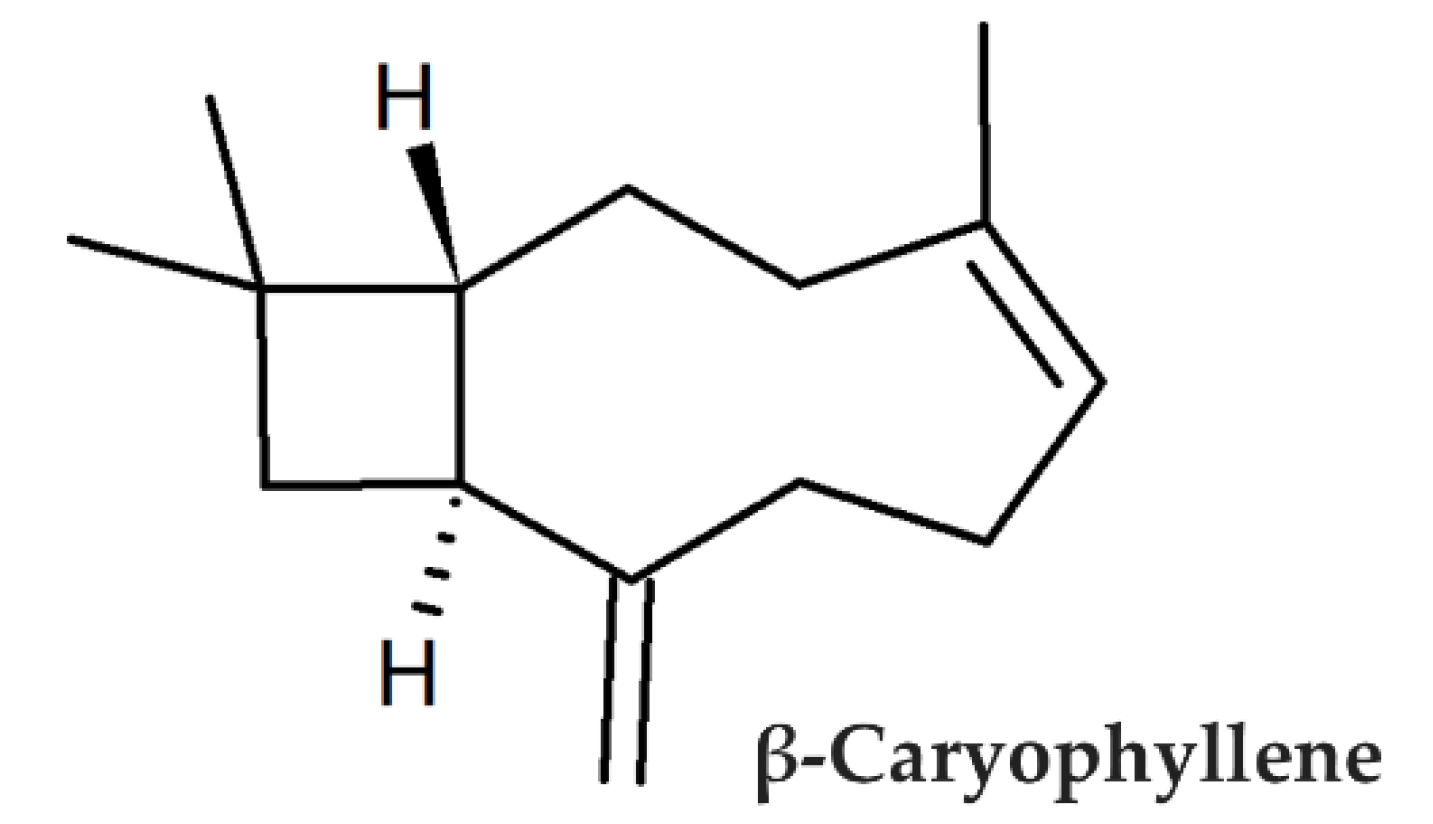
β-Caryophyllene Counteracts Chemoresistance Induced by Cigarette Smoke in Triple-Negative Breast Cancer MDA-MB-468 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Chemical Analysis of Cigarette Smoke Condensate (CSC)
2.2.1. Solid-Phase Micro Extraction (SPME)
2.2.2. Gas Chromatography and Mass Spectrometry (GC-MS) Analysis of CSC
2.3. Cell Line
2.4. Trypan Blue Exclusion Test of Cell Viability
2.5. Cytotoxicity Assay
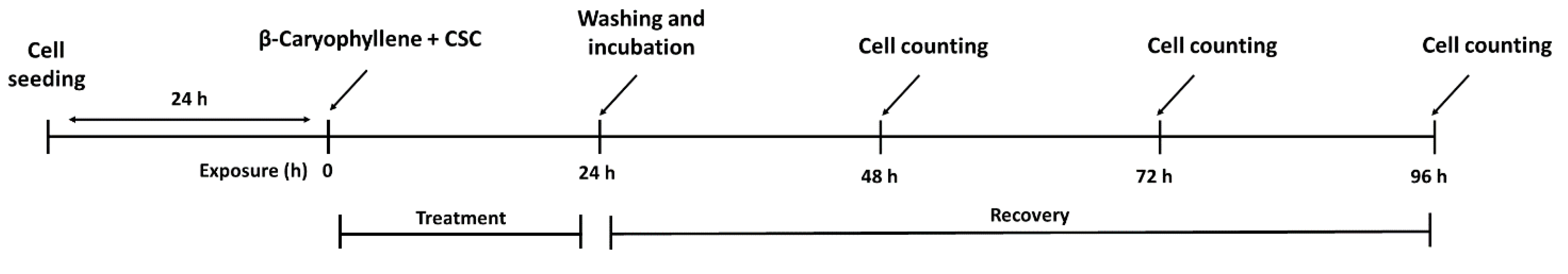
2.6. Treatment Schedules Applied to Evaluate the Chemopreventive Properties of β-Caryophyllene towards CSC
2.7. Determination of Lactate Dehydrogenase (LDH) Release
2.8. Intracellular Reactive Oxygen Species (ROS) Determination
2.9. Determination of Intracellular Glutathione Levels
2.10. Detection of Phosphorylated Histone H2AX (γH2AX)
2.11. Cell Cycle Analysis
2.12. Apoptosis Detection
2.13. Autophagy Detection
2.14. Immunofluorescence Analysis of Autophagy Factors LC3 and Beclin-1
2.15. Cell Migration through Wound Healing Assay
2.16. RT-qPCR Analysis of Gene Expression of Cell Migration Factors
2.17. Western Blotting Analysis of STAT3
2.18. Statistical Analysis
3. Results
3.1. Volatile Chemical Profile of Cigarette Smoke Condensate (CSC)
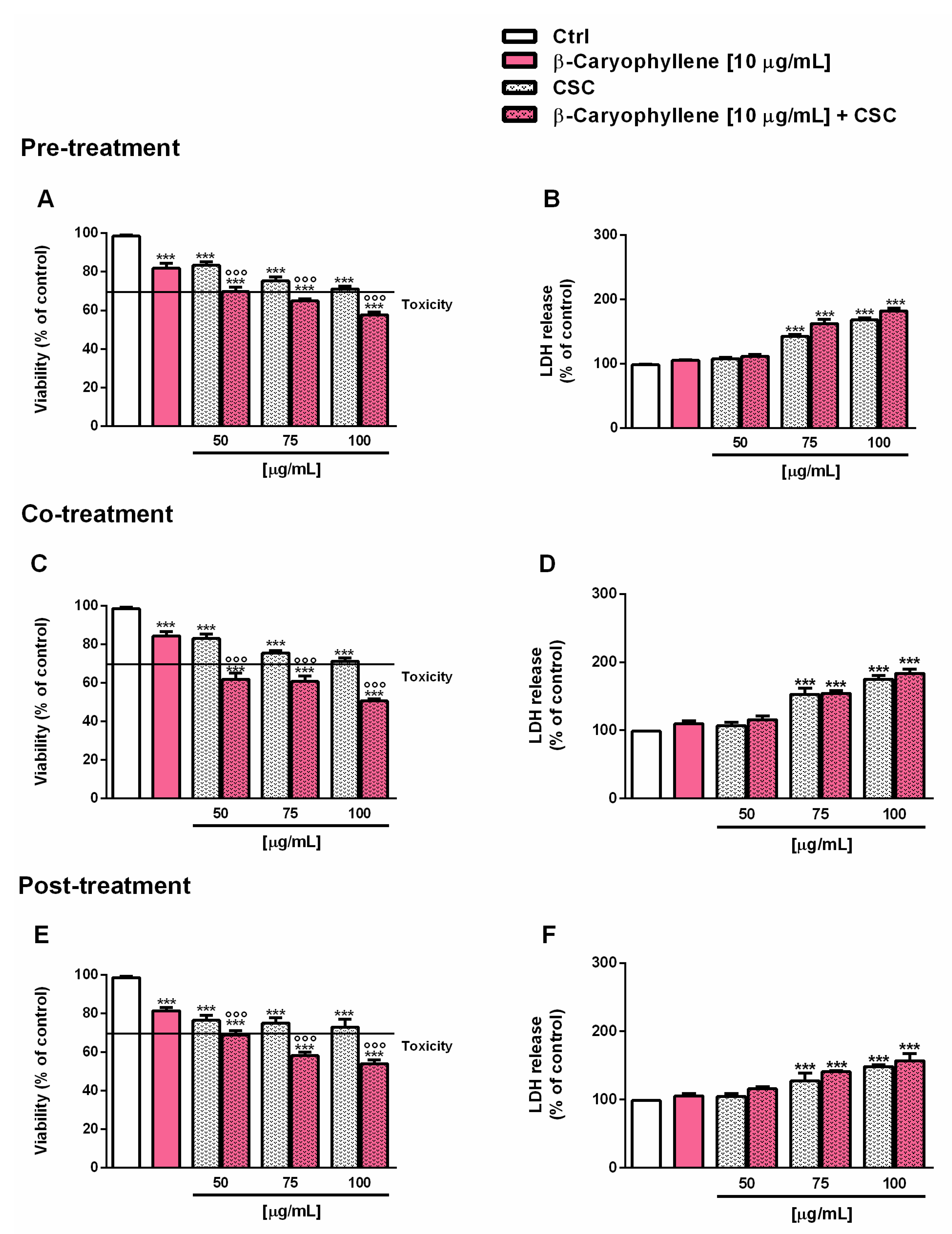
3.2. Effects of β-Caryophyllene and Cigarette Smoke Condensate (CSC) on Viability and Recovery of Triple Negative Breast Cancer MDA-MB-468 Cells
3.3. Effects of β-Caryophyllene and Cigarette Smoke Condensate (CSC) on Intracellular Oxidative Stress
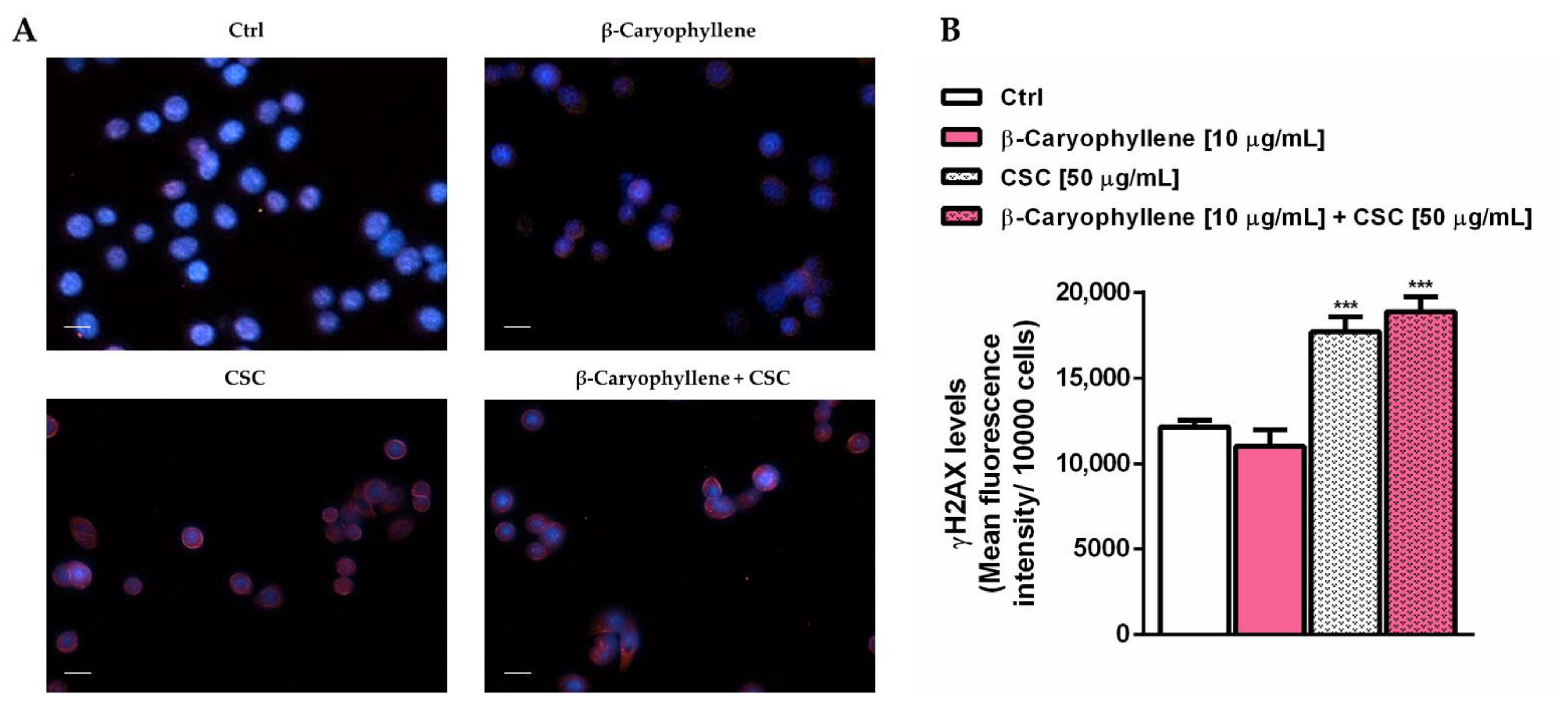
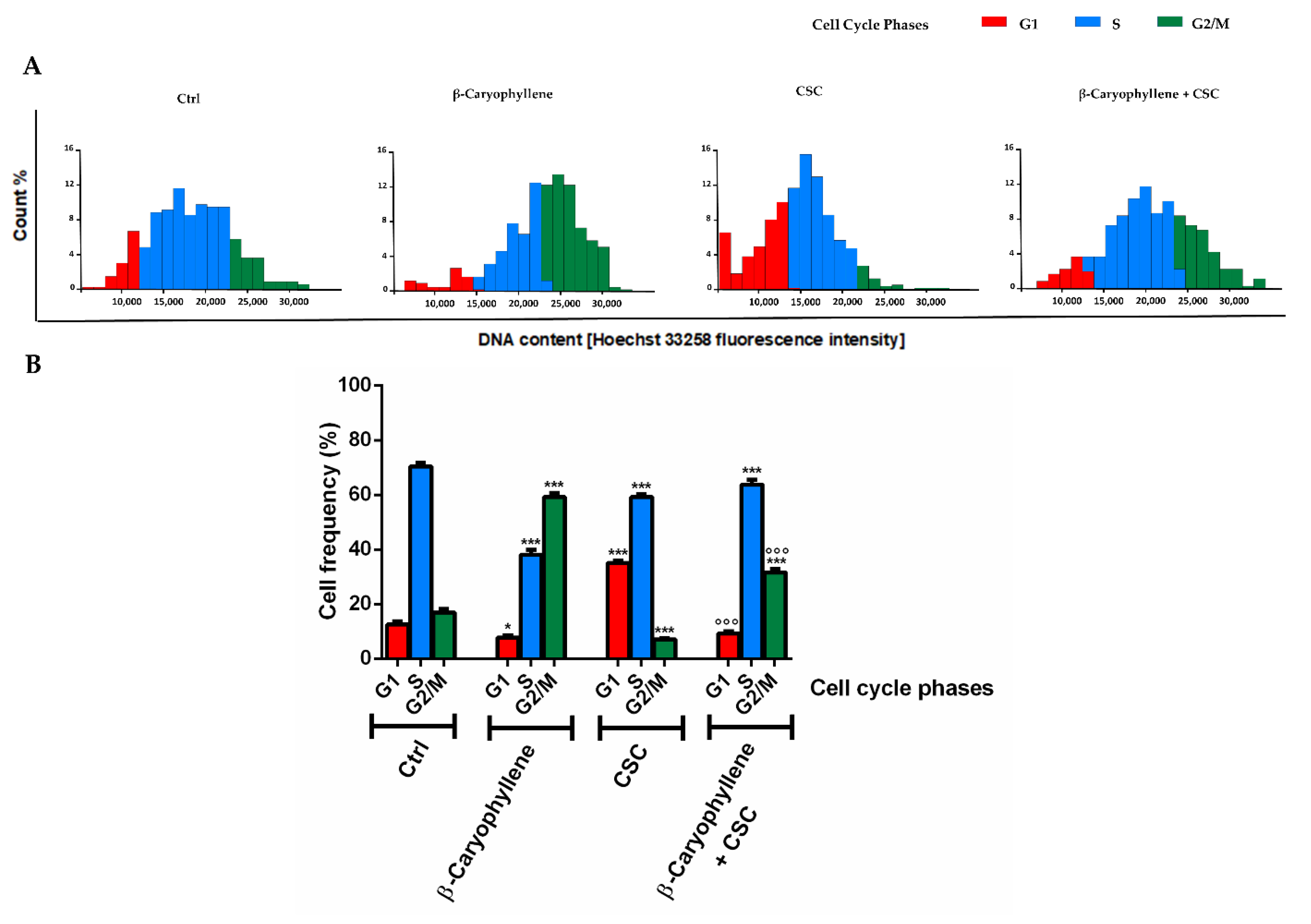
3.4. Effects of β-Caryophyllene and Cigarette Smoke Condensate (CSC) on H2AX Histone Phosphorylation and Cell Cycle Modulation
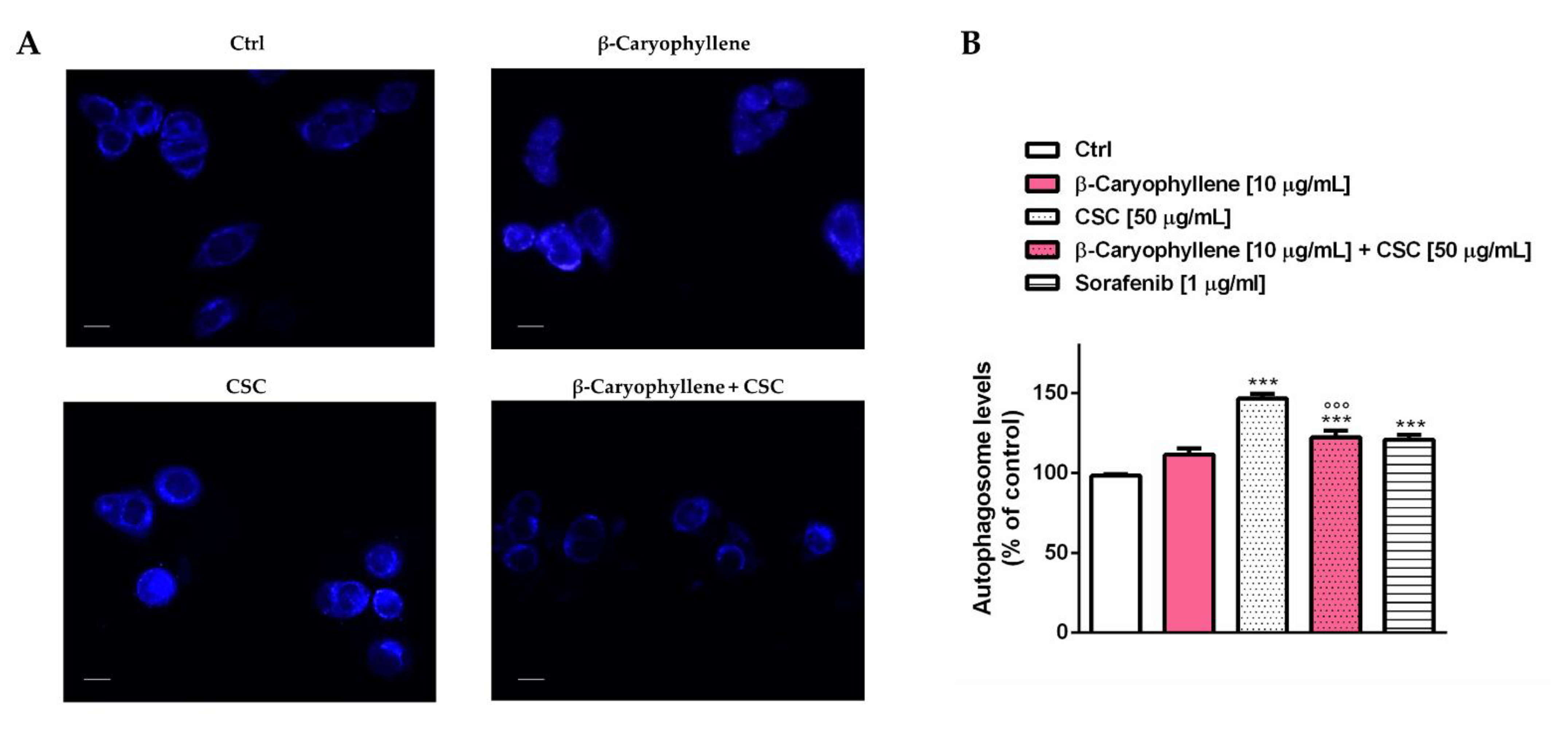
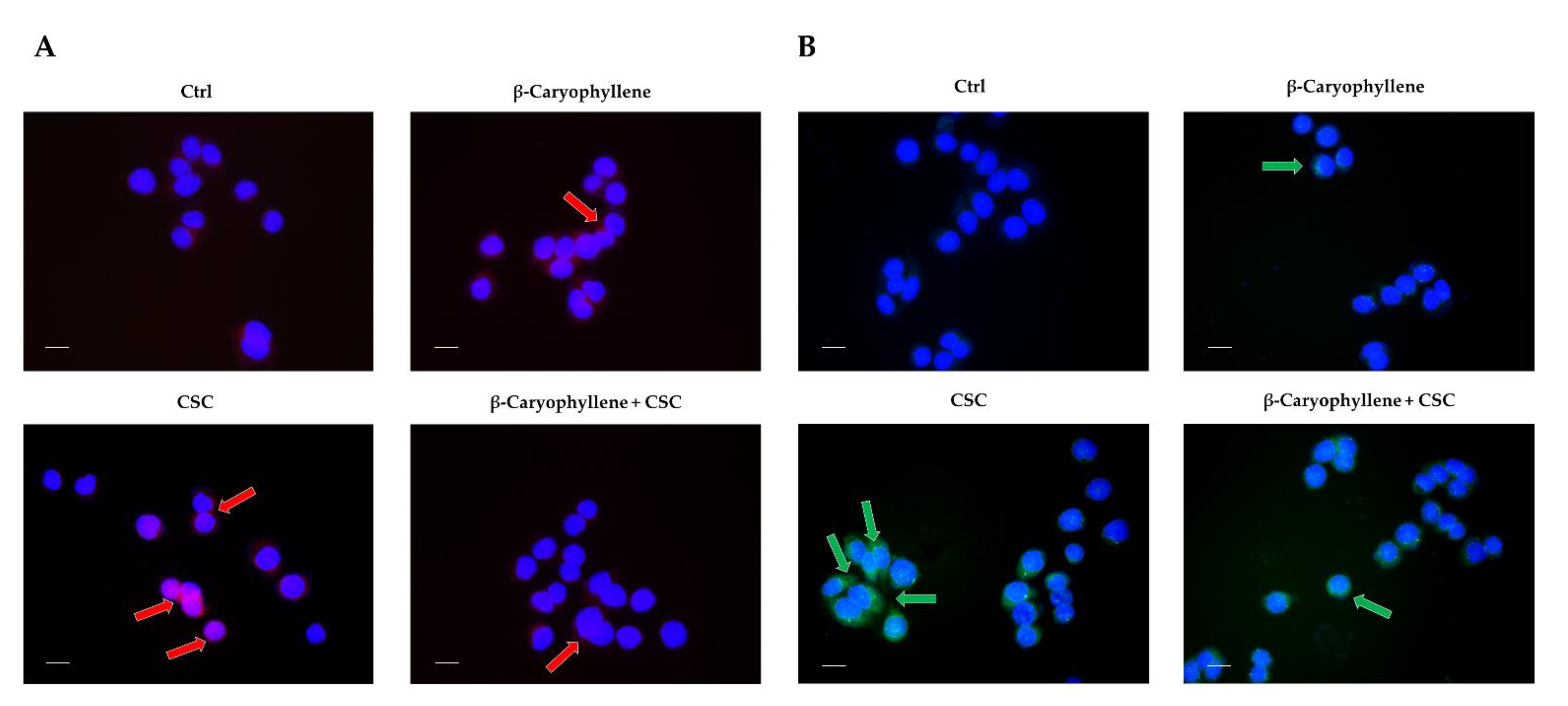
3.5. Effects of β-Caryophyllene and Cigarette Smoke Condensate (CSC) on Apoptosis and Autophagic Cell Death
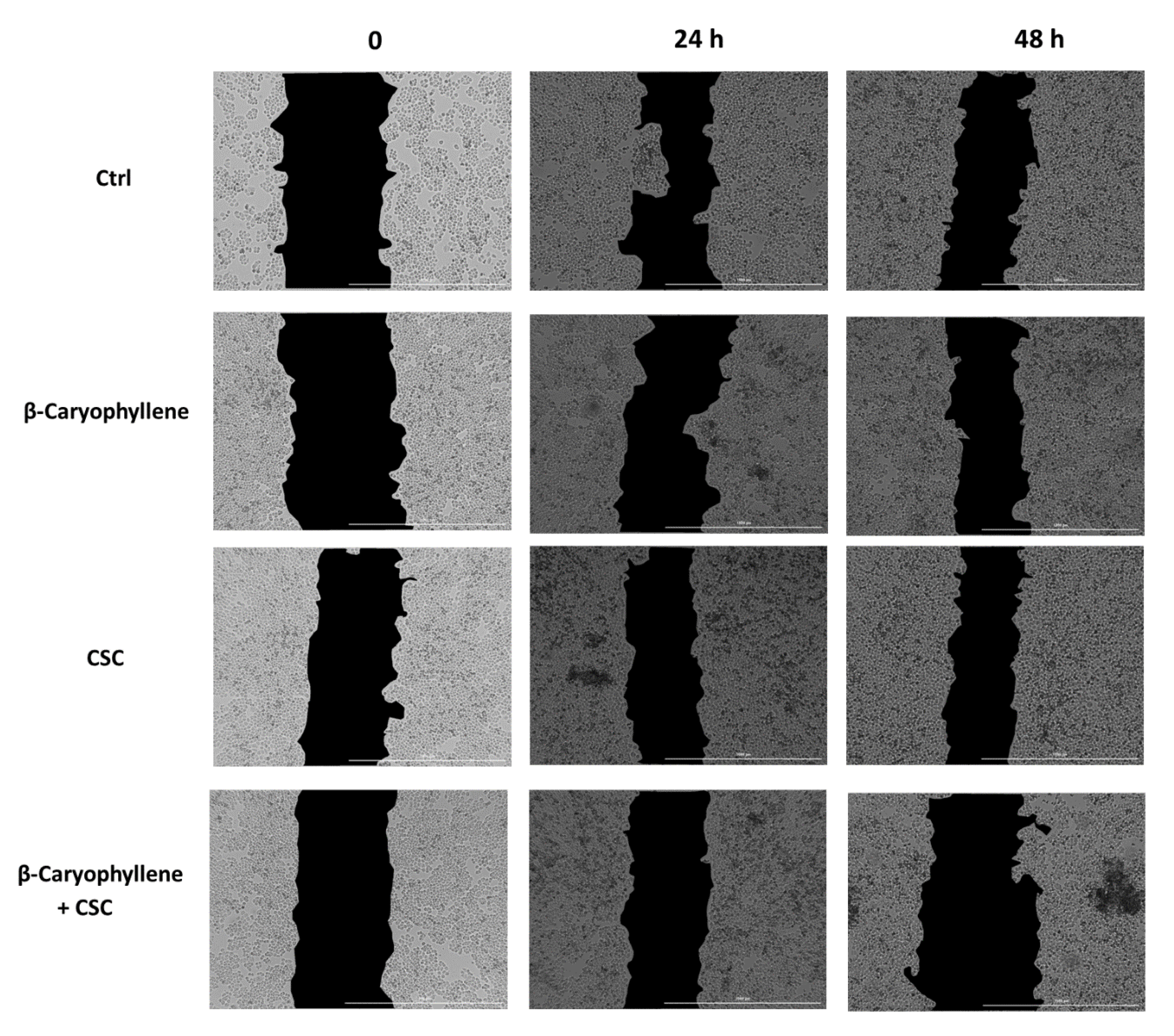
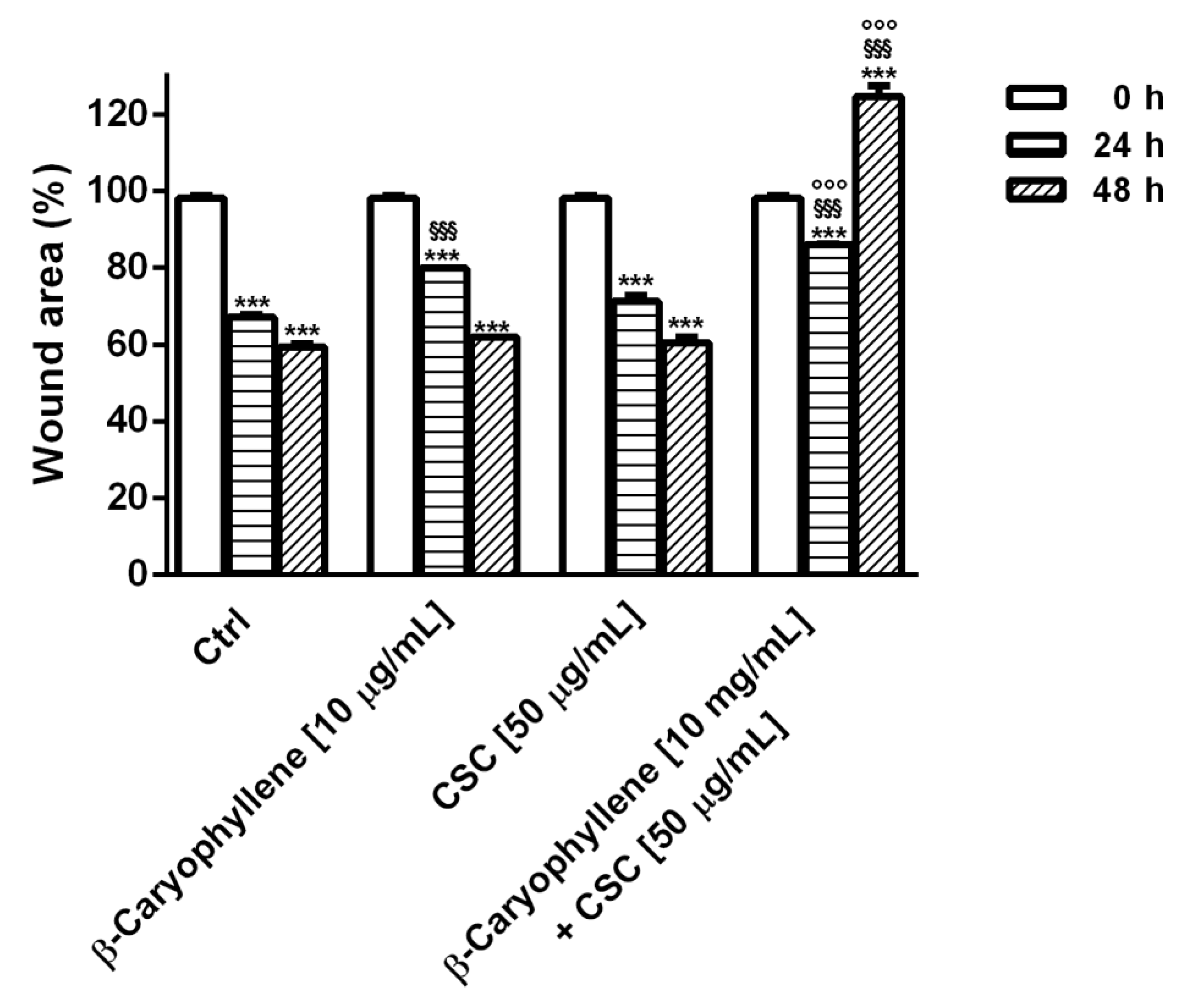
3.6. Effects of β-Caryophyllene and CSC on Cell Migration and on Cell Migration Factors
3.7. Modulation of STAT3 Activation
4. Discussion
