Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Immunofluorescence
2.3. Western Blot Analysis
2.4. Formation of p-FAK/GNP Conjugates and Its Treatment
2.5. NCP Device
2.6. Live/Dead Cell Staining
2.7. Animal Experiments
2.8. Anti-OSCC Activity Evaluation in a Mice Xenograft Model
2.9. Histological Assays
2.10. Statistical Analysis
3. Results
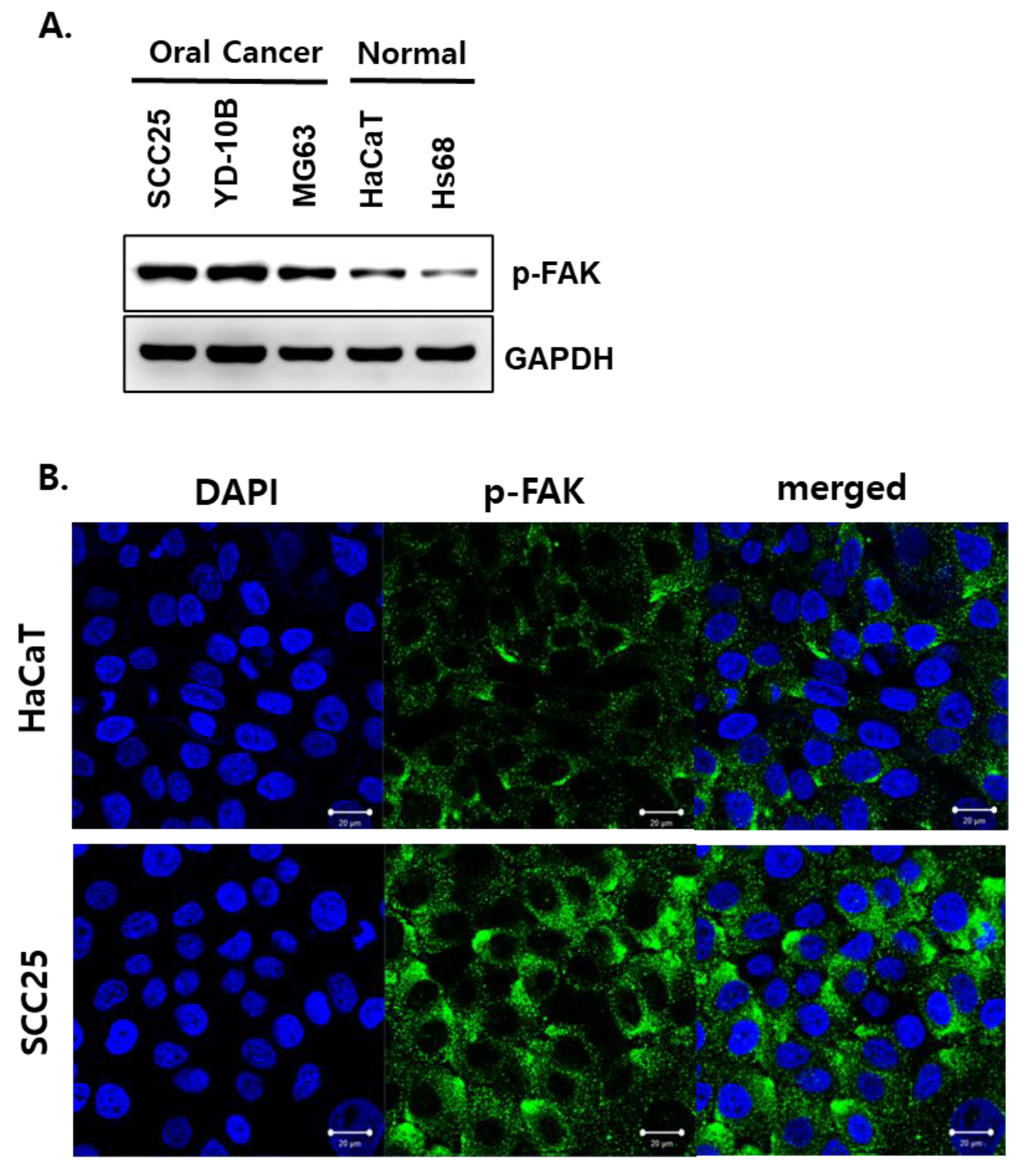
3.1. Phosphorylated FAK Protein Was Highly Expressed in OSCC Cells
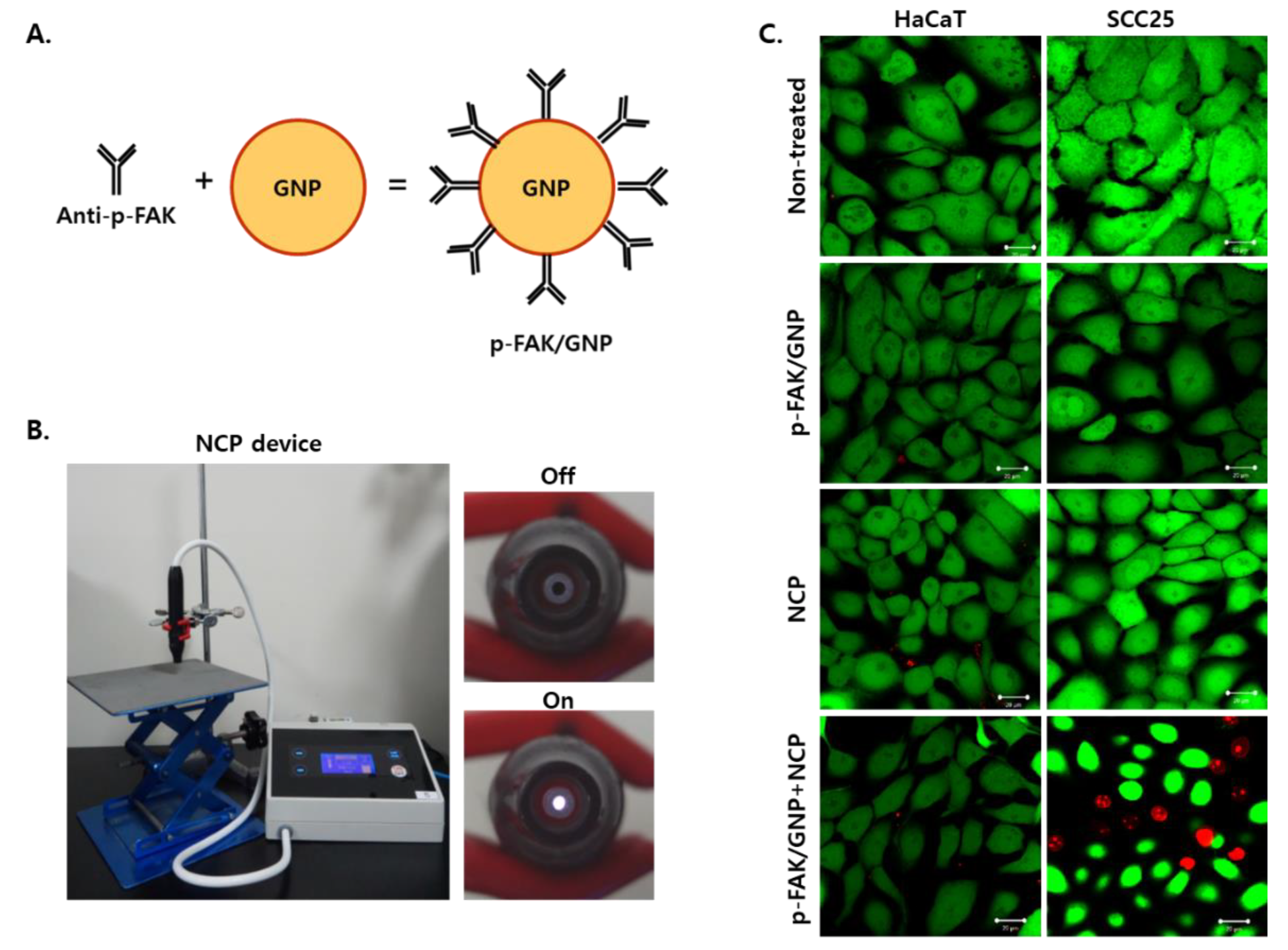
3.2. The Combinational Treatment of NCP with p-FAK/GNP Selectively Kills SCC25 Cells Immediately after Treatment
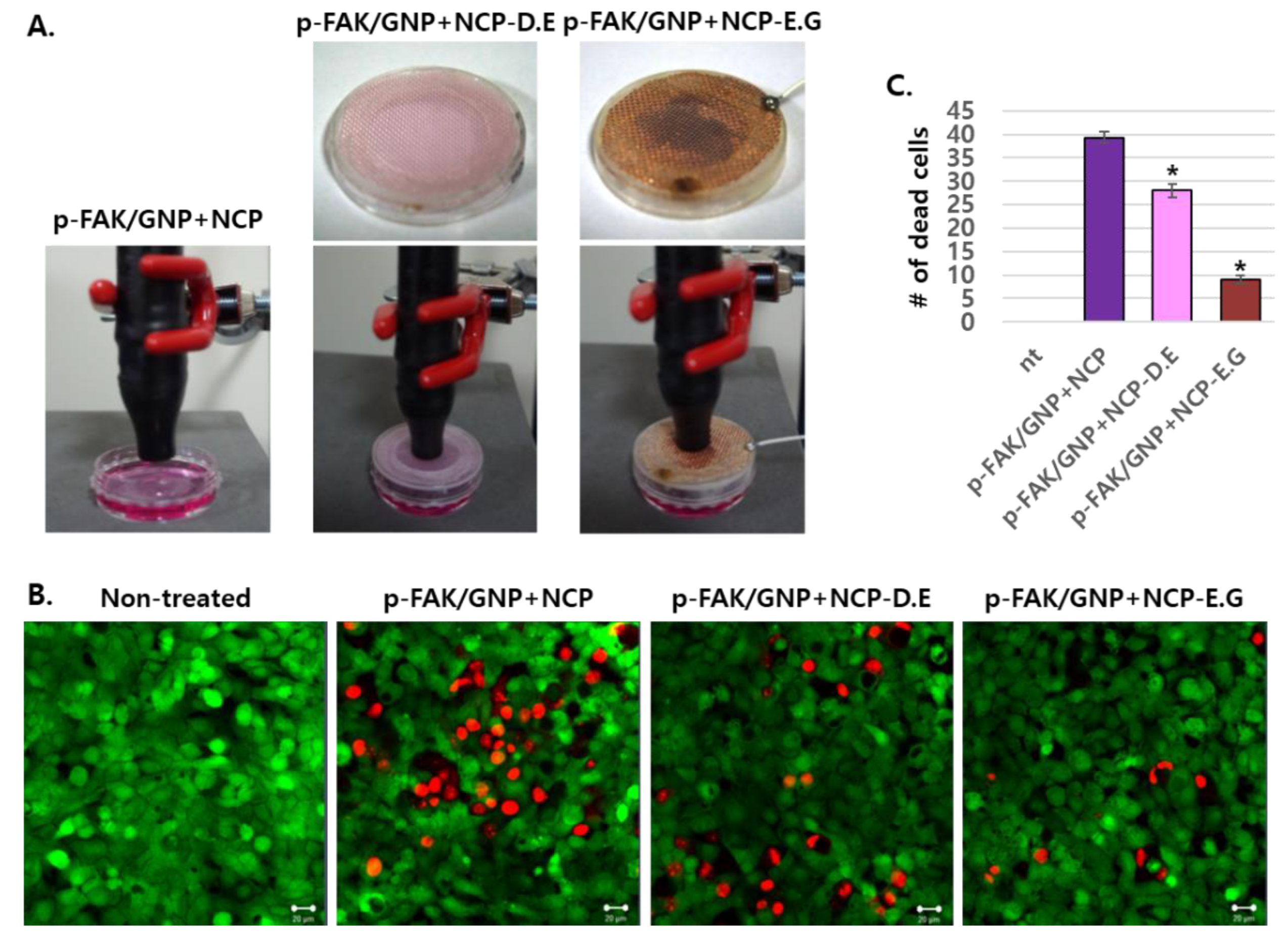
3.3. The Effects of Plasma-Activated Media Treatment on p-FAK/GNP-Treated SCC25 Cells Was Weaker Than the Direct NCP Treatment
3.4. The Placement of Electronic Grounded Mesh during NCP Treatment Hindersp-FAK/GNP+NCP-Mediated SCC25 Cell Death
3.5. The Synergistic Anti-Tumor Effect of the Combinational Treatment of NCP and p-FAK/GNP in Mice Xenograft Models
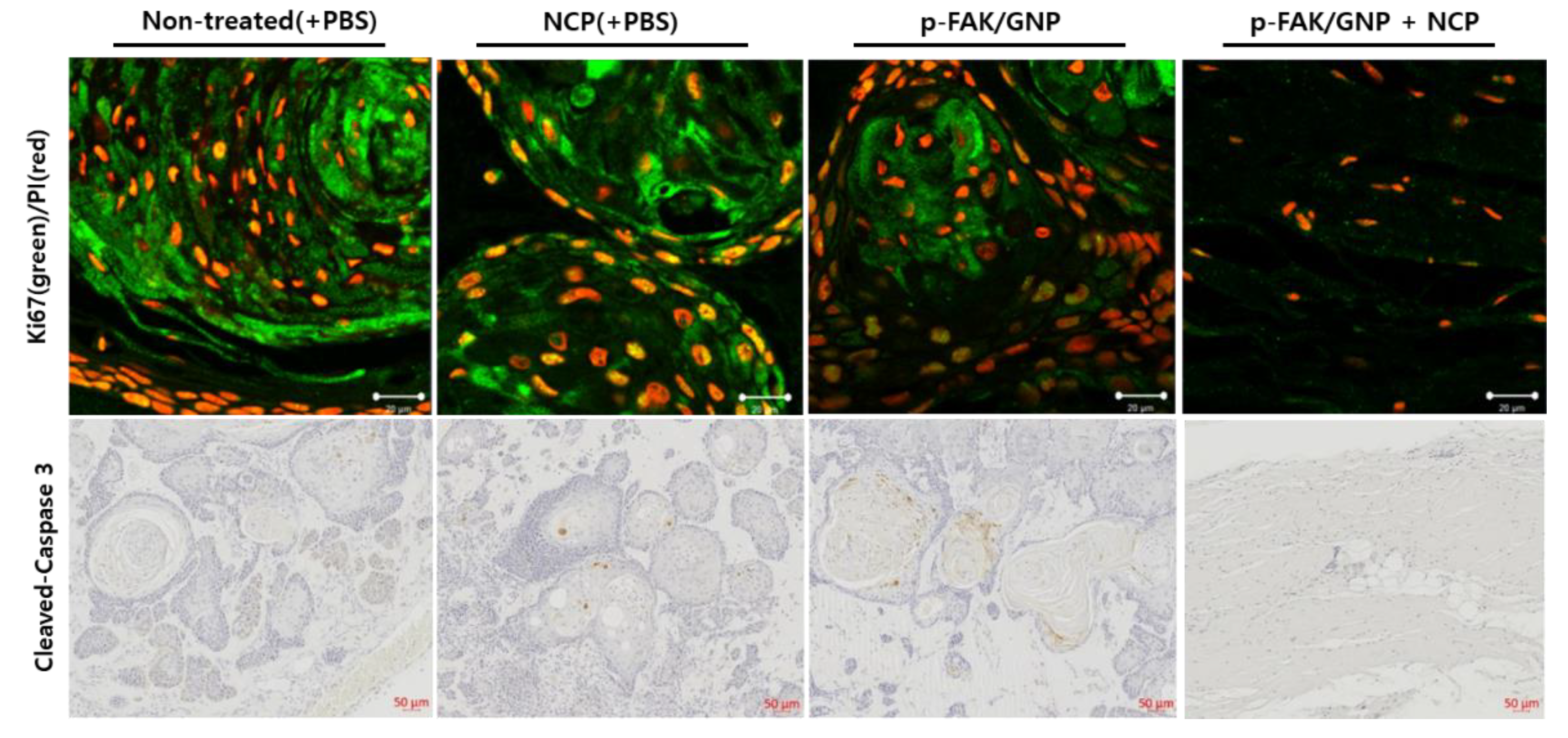
3.6. The Tissue Isolated from the Mice Treated with pFAK/GNP in Combination with NCP Was Not a Tumor
3.7. Apoptotic Cell Death Was Not Monitored in the Tumors Treated with NCP and p-FAK/GNP in Combination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Usman, S.; Jamal, A.; Teh, M.T.; Waseem, A. Major Molecular Signaling Pathways in Oral Cancer Associated With Therapeutic Resistance. Front. Oral Health 2020, 1, 603160. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, R.; Acharya, A.K.; Patthi, B.; Goud, V.; Reddy, S.; Garg, A.; Singla, A. Changing Trends in oral cancer—A global scenario. Nepal J. Epidemiol. 2016, 6, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Seoane, J.; Takkouche, B.; Varela-Centelles, P.; Tomas, I.; Seoane-Romero, J.M. Impact of delay in diagnosis on survival to head and neck carcinomas: A systematic review with meta-analysis. Clin. Otolaryngol. 2012, 37, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e233–e240. [Google Scholar] [CrossRef]
- Kumar, M.S.; Masthan, K.M.; Babu, N.A.; Dash, K.C. Gene therapy in oral cancer: A review. J. Clin. Diagn. Res. 2013, 7, 1261–1263. [Google Scholar] [CrossRef]
- Mohan, S.P.; Bhaskaran, M.K.; George, A.L.; Thirutheri, A.; Somasundaran, M.; Pavithran, A. Immunotherapy in Oral Cancer. J. Pharm. Bioallied Sci. 2019, 11, S107–S111. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma Medicine: A Field of Applied Redox Biology. Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Babaeva, N.Y.; Naidis, G.V. Modeling of Plasmas for Biomedicine. Trends Biotechnol. 2018, 36, 603–614. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Emmert, S.; Metelmann, H.-R.; Rupf, S.; Weltmann, K.-D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Phys. Plasmas 2020, 27, 070601. [Google Scholar] [CrossRef]
- Machala, Z.; Graves, D.B. Frugal Biotech Applications of Low-Temperature Plasma. Trends Biotechnol. 2018, 36, 579–581. [Google Scholar] [CrossRef]
