossRef] [PubMed]
- Chourmouzi, D.; Papadopoulou, E.; Marias, K.; Drevelegas, A. Imaging of Brain Tumors. Surg. Oncol. Clin. North Am. 2014, 23, 629–684. [Google Scholar] [CrossRef] [PubMed]
- van de Weijer, T.; Broen, M.P.G.; Moonen, R.P.M.; Hoeben, A.; Anten, M.; Hovinga, K.; Compter, I.; van der Pol, J.A.J.; Mitea, C.; Lodewick, T.M.; et al. The Use of 18F-FET-PET-MRI in Neuro-Oncology: The Best of Both Worlds—A Narrative Review. Diagnostics 2022, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Cha, S. Perfusion MR Imaging of Brain Tumors. Top. Magn. Reson. Imaging 2004, 15, 279–289. [Google Scholar] [CrossRef]
- Lasocki, A.; Gaillard, F. Non-Contrast-Enhancing Tumor: A New Frontier in Glioblastoma Research. Am. J. Neuroradiol. 2019, 40, 758–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichberg, D.G.; Di, L.; Morell, A.A.; Shah, A.H.; Semonche, A.M.; Chin, C.N.; Bhatia, R.G.; Jamshidi, A.M.; Luther, E.M.; Komotar, R.J.; et al. Incidence of high grade gliomas presenting as radiographically non-enhancing lesions: Experience in 111 surgically treated non-enhancing gliomas with tissue diagnosis. J. Neuro-Oncology 2020, 147, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Gempt, J.; Bette, S.; Ryang, Y.