5. Strengths, Limits and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mladěnka, P.; Applová, L.; Patočka, J.; Costa, V.M.; Remiao, F.; Pourová, J.; Mladěnka, A.; Karlíčková, J.; Jahodář, L.; Vopršalová, M.; et al. Tox-oer and Cardiotox Hradec Králové researchers and collaborators. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev. 2018, 38, 1332–1403. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A.; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef] [PubMed]
- Koual, M.; Tomkiewicz, C.; Cano-Sancho, G.; Antignac, J.P.; Bats, A.S.; Coumoul, X. Environmental chemicals, breast cancer progression and drug resistance. Environ. Health 2020, 19, 117. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Chen, G.G. Cigarette smoking, cyclooxygenase-2 pathway and cancer. Biochim. Biophys. Acta Rev. Cancer 2011, 1815, 158–169. [Google Scholar] [CrossRef]
- Lauseker, M.; Hasford, J.; Saussele, S.; Kremers, S.; Kraemer, D.; Lindemann, W.; Helhmann, R.; Pfirrmann, M. Smokers with chronic myeloid leukemia are at a higher risk of disease progression and premature death. Cancer 2017, 123, 2467–2471. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Cortijo, J. Tobacco, inflammation, and respiratory tract cancer. Curr. Pharm. Des. 2012, 18, 3901–3938. [Google Scholar] [CrossRef]
- Warren, G.W.; Cummings, K.M. Tobacco and lung cancer: Risks, trends, and outcomes in patients with cancer. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 359–364. [Google Scholar] [CrossRef]
- Ochieng, J.; Nangami, G.N.; Ogunkua, O.; Miousse, I.R.; Koturbash, I.; Odero-Marah, V.; McCawley, L.J.; Nangia-Makker, P.; Ahmed, N.; Luqmani, Y.; et al. The impact of low-dose carcinogens and environmental disruptors on tissue invasion and metastasis. Carcinogenesis 2015, 36, 128–159. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Liu, W.; Schiöth, H.B. Can Exposure to Environmental Pollutants Be Associated with Less Effective Chemotherapy in Cancer Patients? Int. J. Environ. Res. Public Health 2022, 19, 2064. [Google Scholar] [CrossRef]
- Calle, E.E.; Miracle-McMahill, H.L.; Thun, M.J.; Heath, C.W. Cigarette smoking and risk of fatal breast cancer. Am. J. Epidemiol. 1994, 139, 1001–1007. [Google Scholar] [CrossRef]
