Chloride Ions, Vascular Function and Hypertension
Abstract
1. Introduction
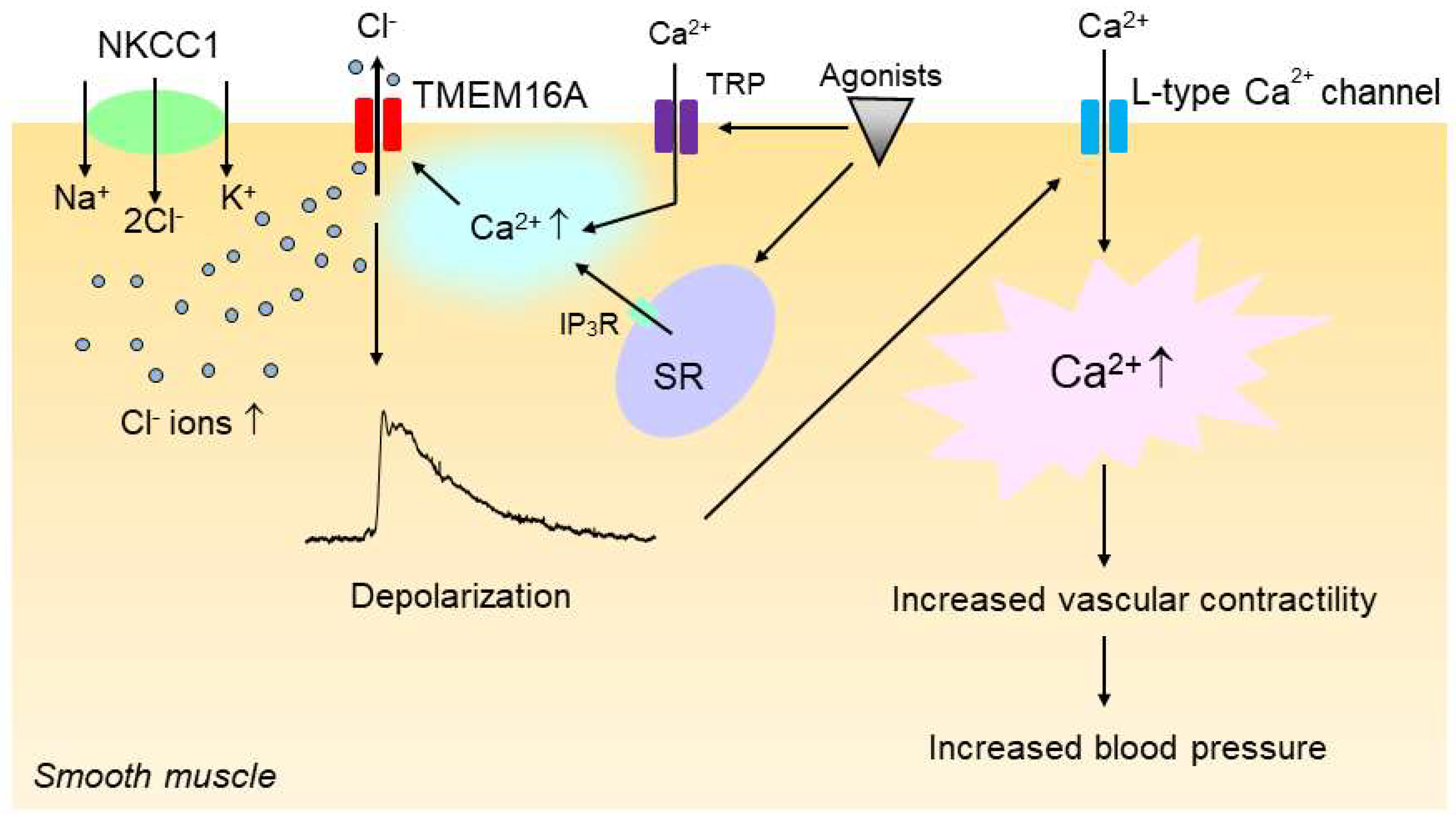
2. Role of Chloride Ions in Regulation of Vascular Tone and Blood Pressure
3. Alterations in Vascular Chloride Channels and Transporters in Hypertension
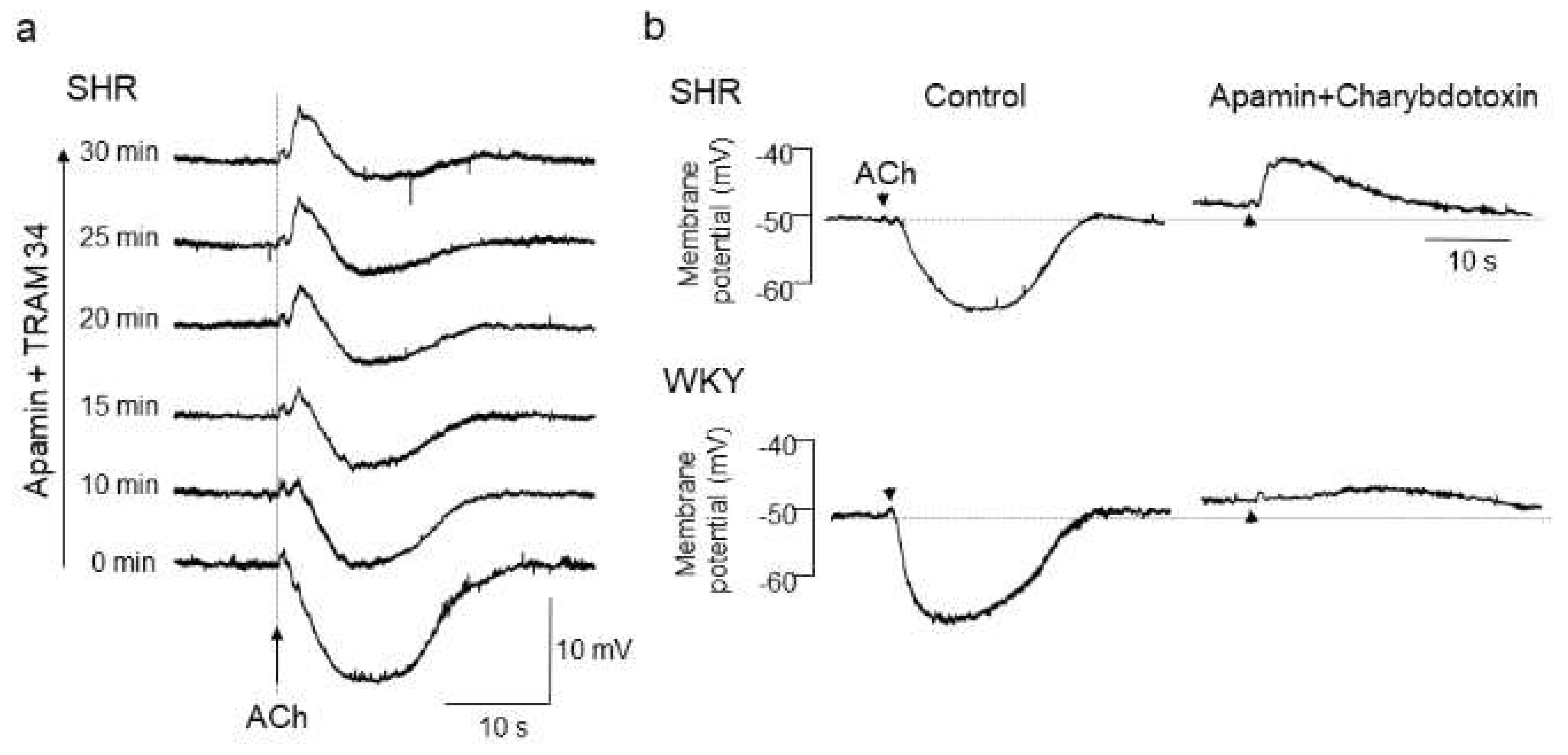
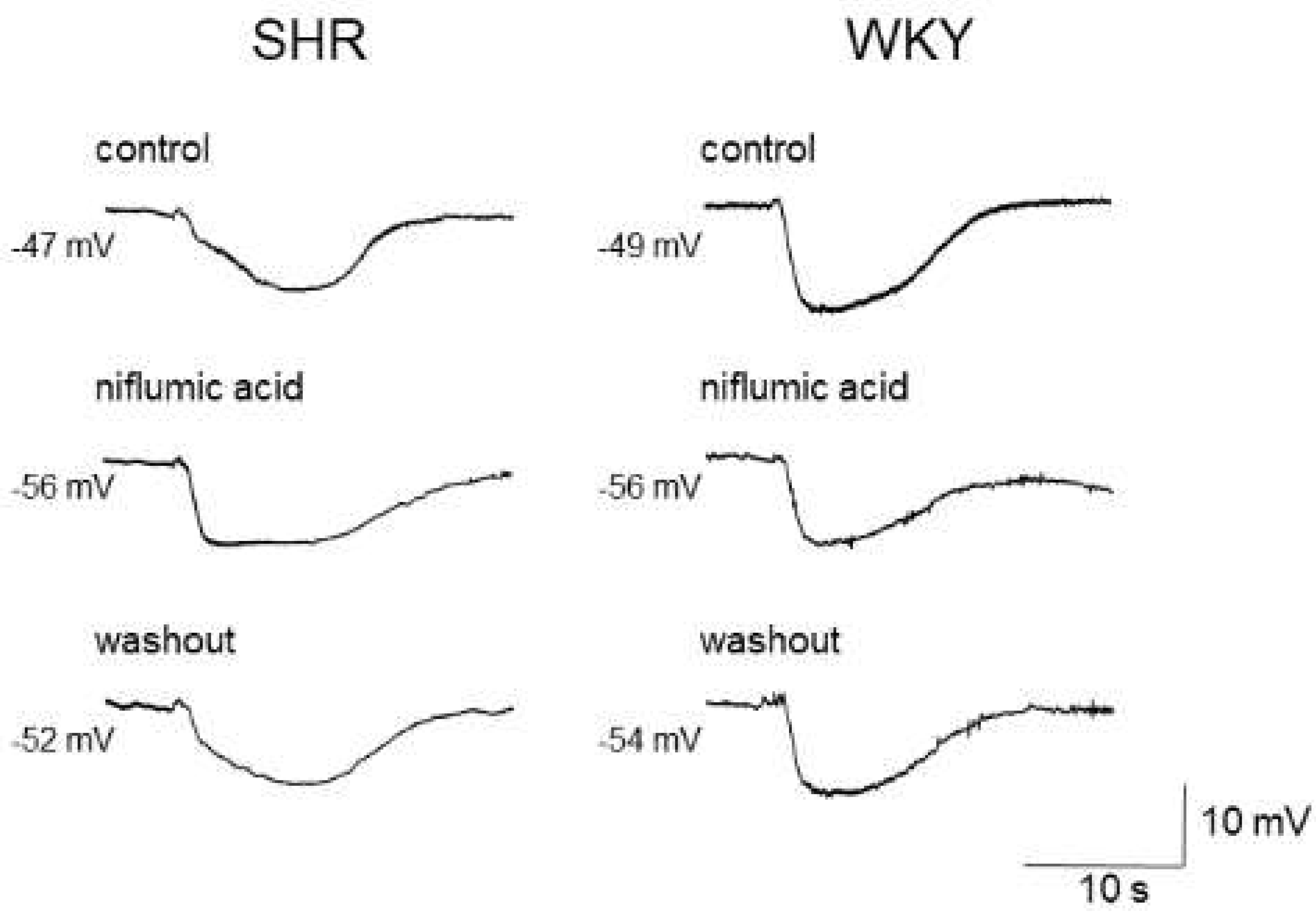
3.1. Ca2+-Activated Chloride Channels (CaCCs) in Vascular Smooth Muscle Cells
3.2. Ca2+-Activated Chloride Channels (CaCCs) in Vascular Endothelial Cells
3.3. Na+–K+–2Cl− Cotransporter1 (NKCC1)
4. Clinical Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawes, C.M.; Hoorn, S.V.; Rodgers, A. Global Burden of Blood-Pressure-Related Disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global Epidemiology, Health Burden and Effective Interventions for Elevated Blood Pressure and Hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, T. Dietary Salt Intake in Japan-Past, Present, and Future. Hypertens. Res. 2022, 45, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Veronesi, M.; Fogacci, F. Dietary Intervention to Improve Blood Pressure Control: Beyond Salt Restriction. High Blood Press. Cardiovasc. Prev. 2021, 28, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Intersalt. Intersalt: An International Study of Electrolyte Excretion and Blood. BMJ 1988, 297, 319–328. [Google Scholar]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef]
- Meneton, P.; Jeunemaitre, X.; De Wardener, H.E.; Macgregor, G.A. Links between Dietary Salt Intake, Renal Salt Handling, Blood Pressure, and Cardiovascular Diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef]
- Dahl, L.K.; Love, R.A. Evidence for Relationship between Sodium (Chloride) Intake and Human Essential Hypertension. AMA Arch. Intern. Med. 1954, 94, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kempner, W. Treatment of Hypertensive Vascular Disease with Rice Diet. Am. J. Med. 1948, 4, 545–577. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Morris, R.C. Dietary Chloride as a Determinant of “Sodium-Dependent” Hypertension. Science 1983, 222, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Kotchen, T.A.; Luke, R.G.; Ott, C.E.; Galla, J.H.; Whitescarver, S. Effect of Chloride on Renin and Blood Pressure Responses to Sodium Chloride. Ann. Intern. Med. 1983, 98 Pt 2, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Whitescarver, S.A.; Holtzclaw, B.J.; Downs, J.H.; Ott, C.E.; Sowers, J.R.; Kotchen, T.A. Effect of Dietary Chloride on Salt-Sensitive and Renin-Dependent Hypertension. Hypertension 1986, 8, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Schmidlin, O.; Yi, S.L.; Bollen, A.W.; Morris, R.C. Genetically Determined Chloride-Sensitive Hypertension and Stroke. Proc. Natl. Acad. Sci. USA 1997, 94, 14748–14752. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, T.W.; Al-Bander, H.A.; Morris, R.C. “Salt-Sensitive” Essential Hypertension in Men. Is the Sodium Ion Alone Important? N. Engl. J. Med. 1987, 317, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C.; Zemel, M.B.; Sowers, J.A.; Fine Berg, N.S.; Weinberger, M.H. Sodium Bicarbonate and Sodium Chloride: Effects on Blood Pressure and Electrolyte Homeostasis in Normal and Hypertensive Man. J. Hypertens. 1990, 8, 663–670. [Google Scholar] [CrossRef]
- Shore, A.C.; Markandu, N.D.; MacGregor, G.A. A Randomized Crossover Study to Compare the Blood Pressure Response to Sodium Loading with and without Chloride in Patients with Essential Hypertension. J. Hypertens. 1988, 6, 613–617. [Google Scholar] [CrossRef] [PubMed]