-M.; Buchmann, N.; Peschke, P.; Pyka, T.; Wester, H.-J.; Förster, S.; Meyer, B.; Ringel, F. 18F-fluoro-ethyl-tyrosine positron emission tomography for grading and estimation of prognosis in patients with intracranial gliomas. Eur. J. Radiol. 2015, 84, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Dunzinger, A.; Wurm, G.; Pichler, J.; Weis, S.; Nußbaumer, K.; Topakian, R.; Aigner, R.M. Is there a place for FET PET in the initial evaluation of brain lesions with unknown significance? Eur. J. Nucl. Med. 2010, 37, 1521–1528. [Google Scholar] [CrossRef]
- Rapp, M.; Heinzel, A.; Galldiks, N.; Stoffels, G.; Felsberg, J.; Ewelt, C.; Sabel, M.; Steiger, H.J.; Reifenberger, G.; Beez, T.; et al. Diagnostic Performance of 18F-FET PET in Newly Diagnosed Cerebral Lesions Suggestive of Glioma. J. Nucl. Med. 2012, 54, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Poulsen, S.H.; Urup, T.; Grunnet, K.; Christensen, I.J.; Larsen, V.A.; Jensen, M.L.; Af Rosenschöld, P.M.; Poulsen, H.S.; Law, I. The prognostic value of FET PET at radiotherapy planning in newly diagnosed glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 373–381. [Google Scholar] [CrossRef] [PubMed]