- Choi, J.H.; Song, Y.S.; Lee, H.J.; Hong, J.W.; Kim, G.C. Inhibition of inflammatory reactions in 2,4-Dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Sci. Rep. 2016, 6, 27376. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Song, Y.S.; Lee, H.J.; Kim, G.C.; Hong, J.W. The topical application of low-temperature argon plasma enhances the anti-inflammatory effect of Jaun-ointment on DNCB-induced NC/Nga mice. BMC Complement. Altern. Med. 2017, 17, 340. [Google Scholar] [CrossRef]
- Haertel, B.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Song, Y.S.; Song, K.; Lee, H.J.; Hong, J.W.; Kim, G.C. Skin renewal activity of non-thermal plasma through the activation of beta-catenin in keratinocytes. Sci. Rep. 2017, 7, 6146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Choi, J.H.; Jang, Y.S.; Kim, U.K.; Kim, G.C.; Hwang, D.S. Non-thermal plasma accelerates the healing process of peripheral nerve crush injury in rats. Int. J. Med. Sci. 2020, 17, 1112–1120. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Ramireddy, L.; Lai, C.H.; Low, B.S.; Li, C.; Hsieh, J.H.; Lee, J.W.; Wu, H.Y. Induction of Apoptosis by Cold Atmospheric Pressure Plasma for Oral Squamous Cell Carcinoma Cells. Plasma Med. 2018, 8, 411–418. [Google Scholar] [CrossRef]
- Lee, J.H.; Om, J.Y.; Kim, Y.H.; Kim, K.M.; Choi, E.H.; Kim, K.N. Selective Killing Effects of Cold Atmospheric Pressure Plasma with NO Induced Dysfunction of Epidermal Growth Factor Receptor in Oral Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0150279. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective Killing of Melanoma Cells With Non-Thermal Atmospheric Pressure Plasma and p-FAK Antibody Conjugated Gold Nanoparticles. Int. J. Med. Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef]