- McCallum, L.; Lip, S.; Padmanabhan, S. The Hidden Hand of Chloride in Hypertension. Pflug. Arch. 2015, 467, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Chipperfield, A.R.; Harper, A.A. Chloride in Smooth Muscle. Prog. Biophys. Mol. Biol. 2000, 74, 175–221. [Google Scholar] [CrossRef]
- Bulley, S.; Jaggar, J.H. Cl− Channels in Smooth Muscle Cells. Pflug. Arch. 2014, 466, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.J.; Harder, D.R.; Pamnani, M.B.; Haddy, F.J. In Vivo Membrane Potentials of Smooth Muscle Cells in the Caudal Artery of the Rat. Am. J. Physiol. 1985, 249 Pt 1, C78–C83. [Google Scholar] [CrossRef]
- Aickin, C.C.; Brading, A.F. Measurement of Intracellular Chloride in Guinea-pig Vas Deferens by Ion Analysis, 36chloride and Micro-electrodes. J. Physiol. 1982, 326, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Large, W.A.; Wang, Q. Characteristics and Physiological Role of the Ca(2+)-Activated Cl− Conductance in Smooth Muscle. Am. J. Physiol. 1996, 271 Pt 1, C435–C454. [Google Scholar] [CrossRef]
- Goto, K.; Fujii, K.; Onaka, U.; Abe, I.; Fujishima, M. Effects of Adrenomedullin and PAMP on Membrane Potential and Neurotransmission. Peptides 2000, 21, 257–263. [Google Scholar] [CrossRef]
- Brock, M.A.; Cunnane, T.C. Effects of Ca2+ Concentration and Ca2+ Channel Blockers on Noradrenaline Release and Purinergic Neuroeffector Transmission in Rat Tail Artery. Br. J. Pharmacol. 1999, 126, 11–18. [Google Scholar] [CrossRef][Green Version]
- Burnstock, G.; Ralevic, V. New Insights into the Local Regulation of Blood Flow by Perivascular Nerves and Endothelium. Br. J. Plast. Surg. 1994, 47, 527–543. [Google Scholar] [CrossRef]
- Hill, C.E.; Phillips, J.K.; Sandow, S.L. Heterogeneous Control of Blood Flow amongst Different Vascular Beds. Med. Res. Rev. 2001, 21, 1–60. [Google Scholar] [CrossRef]
- Plane, F.; Garland, C.J. Electrophysiology of Cerebral Blood Vessels. Pharmacol. Ther. 1992, 56, 341–358. [Google Scholar] [CrossRef]
- Gould, D.J.; Hill, C.E. Alpha-Adrenoceptor Activation of a Chloride Conductance in Rat Iris Arterioles. Am. J. Physiol. 1996, 271 Pt 2, H2469–H2476. [Google Scholar] [CrossRef]
- Kitamura, K.; Yamazaki, J. Chloride Channels and Their Functional Roles in Smooth Muscle Tone in the Vasculature. Jpn. J. Pharmacol. 2001, 85, 351–357. [Google Scholar] [CrossRef]
- Leblanc, N.; Ledoux, J.; Saleh, S.; Sanguinetti, A.; Angermann, J.; O’Driscoll, K.; Britton, F.; Perrino, B.A.; Greenwood, I.A. Regulation of Calcium-Activated Chloride Channels in Smooth Muscle Cells: A Complex Picture Is Emerging. Can. J. Physiol. Pharmacol. 2005, 83, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Hill, M.A. Signaling Mechanisms Underlying the Vascular Myogenic Response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Conway, M.A.; Knot, H.J.; Brayden, J.E. Chloride Channel Blockers Inhibit Myogenic Tone in Rat Cerebral Arteries. J. Physiol. 1997, 502 Pt 2, 259–264. [Google Scholar] [CrossRef]
- Doughty, J.M.; Langton, P.D. Measurement of Chloride Flux Associated with the Myogenic Response in Rat Cerebral Arteries. J. Physiol. 2001, 534 Pt 3, 753–761. [Google Scholar] [CrossRef]
- Doughty, J.M.; Miller, A.L.; Langton, P.D. Non-Specificity of Chloride Channel Blockers in Rat Cerebral Arteries: Block of the L-Type Calcium Channel. J. Physiol. 1998, 507 Pt 2, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.G.; Nelson, M.T.; Eckman, D.M.; Brayden, J.E. Swelling-Activated Cation Channels Mediate Depolarization of Rat Cerebrovascular Smooth Muscle by Hyposmolarity and Intravascular Pressure. J. Physiol. 2000, 527 Pt 1, 139–148. [Google Scholar] [CrossRef]