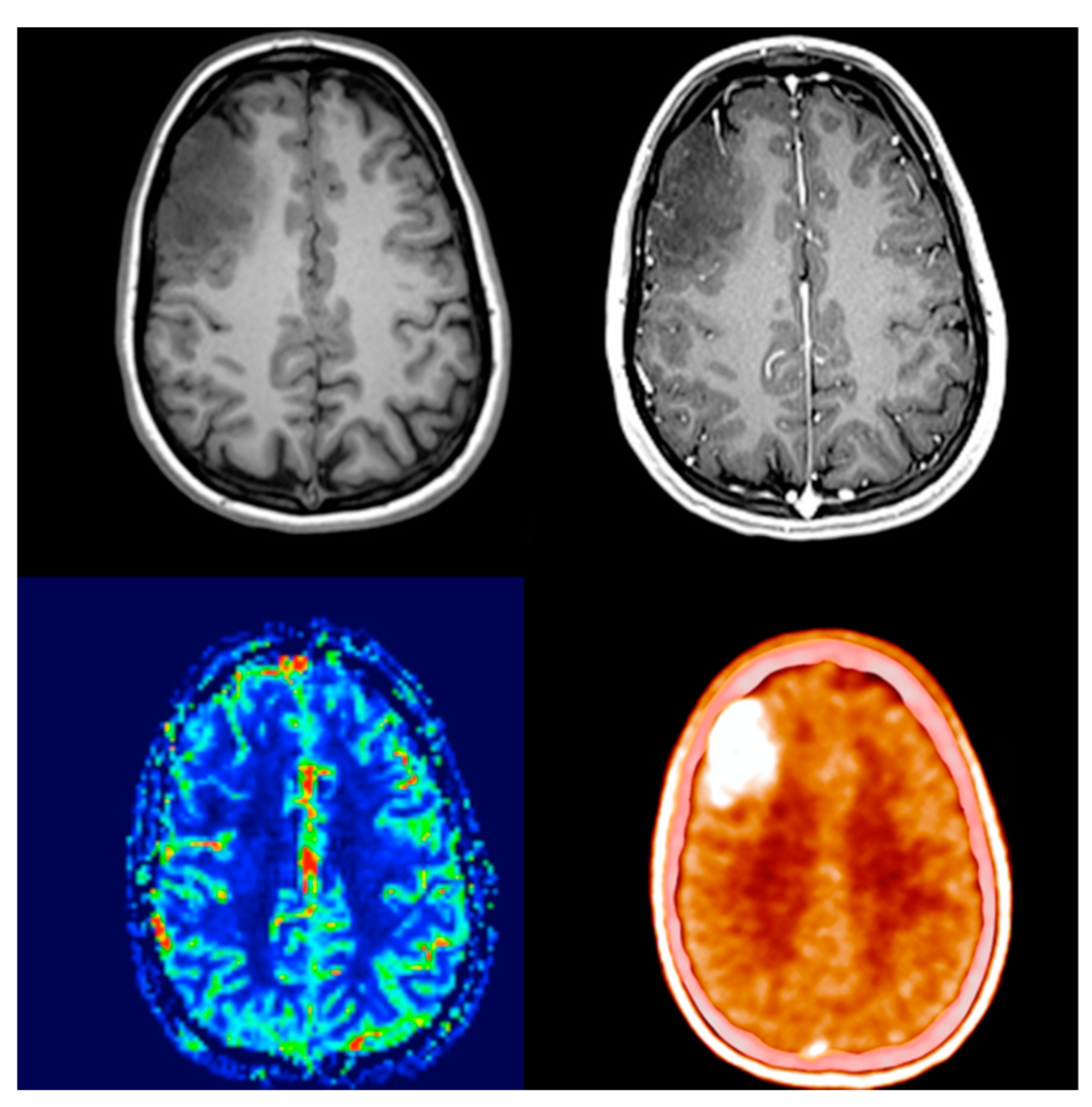



| Characteristics | Cohort (n = 29) | |
|---|---|---|
| Gender | Male | 16 (55.3%) |
| Female | 13 (44.8%) | |
| Age (year) (median; minimum-maximum) | 42 (26–79) | |
| Survival status | Alive | 17 (58.6%) |
| Dead | 10 (34.5%) | |
| NA | 2 (6.9%) | |
| Survival (month) (median; minimum-maximum) | 17 (2–108) | |
| Laterality | Left | 12 (41.4%) |
| Right | 17 (58.6%) | |
| Localization | Frontal | 18 (62.1%) |
| Temporal | 1 (3.4%) | |
| Occipital | 1 (3.4%) | |
| Parietal | 5 (17.2%) | |
| Insular | 4 (13.8%) | |
| Post 18F-FET histology | A2 | 2 (6.9%) |
| A3 | 2 (6.9%) | |
| A4 | 7 (24.1%) | |
| GBM | 6 (20.7%) | |
| E3 | 1 (3.3%) | |
| O2 | 3 (10.3%) | |
| O3 | 7 (24.1%) | |
| ETMR | 1 (3.4%) | |
| TBRmax (median; minimum-maximum) | 2.66 (0.81–5.3) | |
| TBRmax | <1.6 | 3 (10.3%) |
| ≥1.6 | 26 (89.7%) | |
| Ki67 (%) (median; minimum-maximum) | 10 (3–80) | |
| Grade | 2 | 5 (17.2%) |
| 3–4 | 24 (82.8%) | |
| IDH-status | wild-type | 10 (34.5%) |
| mutant | 18 (62.1%) | |
| NA | 1 (3.4%) | |
| MRI contrast enhancement | no | 15 (51.7%) |
