Synuclein Proteins in MPTP-Induced Death of Substantia Nigra Pars Compacta Dopaminergic Neurons
Abstract
1. Introduction
2. Synuclein Structure and Functions
3. Parkinson’s Disease Is a Form of Synucleinopathy
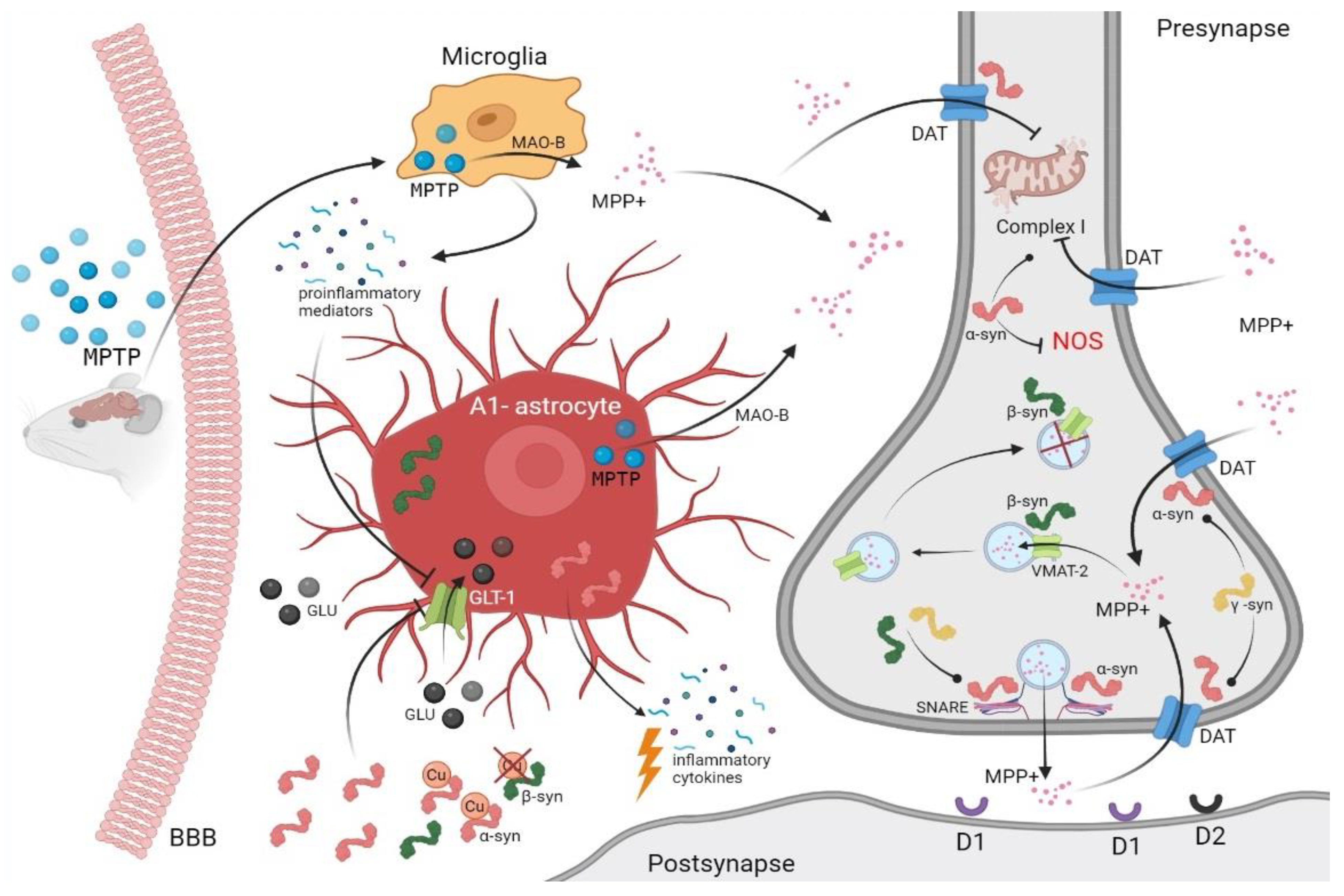
3.1. Toxic Animal Modeling of Parkinsonism Using MPTP
3.2. Synucleins and MPTP Toxicity
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| DA | Dopamine |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| DA neurons | Dopaminergic neurons |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MPP+ | 1-methyl-4-phenylpyridine in ionic form |
| SNpc | Substantia nigra pars compacta |
| NAC | Non-beta-amyloid component |
| CNS | Central nervous system |
| SNARE | Soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| VAMP-2 | Protein synaptobrevin-2 |
| VMAT-2 | Vesicular monoamine transporter-2 |
| DAT | Dopamine transporter |
| TH | Tyrosine hydroxylase |
| VDAC | Voltage-dependent anion channels |
| VTA | Ventral tegmental area |
| CBD | Corticobasal degeneration |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IFN-γ | Interferon gamma |
| TNF-α | Tumor necrosis factor-α |
| MAO-B | Monoamine oxidase B |
| iNOS | Inducible NO synthase |
| NO | Nitric oxide |
| ONOO | Peroxynitrite |
| COX2 | Cyclooxygenase-2 enzyme |
| NMDA | N-methyl-D-aspartate |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ROS | Reactive oxygen species |
References
- Bras, J.; Gibbons, E.; Guerreiro, R. Genetics of synucleins in neurodegenerative diseases. Acta Neuropathol. 2021, 141, 471–490. [Google Scholar] [CrossRef]
- Mahoney-Sanchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2021, 196, 101890. [Google Scholar] [CrossRef]
- Schluter, O.M.; Fornai, F.; Alessandri, M.G.; Takamori, S.; Geppert, M.; Jahn, R.; Sudhof, T.C. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience 2003, 118, 985–1002. [Google Scholar] [CrossRef]
- Ninkina, N.; Millership, S.J.; Peters, O.M.; Connor-Robson, N.; Chaprov, K.; Kopylov, A.T.; Montoya, A.; Kramer, H.; Withers, D.J.; Buchman, V.L. Beta-synuclein potentiates synaptic vesicle dopamine uptake and rescues dopaminergic neurons from MPTP-induced death in the absence of other synucleins. J. Biol. Chem. 2021, 297, 101375. [Google Scholar] [CrossRef]
- Robertson, D.C.; Schmidt, O.; Ninkina, N.; Jones, P.A.; Sharkey, J.; Buchman, V.L. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J. Neurochem. 2004, 89, 1126–1136. [Google Scholar] [CrossRef]
- Merghani, M.M.; Ardah, M.T.; Al Shamsi, M.; Kitada, T.; Haque, M.E. Dose-related biphasic effect of the Parkinson’s disease neurotoxin MPTP, on the spread, accumulation, and toxicity of alpha-synuclein. Neurotoxicology 2021, 84, 41–52. [Google Scholar] [CrossRef]
- Chaprov, K.D.; Teterina, E.V.; Roman, A.Y.; Ivanova, T.A.; Goloborshcheva, V.V.; Kucheryanu, V.G.; Morozov, S.G.; Lysikova, E.A.; Lytkina, O.A.; Koroleva, I.V.; et al. Comparative Analysis of MPTP Neurotoxicity in Mice with a Constitutive Knockout of the alpha-Synuclein Gene. Mol. Biol. 2021, 55, 152–163. [Google Scholar] [CrossRef]
- Patel, D.; Bordoni, B. Physiology, Synuclein; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Burre, J.; Sharma, M.; Sudhof, T.C. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harb. Perspect Med. 2018, 8, a024091. [Google Scholar] [CrossRef]
- Carija, A.; Pinheiro, F.; Pujols, J.; Bras, I.C.; Lazaro, D.F.; Santambrogio, C.; Grandori, R.; Outeiro, T.F.; Navarro, S.; Ventura, S. Biasing the native alpha-synuclein conformational ensemble towards compact states abolishes aggregation and neurotoxicity. Redox Biol. 2019, 22, 101135. [Google Scholar] [CrossRef]
- Hayashi, J.; Carver, J.A. beta-Synuclein: An Enigmatic Protein with Diverse Functionality. Biomolecules 2022, 12, 142. [Google Scholar] [CrossRef]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Fukushima, H.; Masliah, E.; Xia, Y.; Iwai, A.; Yoshimoto, M.; Otero, D.A.; Kondo, J.; Ihara, Y.; Saitoh, T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 11282–11286. [Google Scholar] [CrossRef]