- Byrne, N.G.; Large, W.A. Membrane Mechanism Associated with Muscarinic Receptor Activation in Single Cells Freshly Dispersed from the Rat Anococcygeus Muscle. Br. J. Pharmacol. 1987, 92, 371–379. [Google Scholar] [CrossRef]
- Matchkov, V.V. Mechanisms of Cellular Synchronization in the Vascular Wall. Mechanisms of Vasomotion. Dan. Med. Bull. 2010, 57, B4191. [Google Scholar]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A Confers Receptor-Activated Calcium-Dependent Chloride Conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J.V. TMEM16A, a Membrane Protein Associated with Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression Cloning of TMEM16A as a Calcium-Activated Chloride Channel Subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Dam, V.S.; Boedtkjer, D.M.B.; Aalkjaer, C.; Matchkov, V. The Bestrophin- and TMEM16A-Associated Ca(2+)- Activated Cl(–) Channels in Vascular Smooth Muscles. Channels 2014, 8, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Hawn, M.B.; Akin, E.; Hartzell, H.C.; Greenwood, I.A.; Leblanc, N. Molecular Mechanisms of Activation and Regulation of ANO1-Encoded Ca2+-Activated Cl− Channels. Channels 2021, 15, 569–603. [Google Scholar] [CrossRef]
- Wray, S.; Prendergast, C.; Arrowsmith, S. Calcium-Activated Chloride Channels in Myometrial and Vascular Smooth Muscle. Front. Physiol. 2021, 12, 1805. [Google Scholar] [CrossRef]
- Bulley, S.; Neeb, Z.P.; Burris, S.K.; Bannister, J.P.; Thomas-Gatewood, C.M.; Jangsangthong, W.; Jaggar, J.H. TMEM16A/ANO1 Channels Contribute to the Myogenic Response in Cerebral Arteries. Circ. Res. 2012, 111, 1027–1036. [Google Scholar] [CrossRef]
- Yip, K.P.; Balasubramanian, L.; Kan, C.; Wang, L.; Liu, R.; Ribeiro-Silva, L.; Sham, J.S.K. Intraluminal Pressure Triggers Myogenic Response via Activation of Calcium Spark and Calcium-Activated Chloride Channel in Rat Renal Afferent Arteriole. Am. J. Physiol. Renal Physiol. 2018, 315, F1592–F1600. [Google Scholar] [CrossRef]
- Heinze, C.; Seniuk, A.; Sokolov, M.V.; Huebner, A.K.; Klementowicz, A.E.; Szijártó, I.A.; Schleifenbaum, J.; Vitzthum, H.; Gollasch, M.; Ehmke, H.; et al. Disruption of Vascular Ca2+-Activated Chloride Currents Lowers Blood Pressure. J. Clin. Investig. 2014, 124, 675–686. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Halpern, W. Contractile Properties of Small Arterial Resistance Vessels in Spontaneously Hypertensive and Normotensive Rats. Circ. Res. 1977, 41, 19–26. [Google Scholar] [CrossRef]
- Pintérová, M.; Kuneš, J.; Zicha, J. Altered Neural and Vascular Mechanisms in Hypertension. Physiol. Res. 2011, 60, 381–402. [Google Scholar] [CrossRef]
- Goto, K.; Ohtsubo, T.; Kitazono, T. Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels. Int. J. Mol. Sci. 2018, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Stekiel, W.J.; Contney, S.J.; Lombard, J.H. Small Vessel Membrane Potential, Sympathetic Input, and Electrogenic Pump Rate in SHR. Am. J. Physiol. 1986, 250 Pt 1, C547–C556. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Tominaga, M.; Ohmori, S.; Kobayashi, K.; Koga, T.; Takata, Y.; Fujishima, M. Decreased Endothelium-Dependent Hyperpolarization to Acetylcholine in Smooth Muscle of the Mesenteric Artery of Spontaneously Hypertensive Rats. Circ. Res. 1992, 70, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Fujii, K.; Abe, I. Impaired β-Adrenergic Hyperpolarization in Arteries from Prehypertensive Spontaneously Hypertensive Rats. Hypertension 2001, 37, 609–613. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, Q.; Liu, Z.; Liu, D.; Chen, L. Chloride Channel Activity of Vascular Smooth Muscle in the Spontaneous Hypertensive Rats. Chin. J. Physiol. 2004, 47, 129–135. [Google Scholar] [PubMed]