- Choi, B.B.; Choi, J.H.; Kang, T.H.; Lee, S.J.; Kim, G.C. Enhancement of Osteoblast Differentiation Using No-Ozone Cold Plasma on Human Periodontal Ligament Cells. Biomedicines 2021, 9, 1542. [Google Scholar] [CrossRef]
- Park, N.S.; Yun, S.E.; Lee, H.Y.; Lee, H.J.; Choi, J.H.; Kim, G.C. No-ozone cold plasma can kill oral pathogenic microbes in H2O2-dependent and independent manner. Scientific reports 2022, 12, 7597. [Google Scholar] [CrossRef]
- Ni, J.; Cozzi, P.; Hung, T.T.; Hao, J.; Graham, P.; Li, Y. Monitoring Prostate Tumor Growth in an Orthotopic Mouse Model Using Three-Dimensional Ultrasound Imaging Technique. Transl. Oncol. 2016, 9, 41–45. [Google Scholar] [CrossRef]
- Yadav, D.K.; Adhikari, M.; Kumar, S.; Ghimire, B.; Han, I.; Kim, M.H.; Choi, E.H. Cold atmospheric plasma generated reactive species aided inhibitory effects on human melanoma cells: An in vitro and in silico study. Sci. Rep. 2020, 10, 3396. [Google Scholar] [CrossRef]
- Tanaka, H.; Bekeschus, S.; Yan, D.; Hori, M.; Keidar, M.; Laroussi, M. Plasma-Treated Solutions (PTS) in Cancer Therapy. Cancers 2021, 13, 1737. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. The Application of the Cold Atmospheric Plasma-Activated Solutions in Cancer Treatment. Anti-Cancer Agents Med. Chem. 2018, 18, 769–775. [Google Scholar] [CrossRef]