- Nakajo, S.; Omata, K.; Aiuchi, T.; Shibayama, T.; Okahashi, I.; Ochiai, H.; Nakai, Y.; Nakaya, K.; Nakamura, Y. Purification and characterization of a novel brain-specific 14-kDa protein. J. Neurochem. 1990, 55, 2031–2038. [Google Scholar] [CrossRef]
- Tobe, T.; Nakajo, S.; Tanaka, A.; Mitoya, A.; Omata, K.; Nakaya, K.; Tomita, M.; Nakamura, Y. Cloning and characterization of the cDNA encoding a novel brain-specific 14-kDa protein. J. Neurochem. 1992, 59, 1624–1629. [Google Scholar] [CrossRef]
- Ji, H.; Liu, Y.E.; Jia, T.; Wang, M.; Liu, J.; Xiao, G.; Joseph, B.K.; Rosen, C.; Shi, Y.E. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997, 57, 759–764. [Google Scholar]
- Buchman, V.L.; Adu, J.; Pinon, L.G.; Ninkina, N.N.; Davies, A.M. Persyn, a member of the synuclein family, influences neurofilament network integrity. Nat. Neurosci. 1998, 1, 101–103. [Google Scholar] [CrossRef]
- Iwai, A.; Masliah, E.; Yoshimoto, M.; Ge, N.; Flanagan, L.; de Silva, H.A.; Kittel, A.; Saitoh, T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 1995, 14, 467–475. [Google Scholar] [CrossRef]
- Jakes, R.; Spillantini, M.G.; Goedert, M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994, 345, 27–32. [Google Scholar] [CrossRef]
- Tanji, K.; Mori, F.; Nakajo, S.; Imaizumi, T.; Yoshida, H.; Hirabayashi, T.; Yoshimoto, M.; Satoh, K.; Takahashi, H.; Wakabayashi, K. Expression of beta-synuclein in normal human astrocytes. Neuroreport 2001, 12, 2845–2848. [Google Scholar] [CrossRef]
- Le, T.; Winham, C.L.; Andromidas, F.; Silver, A.C.; Jellison, E.R.; Levesque, A.A.; Koob, A.O. Chimera RNA interference knockdown of gamma-synuclein in human cortical astrocytes results in mitotic catastrophe. Neural. Regen. Res. 2020, 15, 1894–1902. [Google Scholar] [CrossRef]
- Butler, B.; Sambo, D.; Khoshbouei, H. Alpha-synuclein modulates dopamine neurotransmission. J. Chem. Neuroanat. 2017, 83–84, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Conde, L.D.; Ramos-Acevedo, R.; Reyes-Hernandez, M.A.; Balbuena-Olvera, A.J.; Morales-Moreno, I.D.; Arguero-Sanchez, R.; Schule, B.; Guerra-Crespo, M. Alpha-Synuclein Physiology and Pathology: A Perspective on Cellular Structures and Organelles. Front. Neurosci. 2019, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Yang, Y.; Paslawski, W.; Svenningsson, P. alpha-Synuclein induced cholesterol lowering increases tonic and reduces depolarization-evoked synaptic vesicle recycling and glutamate release. NPJ Parkinsons Dis. 2022, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Kachappilly, N.; Srivastava, J.; Swain, B.P.; Thakur, P. Interaction of alpha-synuclein with lipids. Methods Cell Biol. 2022, 169, 43–66. [Google Scholar] [CrossRef]
- Uceda, A.B.; Frau, J.; Vilanova, B.; Adrover, M. Glycation of alpha-synuclein hampers its binding to synaptic-like vesicles and its driving effect on their fusion. Cell Mol. Life Sci. 2022, 79, 342. [Google Scholar] [CrossRef]
- Sulzer, D.; Edwards, R.H. The physiological role of alpha-synuclein and its relationship to Parkinson’s Disease. J. Neurochem. 2019, 150, 475–486. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Sudhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Guo, J.T.; Chen, A.Q.; Kong, Q.; Zhu, H.; Ma, C.M.; Qin, C. Inhibition of vesicular monoamine transporter-2 activity in alpha-synuclein stably transfected SH-SY5Y cells. Cell Mol. Neurobiol. 2008, 28, 35–47. [Google Scholar] [CrossRef]
- Carnazza, K.E.; Komer, L.E.; Xie, Y.X.; Pineda, A.; Briano, J.A.; Gao, V.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; et al. Synaptic vesicle binding of alpha-synuclein is modulated by beta- and gamma-synucleins. Cell Rep. 2022, 39, 110675. [Google Scholar] [CrossRef]
- Carnazza, K.E.; Komer, L.E.; Pineda, A.; Na, Y.; Ramlall, T.; Buchman, V.L.; Eliezer, D.; Sharma, M.; Burre, J. Beta- and gamma-synucleins modulate synaptic vesicle-binding of alpha-synuclein. bioRxiv 2020. [Google Scholar] [CrossRef]