- Wang, B.; Li, C.; Huai, R.; Qu, Z. Overexpression of ANO1/TMEM16A, an Arterial Ca2+-Activated Cl− Channel, Contributes to Spontaneous Hypertension. J. Mol. Cell. Cardiol. 2015, 82, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Askew Page, H.R.; Dalsgaard, T.; Baldwin, S.N.; Jepps, T.A.; Povstyan, O.; Olesen, S.P.; Greenwood, I.A. TMEM16A Is Implicated in the Regulation of Coronary Flow and Is Altered in Hypertension. Br. J. Pharmacol. 2019, 176, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Cil, O.; Chen, X.; Askew Page, H.R.; Baldwin, S.N.; Jordan, M.C.; Myat Thwe, P.; Anderson, M.O.; Haggie, P.M.; Greenwood, I.A.; Roos, K.P.; et al. A Small Molecule Inhibitor of the Chloride Channel TMEM16A Blocks Vascular Smooth Muscle Contraction and Lowers Blood Pressure in Spontaneously Hypertensive Rats. Kidney Int. 2021, 100, 311–320. [Google Scholar] [CrossRef]
- Li, R.S.; Wang, Y.; Chen, H.S.; Jiang, F.Y.; Tu, Q.; Li, W.J.; Yin, R.X. TMEM16A Contributes to Angiotensin II-Induced Cerebral Vasoconstriction via the RhoA/ROCK Signaling Pathway. Mol. Med. Rep. 2016, 13, 3691–3699. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Zheng, L.Y.; Zhang, Z.; Tang, Y.B.; Wang, G.L.; Du, Y.H.; Lv, X.F.; Liu, J.; Zhou, J.G.; et al. Downregulation of TMEM16A Calcium-Activated Chloride Channel Contributes to Cerebrovascular Remodeling during Hypertension by Promoting Basilar Smooth Muscle Cell Proliferation. Circulation 2012, 125, 697–707. [Google Scholar] [CrossRef]
- Shiono, K.; Sokabe, H. Renin-Angiotensin System in Spontaneously Hypertensive Rats. Am. J. Physiol. 1976, 231, 1295–1299. [Google Scholar] [CrossRef]
- Okamura, T.; Miyazaki, M.; Inagami, T.; Toda, N. Vascular Renin-Angiotensin System in Two-Kidney, One Clip Hypertensive Rats. Hypertension 1986, 8, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zheng, B.; Yang, Z.; He, M.; Yue, L.Y.; Zhang, R.N.; Zhang, M.; Zhang, W.; Zhang, X.; Wen, J.K. TMEM16A and Myocardin Form a Positive Feedback Loop That Is Disrupted by KLF5 during Ang II-Induced Vascular Remodeling. Hypertension 2015, 66, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Abel, P.W.; Hermsmeyer, K. Sympathetic Cross-Innervation of SHR and Genetic Controls Suggests a Trophic Influence on Vascular Muscle Membranes. Circ. Res. 1981, 49, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Kostyunina, D.S.; Zhang, L.; Shvetsova, A.A.; Selivanova, E.K.; Tarasova, O.S.; Matchkov, V.V.; Gaynullina, D.K. Trophic Sympathetic Influence Weakens Pro-Contractile Role of Cl− Channels in Rat Arteries during Postnatal Maturation. Sci. Rep. 2020, 10, 20002. [Google Scholar] [CrossRef] [PubMed]
- Liskova, S.; Petrova, M.; Karen, P.; Behuliak, M.; Zicha, J. Contribution of Ca2+-Dependent Cl− Channels to Norepinephrine-Induced Contraction of Femoral Artery Is Replaced by Increasing EDCF Contribution during Ageing. Biomed Res. Int. 2014, 2014, 289361. [Google Scholar] [CrossRef]
- Jensen, A.B.; Joergensen, H.B.; Dam, V.S.; Kamaev, D.; Boedtkjer, D.; Füchtbauer, E.M.; Aalkjaer, C.; Matchkov, V.V. Variable Contribution of TMEM16A to Tone in Murine Arterial Vasculature. Basic Clin. Pharmacol. Toxicol. 2018, 123, 30–41. [Google Scholar] [CrossRef]
- Dam, V.S.; Boedtkjer, D.M.B.; Nyvad, J.; Aalkjaer, C.; Matchkov, V. TMEM16A Knockdown Abrogates Two Different Ca(2+)-Activated Cl(−) Currents and Contractility of Smooth Muscle in Rat Mesenteric Small Arteries. Pflug. Arch. 2014, 466, 1391–1409. [Google Scholar] [CrossRef]