- Choi, B.B.; Kim, M.S.; Song, K.W.; Kim, U.K.; Hong, J.W.; Lee, H.J.; Kim, G.C. Targeting NEU Protein in Melanoma Cells with Non-Thermal Atmospheric Pressure Plasma and Gold Nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.B.R.; Choi, J.H.; Lee, H.Y.; Lee, H.J.; Song, K.W.; Kim, G.C. Protective effects of non-thermal plasma on triethylene glycol dimethacrylate-induced damage in odontoblast-like MDPC-23 cells. Int. Endod. J. 2021, 54, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Rodriguez, Y.A.R.; Jeong, K.; Ahn, E.-Y.E.; Lim, S.-T.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020, 52, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.J.; Liu, X.J.; Liu, Y.; Liu, W.B.; Li, Y.R.; Yu, G.X.; Tian, X.Y.; Zhang, Y.B.; Song, J.; Jin, C.Y.; et al. Drug Discovery Targeting Focal Adhesion Kinase (FAK) as a Promising Cancer Therapy. Molecules 2021, 26, 4250. [Google Scholar] [CrossRef] [PubMed]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Cance, W.G.; Harris, J.E.; Iacocca, M.V.; Roche, E.; Yang, X.; Chang, J.; Simkins, S.; Xu, L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: Correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. 2000, 6, 2417–2423. [Google Scholar]
- Chiu, Y.W.; Liou, L.Y.; Chen, P.T.; Huang, C.M.; Luo, F.J.; Hsu, Y.K.; Yuan, T.C. Tyrosine 397 phosphorylation is critical for FAK-promoted Rac1 activation and invasive properties in oral squamous cell carcinoma cells. Lab. Investig. 2016, 96, 296–306. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, H.J.; Kim, G.C.; Choi, J.H.; Hong, J.W. Plasma cupping induces VEGF expression in skin cells through nitric oxide-mediated activation of hypoxia inducible factor 1. Sci. Rep. 2019, 9, 3821. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Chen, L.; Gong, X.; Yang, H.; Duan, X.; Zhu, Y. Multifunctional Gold Nanoparticles in Cancer Diagnosis and Treatment. Int. J. Nanomed. 2022, 17, 2041–2067. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Naghdi, N.; Nokhbatolfoghahaei, H.; Bagheri, H. The Combination of Laser Therapy and Metal Nanoparticles in Cancer Treatment Originated From Epithelial Tissues: A Literature Review. J. Lasers Med. Sci. 2016, 7, 62–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Sadeghi-Goughari, M.; Jeon, S.; Kwon, H.J. Enhancing Thermal Effect of Focused Ultrasound Therapy Using Gold Nanoparticles. IEEE Trans. Nanobioscience 2019, 18, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Gu, H.-J.; Park, K.-H.; Hwang, D.-S.; Kim, G.-C. Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice. Biomedicines 2022, 10, 2259. https://doi.org/10.3390/biomedicines10092259
Choi J-H, Gu H-J, Park K-H, Hwang D-S, Kim G-C. Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice. Biomedicines. 2022; 10(9):2259. https://doi.org/10.3390/biomedicines10092259
Chicago/Turabian StyleChoi, Jeong-Hae, Hee-Jin Gu, Kwang-Ha Park, Dae-Seok Hwang, and Gyoo-Cheon Kim. 2022. "Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice" Biomedicines 10, no. 9: 2259. https://doi.org/10.3390/biomedicines10092259
APA StyleChoi, J.-H., Gu, H.-J., Park, K.-H., Hwang, D.-S., & Kim, G.-C. (2022). Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice. Biomedicines, 10(9), 2259. https://doi.org/10.3390/biomedicines10092259