- Scheibe, C.; Karreman, C.; Schildknecht, S.; Leist, M.; Hauser, K. Synuclein Family Members Prevent Membrane Damage by Counteracting alpha-Synuclein Aggregation. Biomolecules 2021, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Yates, D. Processing alpha-synuclein interactions. Nat. Rev. Neurosci. 2022, 23, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Y.; Xi, H.; Jiang, J.; Yu, Y.; Dong, W. The Membrane Interaction of Alpha-Synuclein. Front. Cell Neurosci. 2021, 15, 633727. [Google Scholar] [CrossRef] [PubMed]
- Jeannotte, A.M.; McCarthy, J.G.; Sidhu, A. Desipramine induced changes in the norepinephrine transporter, alpha- and gamma-synuclein in the hippocampus, amygdala and striatum. Neurosci. Lett. 2009, 467, 86–89. [Google Scholar] [CrossRef][Green Version]
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. NPJ Parkinsons Dis. 2021, 7, 22. [Google Scholar] [CrossRef]
- Threlfell, S.; Mohammadi, A.S.; Ryan, B.J.; Connor-Robson, N.; Platt, N.J.; Anand, R.; Serres, F.; Sharp, T.; Bengoa-Vergniory, N.; Wade-Martins, R.; et al. Striatal Dopamine Transporter Function Is Facilitated by Converging Biology of alpha-Synuclein and Cholesterol. Front. Cell Neurosci. 2021, 15, 658244. [Google Scholar] [CrossRef]
- Longhena, F.; Faustini, G.; Missale, C.; Pizzi, M.; Bellucci, A. Dopamine Transporter/alpha-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson’s Disease: An In Situ Proximity Ligation Assay Study. Int. J. Mol. Sci. 2018, 19, 1611. [Google Scholar] [CrossRef]
- Lotharius, J.; Barg, S.; Wiekop, P.; Lundberg, C.; Raymon, H.K.; Brundin, P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J. Biol. Chem. 2002, 277, 38884–38894. [Google Scholar] [CrossRef]
- Ghamgosha, M.; Latifi, A.M.; Meftahi, G.H.; Mohammadi, A. Cellular, Molecular and Non-Pharm.ogical Therapeutic Advances for the Treatment of Parkinson’s Disease: Separating Hope from Hype. Curr. Gene. Ther. 2018, 18, 206–224. [Google Scholar] [CrossRef]
- Wersinger, C.; Sidhu, A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci. Lett. 2003, 340, 189–192. [Google Scholar] [CrossRef]
- Bridi, J.C.; Hirth, F. Mechanisms of alpha-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Yavich, L.; Tanila, H.; Vepsalainen, S.; Jakala, P. Role of alpha-synuclein in presynaptic dopamine recruitment. J. Neurosci. 2004, 24, 11165–11170. [Google Scholar] [CrossRef] [PubMed]
- Surguchov, A. Molecular and cellular biology of synucleins. Int. Rev. Cell Mol. Biol. 2008, 270, 225–317. [Google Scholar] [CrossRef]
- Fanning, S.; Selkoe, D.; Dettmer, U. Parkinson’s disease: Proteinopathy or lipidopathy? NPJ Parkinsons Dis 2020, 6, 3. [Google Scholar] [CrossRef]
- Millership, S.; Ninkina, N.; Rochford, J.J.; Buchman, V.L. gamma-synuclein is a novel player in the control of body lipid metabolism. Adipocyte 2013, 2, 276–280. [Google Scholar] [CrossRef][Green Version]
- da Costa, C.A.; Masliah, E.; Checler, F. Beta-synuclein displays an antiapoptotic p53-dependent phenotype and protects neurons from 6-hydroxydopamine-induced caspase 3 activation: Cross-talk with alpha-synuclein and implication for Parkinson’s disease. J. Biol. Chem. 2003, 278, 37330–37335. [Google Scholar] [CrossRef]
- Brockhaus, K.; Bohm, M.R.R.; Melkonyan, H.; Thanos, S. Age-related Beta-synuclein Alters the p53/Mdm2 Pathway and Induces the Apoptosis of Brain Microvascular Endothelial Cells In Vitro. Cell Transpl. 2018, 27, 796–813. [Google Scholar] [CrossRef]
- Martinez, J.H.; Fuentes, F.; Vanasco, V.; Alvarez, S.; Alaimo, A.; Cassina, A.; Coluccio Leskow, F.; Velazquez, F. Alpha-synuclein mitochondrial interaction leads to irreversible translocation and complex I impairment. Arch. Biochem. Biophys. 2018, 651, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Becker, K.; Levine, N.; Zhang, M.; Lieberman, A.P.; Moore, D.J.; Ma, J. Pathogenic alpha-synuclein aggregates preferentially bind to mitochondria and affect cellular respiration. Acta Neuropathol. Commun. 2019, 7, 41. [Google Scholar] [CrossRef]