- Wang, Q.; Dennis Leo, M.; Narayanan, D.; Kuruvilla, K.P.; Jaggar, J.H. Local Coupling of TRPC6 to ANO1/TMEM16A Channels in Smooth Muscle Cells Amplifies Vasoconstriction in Cerebral Arteries. Am. J. Physiol. Cell Physiol. 2016, 310, C1001–C1009. [Google Scholar] [CrossRef]
- Ohya, Y.; Abe, I.; Fujii, K.; Takata, Y.; Fujishima, M. Voltage-Dependent Ca2+ Channels in Resistance Arteries from Spontaneously Hypertensive Rats. Circ. Res. 1993, 73, 1090–1099. [Google Scholar] [CrossRef]
- Pesic, A.; Madden, J.A.; Pesic, M.; Rusch, N.J. High Blood Pressure Upregulates Arterial L-Type Ca2+ Channels: Is Membrane Depolarization the Signal? Circ. Res. 2004, 94, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.M.; Kim, A.; Lee, Y.J.; Lim, W.; Noh, Y.H.; Kim, E.J.; Kim, J.; Kim, T.K.; Park, S.W.; Kim, B.; et al. Enhancement of Receptor-Operated Cation Current and TRPC6 Expression in Arterial Smooth Muscle Cells of Deoxycorticosterone Acetate-Salt Hypertensive Rats. J. Hypertens. 2007, 25, 809–817. [Google Scholar] [CrossRef]
- Suh, B.C.; Hille, B. PIP2 Is a Necessary Cofactor for Ion Channel Function: How and Why? Annu. Rev. Biophys. 2008, 37, 175–195. [Google Scholar] [CrossRef]
- Ta, C.M.; Acheson, K.E.; Rorsman, N.J.G.; Jongkind, R.C.; Tammaro, P. Contrasting Effects of Phosphatidylinositol 4,5-Bisphosphate on Cloned TMEM16A and TMEM16B Channels. Br. J. Pharmacol. 2017, 174, 2984–2999. [Google Scholar] [CrossRef] [PubMed]
- Tembo, M.; Wozniak, K.L.; Bainbridge, R.E.; Carlson, A.E. Phosphatidylinositol 4,5-Bisphosphate (PIP 2) and Ca2+ Are Both Required to Open the Cl− Channel TMEM16A. J. Biol. Chem. 2019, 294, 12556–12564. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Chen, J. Specific PIP 2 Binding Promotes Calcium Activation of TMEM16A Chloride Channels. Commun. Biol. 2021, 4, 259. [Google Scholar] [CrossRef]
- Pritchard, H.A.T.; Leblanc, N.; Albert, A.P.; Greenwood, I.A. Inhibitory Role of Phosphatidylinositol 4,5-Bisphosphate on TMEM16A-Encoded Calcium-Activated Chloride Channels in Rat Pulmonary Artery. Br. J. Pharmacol. 2014, 171, 4311–4321. [Google Scholar] [CrossRef]
- Ek, T.P.; Campbell, M.D.; Deth, R.C. Reduction of Norepinephrine-Induced Tonic Contraction and Phosphoinositide Turnover in Arteries of Spontaneously Hypertensive Rats. A Possible Role for Protein Kinase C. Am. J. Hypertens. 1989, 2, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shah, S.; Liu, Y.; Zhang, H.; Lees, M.; Fu, Z.; Lippiat, J.D.; Beech, D.J.; Sivaprasadarao, A.; Baldwin, S.A.; et al. Activation of the Cl− Channel ANO1 by Localized Calcium Signals in Nociceptive Sensory Neurons Requires Coupling with the IP3 Receptor. Sci. Signal. 2013, 6, ra73. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Pathan, A.R.; Daalis, A.; Hubrack, S.; Abou-Jassoum, H.; Al-Naeimi, H.; Rusch, N.J.; Machaca, K. Inositol 1,4,5-Trisphosphate (IP3) Receptor up-Regulation in Hypertension Is Associated with Sensitization of Ca2+ Release and Vascular Smooth Muscle Contractility. J. Biol. Chem. 2013, 288, 32941–32951. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Droogmans, G. Ion Channels and Their Functional Role in Vascular Endothelium. Physiol. Rev. 2001, 81, 1415–1459. [Google Scholar] [CrossRef]
- Ma, M.M.; Gao, M.; Guo, K.M.; Wang, M.; Li, X.Y.; Zeng, X.L.; Sun, L.; Lv, X.F.; Du, Y.H.; Wang, G.L.; et al. TMEM16A Contributes to Endothelial Dysfunction by Facilitating Nox2 NADPH Oxidase-Derived Reactive Oxygen Species Generation in Hypertension. Hypertension 2017, 69, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yasumoto, M.; Suzuki, Y.; Asai, K.; Imaizumi, Y.; Yamamura, H. TMEM16A Ca2+-Activated Cl− Channel Regulates the Proliferation and Migration of Brain Capillary Endothelial Cells. Mol. Pharmacol. 2020, 98, 61–71. [Google Scholar] [CrossRef]