- Manjer, J.; Andersson, I.; Berglund, G.; Bondesson, L.; Garne, J.P.; Janzon, L.; Malina, J.; Matson, S. Survival of women with breast cancer in relation to smoking. Eur. J. Surg. Acta Chir. 2000, 166, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.N.; Newcomb, P.A.; Hampton, J.M.; Trentham-Dietz, A.; Titus, L.J.; Egan, K.M.; Baron, J.A.; Willett, W.C. Cigarette smoking before and after breast cancer diagnosis: Mortality from breast cancer and smoking-related diseases. J. Clin. Oncol. 2016, 34, 1315. [Google Scholar] [CrossRef] [PubMed]
- Alkhaifi, M.; Clayton, A.; Kishibe, T.; Simpson, J.S. The association between smoking status and breast cancer recurrence: A systematic review. J. Breast Cancer 2022, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.H.; Li, Z.; Shi, J.; Li, H.M.; Wang, Y.; Fu, L.Y.; Liu, Y.P. Passive smoking exposure from partners as a risk factor for ER+/PR+ double positive breast cancer in never-smoking chinese urban women: A hospital-based matched case control study. PLoS ONE 2014, 9, e97498. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Lu, M.S.; Mo, X.F.; Lin, F.Y.; Ho, S.C.; Zhang, C.X. Passive smoking and breast cancer risk among non-Smoking women: A case-control study in China. PLoS ONE 2015, 10, e0125894. [Google Scholar]
- White, A.J.; D’Aloisio, A.A.; Nichols, H.B.; DeRoo, L.A.; Sandler, D.P. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control 2017, 7, 667–675. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Cheung, W.Y. Impact of smoking history on the outcomes of women with early-stage breast cancer: A secondary analysis of a randomized study. Med. Oncol. 2018, 35, 68. [Google Scholar] [CrossRef]
- Pham, K.; DeFina, S.; Wang, H. E-Cigarettes promote macrophage-tumor cells crosstalk: Focus on breast carcinoma progression and lung metastasis. Explor. Res. Hypothesis Med. 2021, 6, 60–66. [Google Scholar] [CrossRef]
- Kispert, S.; McHowat, J. Recent insights into cigarette smoking as a lifestyle risk factor for breast cancer. Breast Cancer 2017, 9, 127–132. [Google Scholar] [CrossRef]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef]