| yes | 14 (48.3%) | |
| elevated MRI perfusion | no | 17 (58.6%) |
| yes | 6 (20.7%) | |
| NA | 6 (20.7%) |
| 18F-FET PET | MRI Contrast | MRI Perfusion | |
|---|---|---|---|
| Sensitivity | 100% | 52.9% | 36.36% |
| (16/16) | (9/17) | (4/11) | |
| Specificity | 40% | 83.33% | 100% |
| (2/5) | (5/6) | (5/5) | |
| Positive predictive value | 84.21% | 90% | 100% |
| (16/19) | (9/10) | (4/4) | |
| Negative predictive value | 100% | 38.46% | 41.67% |
| (2/2) | (5/13) | (5/7) |
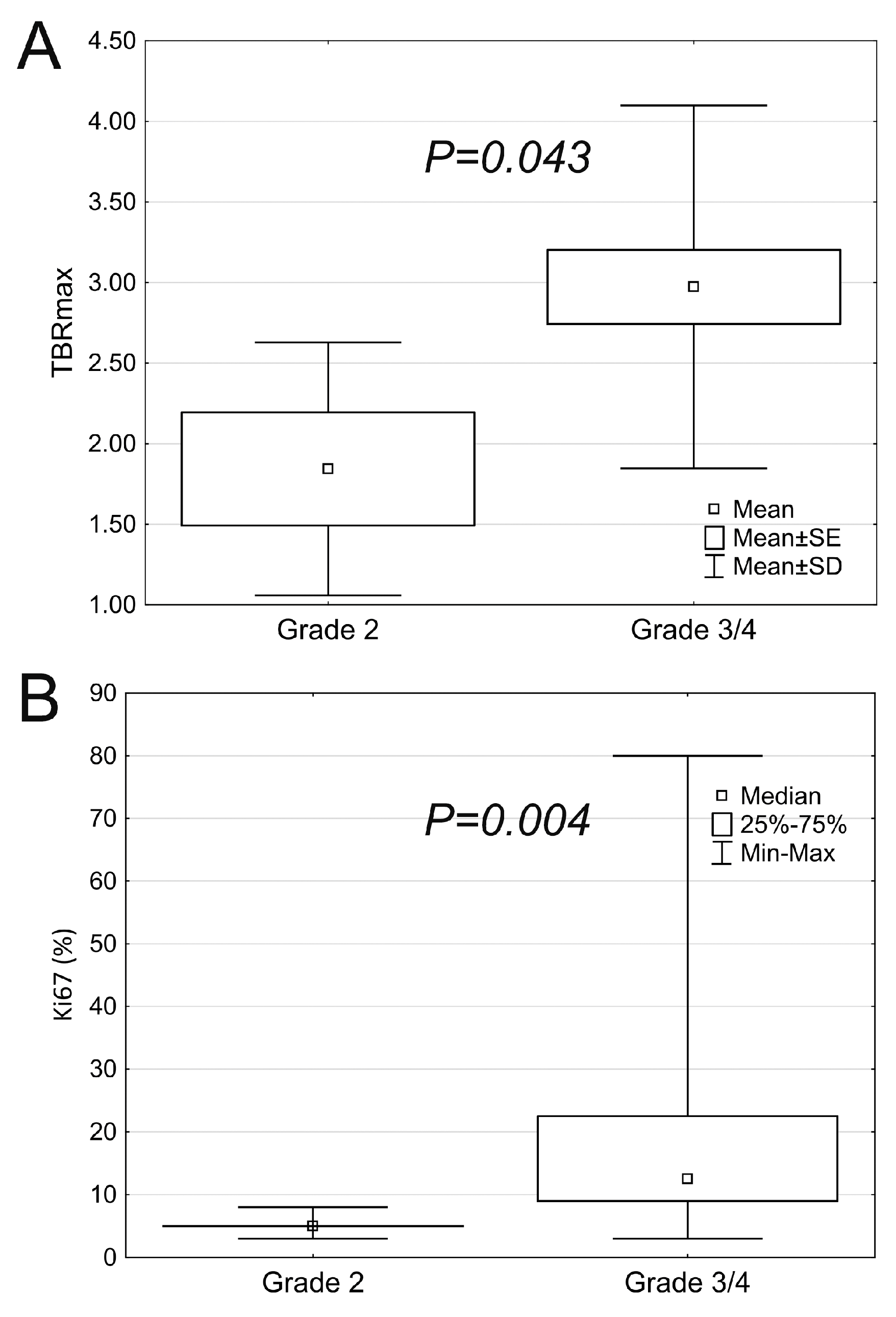
| Grade 2 (n = 5) | Grade 3/4 (n = 24) | p | |
|---|---|---|---|
| Contrast enhancement | 0.042 | ||
| 5 (100%) | 10 (58.3%) | |
| 0 (0%) | 14 (41.7%) | |
| Perfusion elevation | 0.273 | ||
| 5 (100%) | 12 (50%) | |
| 0 (0%) | 6 (25%) | |
| 0 (0%) | 6 (25%) |
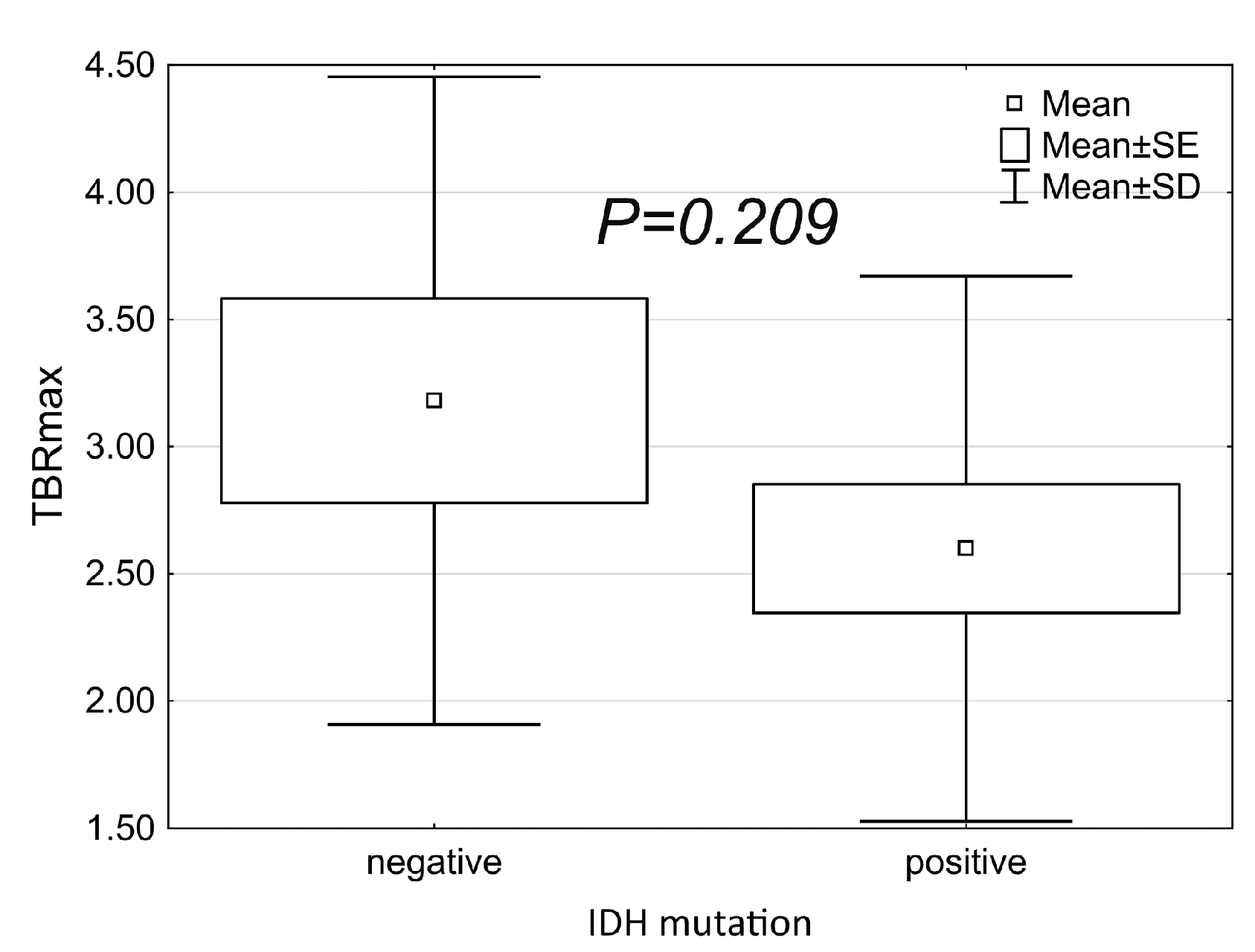
| IDH-Negative (n = 10) | IDH-Positive (n = 18) | p | |
|---|---|---|---|
| Contrast enhancement | 0.114 | ||
| 3 (30%) | 12 (66.7%) | |
| 7 (70%) | 6 (33.3%) | |
| Perfusion elevation | 0.054 | ||
| 3 (30%) | 13 (72.2%) | |
| 4 (40%) | 2 (11.1%) | |