- Skofic Maurer, D.; Zabini, D.; Nagaraj, C.; Sharma, N.; Lengyel, M.; Nagy, B.M.; Frank, S.; Klepetko, W.; Gschwandtner, E.; Enyedi, P.; et al. Endothelial Dysfunction Following Enhanced TMEM16A Activity in Human Pulmonary Arteries. Cells 2020, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Suzuki, H. Effects of Increased Intracellular Cl− Concentration on Membrane Responses to Acetylcholine in the Isolated Endothelium of Guinea Pig Mesenteric Arteries. J. Physiol. Sci. 2007, 57, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Edwards, F.R.; Hill, C.E. Depolarization Evoked by Acetylcholine in Mesenteric Arteries of Hypertensive Rats Attenuates Endothelium-Dependent Hyperpolarizing Factor. J. Hypertens. 2007, 25, 345–359. [Google Scholar] [CrossRef]
- Goto, K.; Rummery, N.M.; Grayson, T.H.; Hill, C.E. Attenuation of Conducted Vasodilation in Rat Mesenteric Arteries during Hypertension: Role of Inwardly Rectifying Potassium Channels. J. Physiol. 2004, 561, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Hirst, G.D.S.; Bramich, N.J.; Teramoto, N.; Suzuki, H.; Edwards, F.R. Regenerative Component of Slow Waves in the Guinea-Pig Gastric Antrum Involves a Delayed Increase in [Ca2+]i and Cl− Channels. J. Physiol. 2002, 540 Pt 3, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Sandow, S.L.; Hill, C.E. Incidence of Myoendothelial Gap Junctions in the Proximal and Distal Mesenteric Arteries of the Rat Is Suggestive of a Role in Endothelium-Derived Hyperpolarizing Factor–Mediated Responses. Circ. Res. 2000, 86, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Fujii, K.; Kansui, Y.; Abe, I.; Iida, M. Critical Role of Gap Junctions in Endothelium-Dependent Hyperpolarization in Rat Mesenteric Arteries. Clin. Exp. Pharmacol. Physiol. 2002, 29, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Mather, S.; Dora, K.A.; Sandow, S.L.; Winter, P.; Garland, C.J. Rapid Endothelial Cell-Selective Loading of Connexin 40 Antibody Blocks Endothelium-Derived Hyperpolarizing Factor Dilation in Rat Small Mesenteric Arteries. Circ. Res. 2005, 97, 399–407. [Google Scholar] [CrossRef]
- Doughty, J.M.; Boyle, J.P.; Langton, P.D. Blockade of Chloride Channels Reveals Relaxations of Rat Small Mesenteric Arteries to Raised Potassium. Br. J. Pharmacol. 2001, 132, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kwan, Y.W.; Chan, S.W.; Lee, S.M.Y.; Leung, G.P.H. Potentiation of EDHF-Mediated Relaxation by Chloride Channel Blockers. Acta Pharmacol. Sin. 2010, 31, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.W.; Flagella, M.; Sutliff, R.L.; Lorenz, J.N.; Nieman, M.L.; Weber, C.S.; Paul, R.J.; Shull, G.E. Decreased Blood Pressure and Vascular Smooth Muscle Tone in Mice Lacking Basolateral Na+-K+-2Cl− Cotransporter. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1846–H1855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Roy, K.; Vidossich, P.; Cancedda, L.; De Vivo, M.; Forbush, B.; Cao, E. Structural Basis for Inhibition of the Cation-Chloride Cotransporter NKCC1 by the Diuretic Drug Bumetanide. Nat. Commun. 2022, 13, 2747. [Google Scholar] [CrossRef]
- Garg, P.; Martin, C.F.; Elms, S.C.; Gordon, F.J.; Wall, S.M.; Garland, C.J.; Sutliff, R.L.; O’Neill, W.C. Effect of the Na-K-2Cl Cotransporter NKCC1 on Systemic Blood Pressure and Smooth Muscle Tone. Am. J. Physiol. Heart Circ. Physiol. 2007, 292. [Google Scholar] [CrossRef]