- Rostovtseva, T.K.; Gurnev, P.A.; Protchenko, O.; Hoogerheide, D.P.; Yap, T.L.; Philpott, C.C.; Lee, J.C.; Bezrukov, S.M. alpha-Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease. J. Biol. Chem. 2015, 290, 18467–18477. [Google Scholar] [CrossRef]
- Hoogerheide, D.P.; Rostovtseva, T.K.; Bezrukov, S.M. Exploring lipid-dependent conformations of membrane-bound alpha-synuclein with the VDAC nanopore. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183643. [Google Scholar] [CrossRef] [PubMed]
- McHugh, P.C.; Wright, J.A.; Brown, D.R. Transcriptional regulation of the beta-synuclein 5′-promoter metal response element by metal transcription factor-1. PLoS ONE 2011, 6, e17354. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.E.; Rios, A.; Trujano-Ortiz, L.G.; Villegas, A.; Castaneda-Hernandez, G.; Fernandez, C.O.; Gonzalez, F.J.; Quintanar, L. Comparing the copper binding features of alpha and beta synucleins. J. Inorg. Biochem. 2022, 229, 111715. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S. The Autophagy Lysosomal Pathway and Neurodegeneration. Cold Spring Harb. Perspect Biol. 2020, 12, a033993. [Google Scholar] [CrossRef] [PubMed]
- Popova, B.; Kleinknecht, A.; Arendarski, P.; Mischke, J.; Wang, D.; Braus, G.H. Sumoylation Protects Against beta-Synuclein Toxicity in Yeast. Front. Mol. Neurosci. 2018, 11, 94. [Google Scholar] [CrossRef]
- Ye, Q.; Peng, Y.; Huang, F.; Chen, J.; Xu, Y.; Li, Y.; Liu, S.; Huang, L. gamma-Synuclein is Closely Involved in Autophagy that Protects Colon Cancer Cell from Endoplasmic Reticulum Stress. Anticancer Agents Med. Chem. 2021, 21, 2385–2396. [Google Scholar] [CrossRef]
- Madsen, D.A.; Schmidt, S.I.; Blaabjerg, M.; Meyer, M. Interaction between Parkin and alpha-Synuclein in PARK2-Mediated Parkinson’s Disease. Cells 2021, 10, 283. [Google Scholar] [CrossRef]
- Tanaka, Y.; Engelender, S.; Igarashi, S.; Rao, R.K.; Wanner, T.; Tanzi, R.E.; Sawa, A.; Dawson, V.L.; Dawson, T.M.; Ross, C.A. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 2001, 10, 919–926. [Google Scholar] [CrossRef]
- Barba, L.; Paolini Paoletti, F.; Bellomo, G.; Gaetani, L.; Halbgebauer, S.; Oeckl, P.; Otto, M.; Parnetti, L. Alpha and Beta Synucleins: From Pathophysiology to Clinical Application as Biomarkers. Mov. Disord. 2022, 37, 669–683. [Google Scholar] [CrossRef]
- Hanson, K.A.; Kim, S.H.; Wassarman, D.A.; Tibbetts, R.S. Ubiquilin modifies TDP-43 toxicity in a Drosophila model of amyotrophic lateral sclerosis (ALS). J. Biol. Chem. 2010, 285, 11068–11072. [Google Scholar] [CrossRef]
- Zhou, R.M.; Huang, Y.X.; Li, X.L.; Chen, C.; Shi, Q.; Wang, G.R.; Tian, C.; Wang, Z.Y.; Jing, Y.Y.; Gao, C.; et al. Molecular interaction of alpha-synuclein with tubulin influences on the polymerization of microtubule in vitro and structure of microtubule in cells. Mol. Biol. Rep. 2010, 37, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tapia, M.L.; Yeh, J.; He, R.C.; Pomerleu, D.; Lee, R.K. Differential Gamma-Synuclein Expression in Acute and Chronic Retinal Ganglion Cell Death in the Retina and Optic Nerve. Mol. Neurobiol. 2020, 57, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, T.V.; Lytkina, O.A.; Goloborshcheva, V.V.; Skuratovskaya, L.N.; Antohin, A.I.; Ovchinnikov, R.K.; Kukharsky, M.S. Genetic inactivation of alpha-synuclein affects embryonic development of dopaminergic neurons of the substantia nigra, but not the ventral tegmental area, in mouse brain. PeerJ 2018, 6, e4779. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.H.; Giasson, B.I.; Lee, V.M. Alpha-synuclein: Normal function and role in neurodegenerative diseases. Curr. Top. Dev. Biol. 2004, 60, 17–54. [Google Scholar] [CrossRef]
- Quilty, M.C.; Gai, W.P.; Pountney, D.L.; West, A.K.; Vickers, J.C. Localization of alpha-, beta-, and gamma-synuclein during neuronal development and alterations associated with the neuronal response to axonal trauma. Exp. Neurol. 2003, 182, 195–207. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Cardoso, F.; Jankovic, J. Movement disorders. Neurol. Clin. 1993, 11, 625–638. [Google Scholar] [CrossRef]