| 3 (30%) | 5 (27.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, D.G.; Fedorcsák, I.; Bagó, A.G.; Gáti, G.; Martos, J.; Szabó, P.; Rajnai, H.; Kenessey, I.; Borbély, K. Therapy Defining at Initial Diagnosis of Primary Brain Tumor—The Role of 18F-FET PET/CT and MRI. Biomedicines 2023, 11, 128. https://doi.org/10.3390/biomedicines11010128
Nagy DG, Fedorcsák I, Bagó AG, Gáti G, Martos J, Szabó P, Rajnai H, Kenessey I, Borbély K. Therapy Defining at Initial Diagnosis of Primary Brain Tumor—The Role of 18F-FET PET/CT and MRI. Biomedicines. 2023; 11(1):128. https://doi.org/10.3390/biomedicines11010128
Chicago/Turabian StyleNagy, Dávid Gergő, Imre Fedorcsák, Attila György Bagó, Georgina Gáti, János Martos, Péter Szabó, Hajnalka Rajnai, István Kenessey, and Katalin Borbély. 2023. "Therapy Defining at Initial Diagnosis of Primary Brain Tumor—The Role of 18F-FET PET/CT and MRI" Biomedicines 11, no. 1: 128. https://doi.org/10.3390/biomedicines11010128
APA StyleNagy, D. G., Fedorcsák, I., Bagó, A. G., Gáti, G., Martos, J., Szabó, P., Rajnai, H., Kenessey, I., & Borbély, K. (2023). Therapy Defining at Initial Diagnosis of Primary Brain Tumor—The Role of 18F-FET PET/CT and MRI. Biomedicines, 11(1), 128. https://doi.org/10.3390/biomedicines11010128