- Orlov, S.N.; Resink, T.J.; Bernhardt, J.; Bühler, F.R. Na(+)-K+ Pump and Na(+)-K+ Co-Transport in Cultured Vascular Smooth Muscle Cells from Spontaneously Hypertensive and Normotensive Rats: Baseline Activity and Regulation. J. Hypertens. 1992, 10, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Denny, T.N.; Aviv, A. 22Na+ and 86Rb+ Transport in Vascular Smooth Muscle of SHR, Wistar Kyoto, and Wistar Rats. J. Cardiovasc. Pharmacol. 1988, 11, 722–729. [Google Scholar] [CrossRef]
- Tokushige, A.; Kino, M.; Tamura, H.; Hopp, L.; Searle, B.M.; Aviv, A. Bumetanide-Sensitive Sodium-22 Transport in Vascular Smooth Muscle Cell of the Spontaneously Hypertensive Rat. Hypertension 1986, 8, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Canessa, M.; Salazar, G.; Werner, E.; Vallega, G.; Gonzalez, A. Cell Growth and Na-K-Cl Cotransport Responses of Vascular Smooth Muscle Cells of Milan Rats. Hypertension 1994, 23 Pt 2, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.L.; Chipperfield, A.R.; Harper, A.A. Accumulation of Intracellular Chloride by (Na-K-Cl) Co-Transport in Rat Arterial Smooth Muscle Is Enhanced in Deoxycorticosterone Acetate (DOCA)/Salt Hypertension. J. Mol. Cell. Cardiol. 1993, 25, 233–237. [Google Scholar] [CrossRef]
- Lee, H.A.; Baek, I.; Seok, Y.M.; Yang, E.; Cho, H.M.; Lee, D.Y.; Hong, S.H.; Kim, I.K. Promoter Hypomethylation Upregulates Na+-K+-2Cl− Cotransporter 1 in Spontaneously Hypertensive Rats. Biochem. Biophys. Res. Commun. 2010, 396, 252–257. [Google Scholar] [CrossRef]
- Cho, H.M.; Lee, H.A.; Kim, H.Y.; Han, H.S.; Kim, I.K. Expression of Na+-K+-2Cl Cotransporter 1 Is Epigenetically Regulated during Postnatal Development of Hypertension. Am. J. Hypertens. 2011, 24, 1286–1293. [Google Scholar] [CrossRef][Green Version]
- Cho, H.M.; Lee, D.Y.; Kim, H.Y.; Lee, H.A.; Seok, Y.M.; Kim, I.K. Upregulation of the Na(+)-K(+)-2Cl(−) Cotransporter 1 via Histone Modification in the Aortas of Angiotensin II-Induced Hypertensive Rats. Hypertens. Res. 2012, 35, 819–824. [Google Scholar] [CrossRef]
- Bergaya, S.; Faure, S.; Baudrie, V.; Rio, M.; Escoubet, B.; Bonnin, P.; Henrion, D.; Loirand, G.; Achard, J.M.; Jeunemaitre, X.; et al. WNK1 Regulates Vasoconstriction and Blood Pressure Response to α 1-Adrenergic Stimulation in Mice. Hypertension 2011, 58, 439–445. [Google Scholar] [CrossRef]
- Yang, S.S.; Lo, Y.F.; Wu, C.C.; Lin, S.W.; Yeh, C.J.; Chu, P.; Sytwu, H.K.; Uchida, S.; Sasaki, S.; Lin, S.H. SPAK-Knockout Mice Manifest Gitelman Syndrome and Impaired Vasoconstriction. J. Am. Soc. Nephrol. 2010, 21, 1868–1877. [Google Scholar] [CrossRef]
- Zeniya, M.; Sohara, E.; Kita, S.; Iwamoto, T.; Susa, K.; Mori, T.; Oi, K.; Chiga, M.; Takahashi, D.; Yang, S.; et al. Dietary Salt Intake Regulates WNK3-SPAK-NKCC1 Phosphorylation Cascade in Mouse Aorta through Angiotensin II. Hypertension 2013, 62, 872–878. [Google Scholar] [CrossRef]
- Murthy, M.; Kurz, T.; O’Shaughnessy, K.M. WNK Signalling Pathways in Blood Pressure Regulation. Cell. Mol. Life Sci. 2017, 74, 1261–1280. [Google Scholar] [CrossRef]
- Jin, H.-S.; Jung, D. Gender-Specific Association of the ANO1 Genetic Variations with Hypertension. Biomed. Sci. Lett. 2015, 21, 144–151. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Hansrivijit, P.; Thirunavukkarasu, S.; Chewcharat, A.; Medaura, J.; Mao, M.A.; Kashani, K. Association of Serum Chloride Level Alterations with In-Hospital Mortality. Postgrad. Med. J. 2020, 96, 731–736. [Google Scholar] [CrossRef]