- Keener, A.M.; Bordelon, Y.M. Parkinsonism. Semin. Neurol. 2016, 36, 330–334. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson disease in 2015: Evolving basic, pathological and clinical concepts in PD. Nat. Rev. Neurol. 2016, 12, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Riederer, P.; Berg, D.; Casadei, N.; Cheng, F.; Classen, J.; Dresel, C.; Jost, W.; Kruger, R.; Muller, T.; Reichmann, H.; et al. alpha-Synuclein in Parkinson’s disease: Causal or bystander? J. Neural. Transm. 2019, 126, 815–840. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017, 7, S51–S69. [Google Scholar] [CrossRef]
- Gadhe, L.; Sakunthala, A.; Mukherjee, S.; Gahlot, N.; Bera, R.; Sawner, A.S.; Kadu, P.; Maji, S.K. Intermediates of alpha-synuclein aggregation: Implications in Parkinson’s disease pathogenesis. Biophys. Chem. 2022, 281, 106736. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Frosch, M.P.; Walsh, D.M.; Hamilton, J.A.; Selkoe, D.J. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 2003, 37, 583–595. [Google Scholar] [CrossRef]
- Paleologou, K.E.; Kragh, C.L.; Mann, D.M.; Salem, S.A.; Al-Shami, R.; Allsop, D.; Hassan, A.H.; Jensen, P.H.; El-Agnaf, O.M. Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain 2009, 132, 1093–1101. [Google Scholar] [CrossRef]
- Park, M.J.; Cheon, S.M.; Bae, H.R.; Kim, S.H.; Kim, J.W. Elevated levels of alpha-synuclein oligomer in the cerebrospinal fluid of drug-naive patients with Parkinson’s disease. J. Clin. Neurol. 2011, 7, 215–222. [Google Scholar] [CrossRef]
- El-Agnaf, O.M.; Salem, S.A.; Paleologou, K.E.; Curran, M.D.; Gibson, M.J.; Court, J.A.; Schlossmacher, M.G.; Allsop, D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J 2006, 20, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gruden, M.A.; Davidova, T.V.; Yanamandra, K.; Kucheryanu, V.G.; Morozova-Roche, L.A.; Sherstnev, V.V.; Sewell, R.D. Nasal inoculation with alpha-synuclein aggregates evokes rigidity, locomotor deficits and immunity to such misfolded species as well as dopamine. Behav. Brain Res. 2013, 243, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Manda, G.; Bosca, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W. The MPTP Story. J. Parkinsons Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef]
- Vivacqua, G.; Biagioni, F.; Busceti, C.L.; Ferrucci, M.; Madonna, M.; Ryskalin, L.; Yu, S.; D’Este, L.; Fornai, F. Motor Neurons Pathology After Chronic Exposure to MPTP in Mice. Neurotox. Res. 2020, 37, 298–313. [Google Scholar] [CrossRef]
- Fox, S.H.; Brotchie, J.M. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog. Brain Res. 2010, 184, 133–157. [Google Scholar] [CrossRef]
- Johannessen, J.N.; Sobotka, T.J.; Weise, V.K.; Markey, S.P. Prolonged alterations in canine striatal dopamine metabolism following subtoxic doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 4′-amino-MPTP are linked to the persistence of pyridinium metabolites. J. Neurochem. 1991, 57, 981–990. [Google Scholar] [CrossRef]
- Aznavour, N.; Cendres-Bozzi, C.; Lemoine, L.; Buda, C.; Sastre, J.P.; Mincheva, Z.; Zimmer, L.; Lin, J.S. MPTP animal model of Parkinsonism: Dopamine cell death or only tyrosine hydroxylase impairment? A study using PET imaging, autoradiography, and immunohistochemistry in the cat. CNS Neurosci. Ther. 2012, 18, 934–941. [Google Scholar] [CrossRef]
- Sokolowski, A.L.; Larsson, B.S.; Lindquist, N.G. Distribution of 1-(3H)-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (3H-MPTP) in the frog: Uptake in neuromelanin. Pharm. Toxicol. 1990, 66, 252–258. [Google Scholar] [CrossRef]
- Ya, V.M. Experimental reproduction of catecholamine deficiency states and the problem of parkinsonism. Neurophysiology 1990, 22, 401–414. (In Russian) [Google Scholar]