- Türker Şener, L.; Güven, C.; Şener, A.; Adin Çinar, S.; Solakoğlu, S.; Albeniz, I. Nicotine reduces effectiveness of doxorubicin chemotherapy and promotes CD44+CD24-cancer stem cells in MCF-7 cell populations. Exp. Ther. Med. 2018, 16, 21–28. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Kiang, A.; Lopez, J.P.; Kuo, S.Z.; Yu, M.A.; Abhold, E.L.; Chen, J.S.; Wang-Rodriguez, J.; Ongkeko, W.M. Cigarette smoke promotes drug resistance and expansion of cancer stem cell-like side population. PLoS ONE 2012, 7, e47919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kamdar, O.; Le, W.; Rosen, G.D.; Upadhyay, D. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am. J. Respir. Cell Mol. Biol. 2009, 40, 135–146. [Google Scholar] [CrossRef]
- Simon, V.; Laot, L.; Laas, E.; Rozette, S.; Guerin, J.; Balezeau, T.; Nicolas, M.; Pierga, J.Y.; Coussy, F.; Laé, M.; et al. No impact of smoking status on breast cancer tumor infiltrating lymphocytes, response to neoadjuvant chemotherapy and prognosis. Cancers 2020, 12, 2943. [Google Scholar] [CrossRef]
- Condoluci, A.; Mazzara, C.; Zoccoli, A.; Pezzuto, A.; Tonini, G. Impact of smoking on lung cancer treatment effectiveness: A review. Future Oncol. 2016, 12, 2149–2161. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mancinelli, R.; Gullì, M.; Eufemi, M.; Mammola, C.L.; Mazzanti, G.; Di Giacomo, S. Chemopreventive potential of caryophyllane sesquiterpenes: An overview of preliminary evidence. Cancers 2020, 12, 3034. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; De Censi, A. Alternate dosing schedules for cancer chemopreventive agents. Semin. Oncol. 2016, 43, 116. [Google Scholar] [CrossRef]
- Van Schooten, F.J.; Besaratinia, A.; De Flora, S.; D’Agostini, F.; Izzotti, A.; Camoirano, A.; Balm, A.J.; Dallinga, J.W.; Bast, A.; Haenen, G.R.; et al. Effects of oral administration of N-acetyl-L-cysteine: A multi-biomarker study in smokers. Cancer Epidemiol. Biom. PRev. 2002, 11, 167–175. [Google Scholar]
- Gilman, E.A.; Pruthi, S.; Hofstatter, E.W.; Mussallem, D.M. Preventing Breast Cancer Through Identification and Pharmacologic Management of High-Risk Patients. Mayo Clin. Proc. 2021, 96, 1033–1040. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Mariano, A.; Gullì, M.; Fraschetti, C.; Vitalone, A.; Filippi, A.; Mannina, L.; Scotto d’Abusco, A.; Di Sotto, A. Role of caryophyllane sesquiterpenes in the entourage effect of Felina 32 hemp inflorescence phytocomplex in triple negative MDA-MB-468 breast cancer cells. Molecules 2021, 26, 6688. [Google Scholar] [CrossRef]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC Liposomes as possible delivery systems for improving bioavailability of the natural sesquiterpene β-Caryophyllene: Lamellarity and drug-loading as key featuRes. for a rational drug delivery design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gullì, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 signaling, cell redox defenses and cell cycle checkpoints by β-Caryophyllene in cholangiocarcinoma cells: Possible mechanisms accounting for Doxorubicin chemosensitization and chemoprevention. Cells 2020, 9, 858. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Abete, L.; Cocchiola, R.; Mazzanti, G.; Eufemi, M.; Di Sotto, A. Caryophyllane sesquiterpenes inhibit DNA-damage by tobacco smoke in bacterial and mammalian cells. Food Chem. Toxicol. 2018, 111, 393–404. [Google Scholar] [CrossRef]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Thu, K.L.; Soria-Bretones, I.; Mak, T.W.; Cescon, D.W. Targeting the cell cycle in breast cancer: Towards the next phase. Cell Cycle 2018, 17, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Wu, A.G.; Huang, Y.Z.; Shao, G.L.; Ji, S.F.; Wang, R.W.; Yuan, H.J.; Fan, X.L.; Zheng, L.H.; Jiao, Q.L. Autophagic regulation of cell growth by altered expression of BeClin. 1 in triple-negative breast cancer. Int. J. Clin. Exp. Med. 2015, 8, 7049–7058. [Google Scholar] [PubMed]
- Maycotte, P.; Gearheart, C.M.; Barnard, R.; Aryal, S.; Mulcahy Levy, J.M.; Fosmire, S.P.; Hansen, R.J.; Morgan, M.J.; Porter, C.C.; Gustafson, D.L.; et al. STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Cancer Res. 2014, 74, 2579–2590. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Vinciguerra, V.; Garzoli, S. A comparative study of the chemical composition by SPME-GC/MS and antiradical activity of less common Citrus species. Molecules 2021, 26, 5378. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- ISO 10993–5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. 2nd ed. International Organization for Standardization/ANSI: Geneva, Switzerland, 2009.