- Takahashi, A.; Maeda, K.; Sasaki, K.; Doi, S.; Nakashima, A.; Doi, T.; Masaki, T. Relationships of Hyperchloremia with Hypertension and Proteinuria in Patients with Chronic Kidney Disease. Clin. Exp. Nephrol. 2022, 26, 880–885. [Google Scholar] [CrossRef] [PubMed]
- McCallum, L.; Jeemon, P.; Hastie, C.E.; Patel, R.K.; Williamson, C.; Redzuan, A.M.; Dawson, J.; Sloan, W.; Muir, S.; Morrison, D.; et al. Serum Chloride Is an Independent Predictor of Mortality in Hypertensive Patients. Hypertension 2013, 62, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bellino, M.C.; Massari, F.; Albanese, M.; Ursi, R.; Angelini, G.; Lisi, F.; Amato, L.; Scicchitano, P.; Guida, P.; Brunetti, N.D.; et al. Baseline and Incident Hypochloremia in Chronic Heart Failure Outpatients: Clinical Correlates and Prognostic Role. Eur. J. Intern. Med. 2021, 84, 32–37. [Google Scholar] [CrossRef] [PubMed]

| Animals | Alterations in Vascular Smooth Muscle CaCCs during Hypertension | Ref. |
|---|---|---|
| SHRs | Increased TMEM16A expression and function in aorta, carotid arteries, hindlimb arteries and mesenteric arteries | [56] |
| Increased TMEM16A expression and function in coronary arteries | [57] | |
| Increased TMEM16A expression and function in renal arterioles | [47] | |
| Knockdown of TMEM16A by siRNA transfection lowered blood pressure | [56] | |
| Inhibition of TMEM16A activity by T16Ainh-A01 lowered blood pressure | [56] | |
| Treatment of mesenteric resistance arteries with TMinh-23 blocked vasoconstriction | [58] | |
| Inhibition of TMEM16A activity by TMinh-23 lowered blood pressure | [58] | |
| 2K2C renal hypertensive rats | Reduced TMEM16A expression and function in basilar arteries during the development of hypertension | [59,60] |
| Animals | Alterations in Endothelial CaCCs during Hypertension | Ref. |
|---|---|---|
| SHRs | Increased CaCC function in endothelium of mesenteric arteries | [86] |
| Increased CaCC function, reduced EDH in mesenteric arteries | [86] | |
| Ang Ⅱ-induced hypertensive mice | Increased TMEM16A expression in endothelium of aorta | [82] |
| Endothelial-specific TMEM16A knockout ameliorated endothelial function and lowered blood pressure | [82] | |
| Endothelial-specific TMEM16A overexpression deteriorated endothelial function and elevated blood pressure | [82] |
| Animals | Alterations in Vascular Smooth Muscle NKCC1 during Hypertension | Ref. |
|---|---|---|
| SHRs | Increased NKCC1 function in aorta and carotid arteries | [97,98,99] |
| Epigenetic upregulation of aorta NKCC1 due to Nkcc1 gene promoter hypomethylation | [102] | |
| Nkcc1 gene promoter hypomethylation resulted from the decreased activity of DNA methyltransferase 3B | [103] | |
| Milan hypertensive rats | Increased NKCC1 function in thoracic aorta | [100] |
| DOCA salt hypertensive rats | Increased NKCC1 function in saphenous branch of femoral arteries | [101] |
| Ang Ⅱ-induced hypertensive rats | Epigenetic upregulation of aorta NKCC1 due to histone modifications | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, K.; Kitazono, T. Chloride Ions, Vascular Function and Hypertension. Biomedicines 2022, 10, 2316. https://doi.org/10.3390/biomedicines10092316
Goto K, Kitazono T. Chloride Ions, Vascular Function and Hypertension. Biomedicines. 2022; 10(9):2316. https://doi.org/10.3390/biomedicines10092316
Chicago/Turabian StyleGoto, Kenichi, and Takanari Kitazono. 2022. "Chloride Ions, Vascular Function and Hypertension" Biomedicines 10, no. 9: 2316. https://doi.org/10.3390/biomedicines10092316
APA StyleGoto, K., & Kitazono, T. (2022). Chloride Ions, Vascular Function and Hypertension. Biomedicines, 10(9), 2316. https://doi.org/10.3390/biomedicines10092316