- Sablin, S.O.; Krueger, M.J.; Bachurin, S.O.; Solyakov, L.S.; Efange, S.M.; Singer, T.P. Oxidation products arising from the action of monoamine oxidase B on 1-methyl-4-benzyl-1,2,3,6-tetrahydropyridine, a nonneurotoxic analogue of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurochem. 1994, 62, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.A.; Kindt, M.V.; Heikkila, R.E. Prevention of the nigrostriatal toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by inhibitors of 3,4-dihydroxyphenylethylamine transport. J. Neurochem. 1986, 47, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Mat Taib, C.N.; Mustapha, M. MPTP-induced mouse model of Parkinson’s disease: A promising direction of therapeutic strategies. Bosn. J. Basic Med. Sci. 2020, 21, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Lu, C.W.; Wang, S.E.; Lin, C.L.; Su, L.Y.; Wu, C.H. MPTP toxicity causes vocal, auditory, orientation and movement defects in the echolocation bat. Neuroreport 2021, 32, 125–134. [Google Scholar] [CrossRef]
- Haga, H.; Matsuo, K.; Yabuki, Y.; Zhang, C.; Han, F.; Fukunaga, K. Enhancement of ATP production ameliorates motor and cognitive impairments in a mouse model of MPTP-induced Parkinson’s disease. Neurochem. Int. 2019, 129, 104492. [Google Scholar] [CrossRef]
- Vyas, I.; Heikkila, R.E.; Nicklas, W.J. Studies on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Inhibition of NAD-linked substrate oxidation by its metabolite, 1-methyl-4-phenylpyridinium. J. Neurochem. 1986, 46, 1501–1507. [Google Scholar] [CrossRef]
- Prasad, E.M.; Hung, S.Y. Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson’s Disease. Antioxidants 2020, 9, 1007. [Google Scholar] [CrossRef]
- Rossetti, Z.L.; Sotgiu, A.; Sharp, D.E.; Hadjiconstantinou, M.; Neff, N.H. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and free radicals in vitro. Biochem. Pharm. 1988, 37, 4573–4574. [Google Scholar] [CrossRef]
- Przedborski, S.; Chen, Q.; Vila, M.; Giasson, B.I.; Djaldatti, R.; Vukosavic, S.; Souza, J.M.; Jackson-Lewis, V.; Lee, V.M.; Ischiropoulos, H. Oxidative post-translational modifications of alpha-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J. Neurochem. 2001, 76, 637–640. [Google Scholar] [CrossRef]
- Baranyi, M.; Porceddu, P.F.; Goloncser, F.; Kulcsar, S.; Otrokocsi, L.; Kittel, A.; Pinna, A.; Frau, L.; Huleatt, P.B.; Khoo, M.L.; et al. Novel (Hetero)arylalkenyl propargylamine compounds are protective in toxin-induced models of Parkinson’s disease. Mol. Neurodegener 2016, 11, 6. [Google Scholar] [CrossRef]
- Wada, M.; Ang, M.J.; Weerasinghe-Mudiyanselage, P.D.E.; Kim, S.H.; Kim, J.C.; Shin, T.; Moon, C. Behavioral characterization in MPTP/p mouse model of Parkinson’s disease. J. Integr. Neurosci. 2021, 20, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Goloborshcheva, V.; Voronina, N.; Ovchinnikov, R.; Kucheryanu, V.; Morozov, S. MPTP-induced Parkinsonism in genetically modified mice. Pathogenesis 2021, 19, 12–23. (In Russian) [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Goloborshcheva, V.V.N.; Ovchinnikov, R.; Kucheryanu, V.; Morozov, S. Morphometric analysis of dopaminergic neurons (substantia nigra) in the brain of MPTP treated alpha synuclein knockout mice. Pathogenesis 2021, 19, 32–37. (In Russian) [Google Scholar]
- Klivenyi, P.; Siwek, D.; Gardian, G.; Yang, L.; Starkov, A.; Cleren, C.; Ferrante, R.J.; Kowall, N.W.; Abeliovich, A.; Beal, M.F. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 2006, 21, 541–548. [Google Scholar] [CrossRef]
- Zharikov, A.; Bai, Q.; De Miranda, B.R.; Van Laar, A.; Greenamyre, J.T.; Burton, E.A. Long-term RNAi knockdown of alpha-synuclein in the adult rat substantia nigra without neurodegeneration. Neurobiol. Dis. 2019, 125, 146–153. [Google Scholar] [CrossRef]
- Goloborshcheva, V.V.; Chaprov, K.D.; Teterina, E.V.; Ovchinnikov, R.; Buchman, V.L. Reduced complement of dopaminergic neurons in the substantia nigra pars compacta of mice with a constitutive “low footprint” genetic knockout of alpha-synuclein. Mol. Brain 2020, 13, 75. [Google Scholar] [CrossRef]
- Lehmensiek, V.; Tan, E.M.; Schwarz, J.; Storch, A. Expression of mutant alpha-synucleins enhances dopamine transporter-mediated MPP+ toxicity in vitro. Neuroreport 2002, 13, 1279–1283. [Google Scholar] [CrossRef]