- Di Giacomo, S.; Gullì, M.; Facchinetti, R.; Minacori, M.; Mancinelli, R.; Percaccio, E.; Scuderi, C.; Eufemi, M.; Di Sotto, A. Sorafenib chemosensitization by caryophyllane sesquiterpenes in liver, biliary, and pancreatic cancer cells: The role of STAT3/ABC transporter axis. Pharmaceutics 2022, 14, 1264. [Google Scholar] [CrossRef]
- Roukos, V.; Pegoraro, G.; Voss, T.C.; Misteli, T. Cell cycle staging of individual cells by fluorescence microscopy. Nat. Protoc. 2015, 10, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Held, P.; 2018 Monitoring Cell Cycle Progression in Cancer Cells. Using Nuclear Staining to Assess Cellular DNA Content. Available online: https://www.biotek.com/resources/application-notes/monitoring-cell-cycle-progression-in-cancer-cells/ (accessed on 14 July 2022).
- Wu, N.; Meng, F.; Invernizzi, P.; Bernuzzi, F.; Venter, J.; Standeford, H.; Onori, P.; Marzioni, M.; Alvaro, D.; Franchitto, A.; et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-β1 biliary secretion in mice. Hepatology 2016, 64, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Rubini, E.; Paglia, G.; Cannella, D.; Macone, A.; Di Sotto, A.; Gullì, M.; Altieri, F.; Eufemi, M. β-Hexachlorocyclohexane: A small molecule with a big impact on human cellular biochemistry. Biomedicines 2020, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Mazzanti, G.; Di Sotto, A. Mutagenicity of cigarette butt waste in the bacterial reverse mutation assay: The protective effects of β-caryophyllene and β-caryophyllene oxide. Environ. Toxicol. 2016, 31, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.M.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar]
- Wang, H.Q.; Man, Q.W.; Huo, F.Y.; Gao, X.; Lin, H.; Li, S.R.; Wang, J.; Su, F.C.; Cai, L.; Shi, Y.; et al. STAT3 pathway in cancers: Past, present, and future. Med. Comm. 2022, 3, 124. [Google Scholar] [CrossRef]
- Molina-Jasso, D.; Alvarez-González, I.; Madrigal-Bujaidar, E. Clastogenicity of beta-caryophyllene in mouse. Biol. Pharm. Bull. 2009, 32, 520–522. [Google Scholar] [CrossRef][Green Version]
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Inhibition by beta-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat Res. 2010, 699, 23–28. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoking; International Agency for Research on Cancer: Lyon, France, 1986; Volume 38. [Google Scholar]
- Wynder, E.L.; Hofmann, D. A study of tobacco carcinogenesis. VII. The role of higher polycyclic hydrocarbons. Cancer 1959, 12, 1079–1086. [Google Scholar] [CrossRef]
- Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Hristeva, T.; Zheljazkov, V.D. GC-MS Composition and olfactory profile of concretes from the flowers of four Nicotiana species. Molecules 2020, 25, 2617. [Google Scholar] [CrossRef]
- Guerin, M.R.; Olerich, G. Gas chromatographic determination of neuphytadiene as a measure of the terpenoid contribution to experimental tobacco smoke carcinogenesis. Environ. Lett. 1975, 10, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Leffingwell, J. Leaf Chemistry. Basic chemical constituents of tobacco leaf and differences among tobacco types. In Tobacco: Production, Chemistry, and Technology; Wiley: Hoboken, NJ, USA, 1999; pp. 265–284. [Google Scholar]
- Berenguer, C.; Pereira, J.; Câmara, J. Fingerprinting the volatile profile of traditional tobacco and e-cigarettes: A comparative study. Microchem. J. 2021, 166, 106196. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria ornata suppresses LPS-Induced inflammatory response in RAW 264.7 macrophages and sprague dawley rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, M.V.; Doran, K.A. Nicotine content and delivery across tobacco products. Handb. Exp. Pharmacol. 2009, 192, 61–82. [Google Scholar]
- Khodabandeh, Z.; Valilo, M.; Velaei, K.; Pirpour Tazehkand, A. The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy. Breast Cancer 2022, 29, 778–789. [Google Scholar] [CrossRef]
- Konno, S.; Chiao, J.W.; Wu, J.M. Effects of nicotine on cellular proliferation, cell cyclephase distribution, and macromolecular synthesis in human promyelocytic HL-60 leukemia cells. Cancer Lett. 1986, 33, 91–97. [Google Scholar] [CrossRef]
- Moldoveanu, S.; Coleman, W.I.; Kulshreshtha, N. Evaluation of the effect of phytol on the formation of PAHs in cigarette smoke. Contrib. Tob. Res. 2010, 24, 10–23. [Google Scholar] [CrossRef][Green Version]
- Graves, B.M.; Johnson, T.J.; Nishida, R.T.; Dias, R.P.; Savareear, B.; Harynuk, J.J.; Kazemimanesh, M.; Olfert, J.S.; Boies, A.M. Comprehensive characterization of mainstream marijuana and tobacco smoke. Sci. Rep. 2020, 10, 7160. [Google Scholar] [CrossRef]
- Ginzkey, C.; Stueber, T.; Friehs, G.; Koehler, C.; Hackenberg, S.; Richter, E.; Hagen, R.; Kleinsasser, N.H. Analysis of nicotine-induced DNA damage in cells of the human respiratory tract. Toxicol. Lett. 2012, 208, 23–29. [Google Scholar] [CrossRef]
- Kim, Y.H.; An, Y.J.; Jo, S.; Lee, S.H.; Lee, S.J.; Choi, S.J.; Lee, K. Comparison of volatile organic compounds between cigarette smoke condensate (CSC) and extract (CSE) samples. Environ. Health Tox 2018, 33, e2018012. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene: What are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef] [PubMed]
- Tso, T.C. Preventive medicine welcomes inquiries which are of general interest to its readers: “Terpenes” in Tobacco. Prev. Med. 1974, 3, 294. [Google Scholar] [CrossRef]
- Gomes-Carneiro, M.R.; Viana, M.E.; Felzenszwalb, I.; Paumgartten, F.J. Evaluation of beta-myrcene, alpha-terpinene and (+)- and (-)-alpha-pinene in the Salmonella/microsome assay. Food Chem. Toxicol. 2005, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zamith, H.P.; Vidal, M.N.; Speit, G.; Paumgartten, F.J. Absence of genotoxic activity of beta-myrcene in the in vivo cytogenetic bone marrow assay. Braz. J. Med. Biol. Res. 1993, 26, 93–98. [Google Scholar]
- Mitić-Culafić, D.; Zegura, B.; Nikolić, B.; Vuković-Gacić, B.; Knezević-Vukcević, J.; Filipic, M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem. Toxicol. 2009, 47, 260–266. [Google Scholar] [CrossRef]
- Orlando, J.B.; Silva, B.O.; Pires-Cunha, C.L.; Hiruma-Lima, C.A.; Gaivão, I.O.M.; Maistro, E.L. Genotoxic effects induced by beta-myrcene following metabolism by liver HepG2/C3A human cells. J. Toxicol. Environ. Health A 2019, 82, 176–185. [Google Scholar] [CrossRef]
- Romero-Estrada, A.; Maldonado, M.A.; González-Christen, J.; Marquina, B.S.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complement Altern. Med. 2016, 16, 422. [Google Scholar] [CrossRef]
- Albino, A.P.; Huang, X.; Jorgensen, E.; Yang, J.; Gietl, D.; Traganos, F.; Darzynkiewicz, Z. Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: A new assay for carcinogens. Cell Cycle 2004, 3, 1062–1068. [Google Scholar] [CrossRef]