- Van Laar, V.S.; Chen, J.; Zharikov, A.D.; Bai, Q.; Di Maio, R.; Dukes, A.A.; Hastings, T.G.; Watkins, S.C.; Greenamyre, J.T.; St Croix, C.M.; et al. alpha-Synuclein amplifies cytoplasmic peroxide flux and oxidative stress provoked by mitochondrial inhibitors in CNS dopaminergic neurons in vivo. Redox. Biol. 2020, 37, 101695. [Google Scholar] [CrossRef]
- Cabin, D.E.; Shimazu, K.; Murphy, D.; Cole, N.B.; Gottschalk, W.; McIlwain, K.L.; Orrison, B.; Chen, A.; Ellis, C.E.; Paylor, R.; et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002, 22, 8797–8807. [Google Scholar] [CrossRef]
- Ninkina, N.; Tarasova, T.V.; Chaprov, K.D.; Roman, A.Y.; Kukharsky, M.S.; Kolik, L.G.; Ovchinnikov, R.; Ustyugov, A.A.; Durnev, A.D.; Buchman, V.L. Alterations in the nigrostriatal system following conditional inactivation of alpha-synuclein in neurons of adult and aging mice. Neurobiol. Aging. 2020, 91, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Ninkina, N.N.; Tarasova, T.V.; Chaprov, K.D.; Goloborshcheva, V.V.; Bachurin, S.O.; Buchman, V.L. Synuclein Deficiency Decreases the Efficiency of Dopamine Uptake by Synaptic Vesicles. Dokl. Biochem. Biophys. 2019, 486, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Connor-Robson, N.; Peters, O.M.; Millership, S.; Ninkina, N.; Buchman, V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behavior and dopamine metabolism. Neurobiol. Aging. 2016, 46, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Al-Wandi, A.; Ninkina, N.; Millership, S.; Williamson, S.J.; Jones, P.A.; Buchman, V.L. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol. Aging. 2010, 31, 796–804. [Google Scholar] [CrossRef]
- Pavia-Collado, R.; Rodriguez-Aller, R.; Alarcon-Aris, D.; Miquel-Rio, L.; Ruiz-Bronchal, E.; Paz, V.; Campa, L.; Galofre, M.; Sgambato, V.; Bortolozzi, A. Up and Down gamma-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice. Int. J. Mol. Sci. 2022, 23, 1807. [Google Scholar] [CrossRef]
- Thomas, B.; Mandir, A.S.; West, N.; Liu, Y.; Andrabi, S.A.; Stirling, W.; Dawson, V.L.; Dawson, T.M.; Lee, M.K. Resistance to MPTP-neurotoxicity in alpha-synuclein knockout mice is complemented by human alpha-synuclein and associated with increased beta-synuclein and Akt activation. PLoS ONE 2011, 6, e16706. [Google Scholar] [CrossRef]
- Hashimoto, M.; Bar-On, P.; Ho, G.; Takenouchi, T.; Rockenstein, E.; Crews, L.; Masliah, E. Beta-synuclein regulates Akt activity in neuronal cells. A possible mechanism for neuroprotection in Parkinson’s disease. J. Biol. Chem. 2004, 279, 23622–23629. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Neuron-Astrocyte Interactions in Parkinson’s Disease. Cells 2020, 9, 2623. [Google Scholar] [CrossRef]
- Gibrat, C.; Saint-Pierre, M.; Bousquet, M.; Levesque, D.; Rouillard, C.; Cicchetti, F. Differences between subacute and chronic MPTP mice models: Investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J. Neurochem. 2009, 109, 1469–1482. [Google Scholar] [CrossRef]
- Fornai, F.; Schluter, O.M.; Lenzi, P.; Gesi, M.; Ruffoli, R.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Pontarelli, F.; Battaglia, G.; et al. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 3413–3418. [Google Scholar] [CrossRef]
- Gu, X.L.; Long, C.X.; Sun, L.; Xie, C.; Lin, X.; Cai, H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol. Brain 2010, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, T.A.; Littlejohn, K.; Yuan, S.; Fernandez, C.; Cilluffo, M.; Koo, B.K.; Whitelegge, J.P.; Watson, J.B. Stimulation of synaptoneurosome glutamate release by monomeric and fibrillated alpha-synuclein. J. Neurosci. Res. 2017, 95, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hertz, E.; Zhang, X.; Leinartaite, L.; Lundius, E.G.; Li, J.; Svenningsson, P. Overexpression of alpha-synuclein simultaneously increases glutamate NMDA receptor phosphorylation and reduces glucocerebrosidase activity. Neurosci. Lett. 2016, 611, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Voronina, N.A.; Lisina, O.Y.; Krasilnikova, I.A.; Kucheryanu, V.G.; Kapitsa, I.G.; Voronina, T.A.; Surin, A.M. Influence of hemantane on changes in Ca 2+ and Na+ caused by activation of NMDA channels in cultured rat brain neurons. Neurochem. J. 2021, 15, 8–17. [Google Scholar] [CrossRef]