- Moktar, A.; Singh, R.; Vadhanam, M.V.; Ravoori, S.; Lillard, J.W.; Gairola, C.G.; Gupta, R.C. Cigarette smoke condensate-induced oxidative DNA damage and its removal in human cervical cancer cells. Int. J. Oncol. 2011, 39, 941–947. [Google Scholar]
- Huang, J.; Okuka, M.; Lu, W.; Tsibris, J.C.; McLean, M.P.; Keefe, D.L.; Liu, L. Telomere shortening and DNA damage of embryonic stem cells induced by cigarette smoke. Reprod. Tox 2013, 35, 89–95. [Google Scholar] [CrossRef]
- Chai, X.M.; Li, Y.L.; Chen, H.; Guo, S.L.; Shui, L.L.; Chen, Y.J. Cigarette smoke extract alters the cell cycle via the phospholipid transfer protein/transforming growth factor-β1/CyclinD1/CDK4 pathway. Eur. J. Pharmacol. 2016, 786, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Krayzler, E.; Nagler, R.M. Cigarette smoke-induced effects on the cell cycle in oral cancer cells: Reduction of G2/M fraction. Cancer Genom. Proteom. 2015, 12, 73–76. [Google Scholar]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant properties dedicated to nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef]
- Horiyama, S.; Hatai, M.; Takahashi, Y.; Date, S.; Masujima, T.; Honda, C.; Ichikawa, A.; Yoshikawa, N.; Nakamura, K.; Kunitomo, M.; et al. Intracellular metabolism of α,β-unsaturated carbonyl compounds, acrolein, crotonaldehyde and methyl vinyl ketone, active toxicants in cigarette smoke: Participation of glutathione conjugation ability and aldehyde-ketone sensitive reductase activity. Chem. Pharm. Bull. 2016, 64, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the potential role of glutathione in multiple forms of cell death in cancer therapy. Oxid. Med. Cell Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef] [PubMed]
- Maycotte, P.; Jones, K.L.; Goodall, M.L.; Thorburn, J.; Thorburn, A. Autophagy supports breast cancer stem cell maintenance by regulating IL6 secretion. Mol. Cancer Res. 2015, 13, 651–658. [Google Scholar] [CrossRef]
- Babaeim, G.; Aziz, S.G.; Jaghi, N.Z.Z. EMT, cancer stem cells and autophagy; the three main axes of metastasis. BioMed. Pharmacother. 2021, 133, 110909. [Google Scholar] [CrossRef]
- Sun, K.; Deng, W.; Zhang, S.; Cai, N.; Jiao, S.; Song, J.; Wei, L. Paradoxical roles of autophagy in different stages of tumorigenesis: Protector for normal or cancer cells. Cell BioSci. 2013, 3, 35. [Google Scholar] [CrossRef]
- Hasan, A.; Rizvi, S.F.; Parveen, S.; Pathak, N.; Nazir, A.; Mir, S.S. Crosstalk between ROS and autophagy in tumorigenesis: Understanding the multifaceted paradox. Front. Oncol. 2022, 12, 852424. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y. Life and death partners in post-PCI restenosis: Apoptosis, autophagy, and the Cross-talk between them. Curr. Drug Targets 2018, 19, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Gannon, A.M.; Stämpfli, M.R.; Foster, W.G. Cigarette smoke exposure elicits increased autophagy and dysregulation of mitochondrial dynamics in murine granulosa cells. Biol. Reprod. 2013, 88, 63. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.F.; Al-Zoubi, M.S.; Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Lamb, R.; Hulit, J.; Howell, A.; Gandara, R.; Sartini, M.; Galbiati, F.; et al. Cigarette smoke metabolically promotes cancer, via autophagy and premature aging in the host stromal microenvironment. Cell Cycle 2013, 12, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene inhibits cell proliferation through a direct modulation of CB2 receptors in glioblastoma cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and tumorigenesis: Molecular mechanisms and therapeutic strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef]
- Xie, T.X.; Wei, D.; Liu, M.; Gao, A.C.; Ali-Osman, F.; Sawaya, R.; Huang, S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 2004, 23, 3550–3560. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Fan, Y.; Xu, Q.; Ji, W.; Tian, R.; Niu, R. Elevated STAT3 signaling-mediated upregulation of MMP-2/9 confers enhanced invasion ability in multidrug-resistant breast cancer cells. Int. J. Mol. Sci. 2015, 16, 24772–24790. [Google Scholar] [CrossRef]
- Miwa, T.; Kokuryo, T.; Yokoyama, Y.; Yamaguchi, J.; Nagino, M. Therapeutic potential of targeting protein for Xklp2 silencing for pancreatic cancer. Cancer Med. 2015, 4, 1091–1100. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.P.; Shen, N.; Yu, X.C.; Li, J.B.; Song, Q.; Zhang, J.H. TPX2 promotes migration and invasion of human breast cancer cells. Asian Pac. J. Trop Med. 2015, 8, 1064–1070. [Google Scholar] [CrossRef]
- Deng, F.; Weng, Y.; Li, X.; Wang, T.; Fan, M.; Shi, Q. Overexpression of IL-8 promotes cell migration via PI3K-Akt signaling pathway and EMT in triple-negative breast cancer. Pathol. Res. Pract. 2020, 216, 152902. [Google Scholar] [CrossRef]
- Singh, J.K.; Simões, B.M.; Howell, S.J.; Farnie, G.; Clarke, R.B. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Cocchiola, R.; Grillo, C.; Altieri, F.; Chichiarelli, S.; Turano, C.; Eufemi, M. Upregulation of TPX2 by STAT3: Identification of a novel STAT3 binding site. PLoS ONE 2014, 9, e113096. [Google Scholar]
- Gharavi, N.M.; Alva, J.A.; Mouillesseaux, K.P.; Lai, C.; Yeh, M.; Yeung, W.; Johnson, J.; Szeto, W.L.; Hong, L.; Fishbein, M.; et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J. Biol. Chem. 2007, 282, 31460–31468. [Google Scholar] [CrossRef]
- Walker, S.R.; Xiang, M.; Frank, D.A. Distinct roles of STAT3 and STAT5 in the pathogenesis and targeted therapy of breast cancer. Mol. Cell Endocrinol. 2014, 382, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Byun, W.S.; Lee, S.; Han, Y.T.; Jeong, Y.S.; Jang, K.; Chung, S.J.; Lee, J.; Suh, Y.G.; Lee, S.K. A novel small molecule STAT3 inhibitor SLSI-1216 suppresses proliferation and tumor growth of triple-negative breast cancer cells through apoptotic induction. Biochem. Pharmacol. 2020, 178, 114053. [Google Scholar] [CrossRef]
- Kim, B.; Lee, K.Y.; Park, B. Minecoside promotes apoptotic progression through STAT3 inactivation in breast cancer cells. Oncol. Lett. 2022, 23, 94. [Google Scholar] [CrossRef]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. BioMed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef]
- Guiu, S.; Michiels, S.; André, F.; Cortes, J.; Denkert, C.; Di Leo, A.; Hennessy, B.T.; Sorlie, T.; Sotiriou, C.; Turner, N.; et al. Molecular subclasses of breast cancer: How do we define them? The IMPAKT 2012 Working Group Statement. Ann. Oncol. 2012, 23, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Zardavas, D.; Irrthum, A.; Swanton, C.; Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 2015, 12, 381–394. [Google Scholar] [CrossRef]
- Januškevičienė, I.; Petrikaitė, V. Heterogeneity of breast cancer: The importance of interaction between different tumor cell populations. Life Sci. 2019, 239, 117009. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou Zerdan, M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple negative breast cancer: Updates on classification and treatment in 2021. Cancers 2022, 14, 1253. [Google Scholar] [CrossRef] [PubMed]