- Bashkatova, V. Metabotropic glutamate receptors and nitric oxide in dopaminergic neurotoxicity. World J. Psychiatry 2021, 11, 830–840. [Google Scholar] [CrossRef]
- Fairless, R.; Bading, H.; Diem, R. Pathophysiological Ionotropic Glutamate Signalling in Neuroinflammatory Disease as a Therapeutic Target. Front. Neurosci. 2021, 15, 741280. [Google Scholar] [CrossRef]
- Huls, S.; Hogen, T.; Vassallo, N.; Danzer, K.M.; Hengerer, B.; Giese, A.; Herms, J. AMPA-receptor-mediated excitatory synaptic transmission is enhanced by iron-induced alpha-synuclein oligomers. J. Neurochem. 2011, 117, 868–878. [Google Scholar] [CrossRef]
- Jensen, P.J.; Alter, B.J.; O’Malley, K.L. Alpha-synuclein protects naive but not dbcAMP-treated dopaminergic cell types from 1-methyl-4-phenylpyridinium toxicity. J. Neurochem. 2003, 86, 196–209. [Google Scholar] [CrossRef]
- Rosencrans, W.M.; Aguilella, V.M.; Rostovtseva, T.K.; Bezrukov, S.M. alpha-Synuclein emerges as a potent regulator of VDAC-facilitated calcium transport. Cell Calcium 2021, 95, 102355. [Google Scholar] [CrossRef]
| Functions | α-syn | β-syn | γ-syn | Ref. |
|---|---|---|---|---|
| Neurotransmission | ✔ | ✔ | ✔ | [22,23,24,25,26,27] |
| Chaperoning | ✔ | ✔ | ✔ | [32,33,34] |
| SNARE assembly | ✔ | Maintenance | Maintenance | [28,29,30,31] |
| DAT transporter delivery to the presynapse | ✔ | ✔ | ✔ | [11,31,35] |
| Regulation of DAT transporter activity | ✔ | NA | Maintenance | [36,37,38] |
| Regulation of dopamine homeostasis | ✔ | ? | NA | [27,39,40,41,42,43,44] |
| Potentiation of vesicular dopamine uptake | NA | ✔ | NA | [4] |
| Lipid structure or morphology changes | ✔ | ✔ | ✔ | [11,31,45] |
| Regulation of lipid metabolism | NA | NA | ✔ | [46] |
| Anti-apoptosis | ✔ | ✔ | NA | [47,48] |
| Mitochondrial regulation | ? | NA | NA | [49,50,51,52] |
| Regulation of cellular metal homeostasis | NA | ✔ | NA | [11,53,54] |
| Regulation of the autophagic–lysosomal pathway | NA | ? | ✔ | [55,56,57] |
| Interaction with proteasomes | ✔ | ✔ | ✔ | [58,59,60] |
| Cytoskeleton stabilization | ? | NA | ✔ | [61,62,63] |
| Regulation of the growth of neurons in SNpc | ✔ | NA | NA | [64] |
| Regeneration of damaged neurons | ? | ? | ? | [65,66] |
| Effect | MPTP * | α-syn KO | β-syn KO | γ-syn KO | αβγ-syn KO |
|---|---|---|---|---|---|
| Clinical manifestation | – | ≈ | ≈ | ≈ | ≈ |
| + | ✔ | NA | NA | ✖ | |
| Striatal dopamine | – | ≈ | ▼ | ≈ | ▼ |
| + | ▼ | ▼ | NA | ▼ | |
| DAT expression | – | ▼ | NA | NA | NA |
| + | NA | NA | NA | NA | |
| SNpc neurons | – | ▼ | ≈ | ▼ | ≈ |
| + | resistant | ▼ | resistant | ▼ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goloborshcheva, V.V.; Kucheryanu, V.G.; Voronina, N.A.; Teterina, E.V.; Ustyugov, A.A.; Morozov, S.G. Synuclein Proteins in MPTP-Induced Death of Substantia Nigra Pars Compacta Dopaminergic Neurons. Biomedicines 2022, 10, 2278. https://doi.org/10.3390/biomedicines10092278
Goloborshcheva VV, Kucheryanu VG, Voronina NA, Teterina EV, Ustyugov AA, Morozov SG. Synuclein Proteins in MPTP-Induced Death of Substantia Nigra Pars Compacta Dopaminergic Neurons. Biomedicines. 2022; 10(9):2278. https://doi.org/10.3390/biomedicines10092278
Chicago/Turabian StyleGoloborshcheva, Valeria V., Valerian G. Kucheryanu, Natalia A. Voronina, Ekaterina V. Teterina, Aleksey A. Ustyugov, and Sergei G. Morozov. 2022. "Synuclein Proteins in MPTP-Induced Death of Substantia Nigra Pars Compacta Dopaminergic Neurons" Biomedicines 10, no. 9: 2278. https://doi.org/10.3390/biomedicines10092278
APA StyleGoloborshcheva, V. V., Kucheryanu, V. G., Voronina, N. A., Teterina, E. V., Ustyugov, A. A., & Morozov, S. G. (2022). Synuclein Proteins in MPTP-Induced Death of Substantia Nigra Pars Compacta Dopaminergic Neurons. Biomedicines, 10(9), 2278. https://doi.org/10.3390/biomedicines10092278