- Chalakur-Ramireddy, N.K.R.; Pakala, S.B. Combined drug therapeutic strategies for the effective treatment of Triple Negative Breast Cancer. BioSci. Rep. 2018, 38, BSR20171357. [Google Scholar] [CrossRef]
- Murin, S.; Pinkerton, K.E.; Hubbard, N.E.; Erickson, K. The effect of cigarette smoke exposure on pulmonary metastatic disease in a murine model of metastatic breast cancer. Chest 2004, 125, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Di Cello, F.; Flowers, V.L.; Li, H.; Vecchio-Pagán, B.; Gordon, B.; Harbom, K.; Shin, J.; Beaty, R.; Wang, W.; Brayton, C.; et al. Cigarette smoke induces epithelial to mesenchymal transition and increases the metastatic ability of breast cancer cells. Molecular. Cancer 2013, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Kispert, S.; Marentette, J.; McHowat, J. Cigarette smoking promotes bladder cancer via increased platelet-activating factor. Physiol Rep. 2019, 7, e13981. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Sharma, S.; Wu, K.; Wu, S.Y.; Xing, F.; Liu, Y.; Zhao, D.; Deshpande, R.P.; D’Agostino, R.B.; Watabe, K. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat. Commun. 2021, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, D.; Izano, M.; Moore, D.H.; Kwan, M.L.; Tammemagi, M.C.; Hiatt, R.A.; Kerlikowske, K.; Kroenke, C.H.; Sweeney, C.; Habel, L.; et al. Smoking and survival after breast cancer diagnosis: A prospective observational study and systematic review. Breast Cancer Res. Treat. 2012, 136, 521–533. [Google Scholar] [CrossRef]
- Izano, M.; Satariano, W.A.; Hiatt, R.A.; Braithwaite, D. Smoking and mortality after breast cancer diagnosis: The health and functioning in women study. Cancer Med. 2015, 4, 315–324. [Google Scholar] [CrossRef]
- Español, A.; Sanchez, Y.; Salem, A.; Obregon, J.; Sales, M.E. Nicotinic receptors modulate antitumor therapy response in triple negative breast cancer cells. World J. Clin. Oncol. 2022, 13, 505–519. [Google Scholar] [CrossRef]
- Rudrapal, M.; Maji, S.; Prajapati, S.K.; Kesharwani, P.; Deb, P.K.; Khan, J.; Mohamed Ismail, R.; Kankate, R.S.; Sahoo, R.K.; Khairnar, S.J.; et al. Protective Effects of Diets Rich in Polyphenols in Cigarette Smoke (CS)-Induced Oxidative Damages and Associated Health Implications. Antioxidants 2022, 11, 1217. [Google Scholar] [CrossRef]
- Izzotti, A.; Balansky, R.; Micale, R.T.; Pulliero, A.; La Maestra, S.; De Flora, S. Modulation of smoke-induced DNA and microRNA alterations in mouse lung by licofelone, a triple COX-1, COX-2 and 5-LOX inhibitor. Carcinogenesis 2020, 41, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Hou, X.; Yuan, J.; Tan, X.; Chen, J.; Yang, N.; Luo, Y.; Jiang, Z.; Jin, P.; Dong, Z.; et al. Wedelolactone protects human bronchial epithelial cell injury against cigarette smoke extract-induced oxidant stress and inflammation responses through Nrf2 pathway. Int. Immunopharmacol. 2015, 29, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Katiyar, S.K. Grape seed proanthocyanidins inhibit cigarette smoke condensate-induced lung cancer cell migration through inhibition of NADPH oxidase and reduction in the binding of p22(phox) and p47(phox) proteins. Mol. Carcinog. 2015, 54 (Suppl. S1), E61–E71. [Google Scholar] [CrossRef]
- La Maestra, S.; D’Agostini, F.; Izzotti, A.; Micale, R.T.; Mastracci, L.; Camoirano, A.; Balansky, R.; Trosko, J.E.; Steele, V.E.; De Flora, S. Modulation by aspirin and naproxen of nucleotide alterations and tumors in the lung of mice exposed to environmental cigarette smoke since birth. Carcinogenesis 2015, 36, 1531–1538. [Google Scholar] [CrossRef]
- Pulliero, A.; Wu, Y.; Fenoglio, D.; Parodi, A.; Romani, M.; Soares, C.P.; Filaci, G.; Lee, J.L.; Sinkam, P.N.; Izzotti, A. Nanoparticles increase the efficacy of cancer chemopreventive agents in cells exposed to cigarette smoke condensate. Carcinogenesis 2015, 36, 368–377. [Google Scholar] [CrossRef] [PubMed]








| Gene | Brand | Primer (5′ → 3′) | Annealing (°C) | Efficiency (%) | R2 | |
|---|---|---|---|---|---|---|
| BIRC-5 | Bio-Rad | Forward | N/A (Cod. qHsaCED0001615) | 60 | 97.0 | 0.999 |
| Reverse | ||||||
| MMP2 | Bio-Rad | Forward | N/A (Cod. qHsaCED0042560) | 60 | 99.0 | 0.999 |
| Reverse | ||||||
| TPX2 | Bio-Rad | Forward | N/A (Cod. qHsaCID0016024) | 60 | 98.0 | 0.997 |
| Reverse | ||||||
| IL-8 | Bio-Rad | Forward | N/A (Cod. qHsaCED0023767) | 60 | 97.0 | 0.999 |
| Reverse | ||||||
| GAPDH | Bio-Rad | Forward | N/A (Cod. qHsaCED0038674) | 60 | 97.0 | 0.999 |
| Reverse | ||||||
| No. | Component 1 | LRI 2 | LRI 3 | Smoke (%) 4 |
|---|---|---|---|---|
| 1 | α-Myrcene | 979 | 981 | 0.8 |
| 2 | Triacetin | 1340 | 1344 | 1.2 |
| 3 | Nicotine | 1366 | 1360 | 26.7 |
| 4 | (Z)-2,6,10-Trimethyl-1,5,9-undecatriene | 1410 | * | 2.4 |
| 5 | 7-Methyl-6-tridecene | 1455 | * | 6.9 |
| 6 | 6,11-Dimethyl-2,6,10-dodecatrien-1-ol | 1665 | 1658 | 3.1 |
| 7 | Neophytadiene | 1912 | 1915 | 56.4 |
| 8 | Urs-12-en-28-ol | 3385 | 3376 | 2.6 |
| SUM (%) | 99.9 |
| Tested Sample | IC50 [µg/mL] (CL) |
|---|---|
| MDA-MB-468 | |
| CSC | 134.8 (119.6–152.7) *** |
| β-Caryophyllene | 18.6 (15.9–21.7) *** |
| Doxorubicin | 4.7 (3.8–6.7) |
| Ctrl | β-Caryophyllene | CSC | β-Caryophyllene + CSC | |
|---|---|---|---|---|
| LC3B | - | +/− | + | +/− |
| Beclin-1 | - | +/− | + | +/− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sotto, A.; Gullì, M.; Minacori, M.; Mancinelli, R.; Garzoli, S.; Percaccio, E.; Incocciati, A.; Romaniello, D.; Mazzanti, G.; Eufemi, M.; et al. β-Caryophyllene Counteracts Chemoresistance Induced by Cigarette Smoke in Triple-Negative Breast Cancer MDA-MB-468 Cells. Biomedicines 2022, 10, 2257. https://doi.org/10.3390/biomedicines10092257
Di Sotto A, Gullì M, Minacori M, Mancinelli R, Garzoli S, Percaccio E, Incocciati A, Romaniello D, Mazzanti G, Eufemi M, et al. β-Caryophyllene Counteracts Chemoresistance Induced by Cigarette Smoke in Triple-Negative Breast Cancer MDA-MB-468 Cells. Biomedicines. 2022; 10(9):2257. https://doi.org/10.3390/biomedicines10092257
Chicago/Turabian StyleDi Sotto, Antonella, Marco Gullì, Marco Minacori, Romina Mancinelli, Stefania Garzoli, Ester Percaccio, Alessio Incocciati, Donatella Romaniello, Gabriela Mazzanti, Margherita Eufemi, and et al. 2022. "β-Caryophyllene Counteracts Chemoresistance Induced by Cigarette Smoke in Triple-Negative Breast Cancer MDA-MB-468 Cells" Biomedicines 10, no. 9: 2257. https://doi.org/10.3390/biomedicines10092257
APA StyleDi Sotto, A., Gullì, M., Minacori, M., Mancinelli, R., Garzoli, S., Percaccio, E., Incocciati, A., Romaniello, D., Mazzanti, G., Eufemi, M., & Di Giacomo, S. (2022). β-Caryophyllene Counteracts Chemoresistance Induced by Cigarette Smoke in Triple-Negative Breast Cancer MDA-MB-468 Cells. Biomedicines, 10(9), 2257. https://doi.org/10.3390/biomedicines10092257










