J-Shaped Relationship of Serum Uric Acid with Unfavorable Short-Term Outcomes among Patients with Acute Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Statement of Ethics
2.3. Stroke Severity, Classification, and Clinical Features
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sennfält, S.; Pihlsgård, M.; Petersson, J.; Norrving, B.; Ullberg, T. Long-term outcome after ischemic stroke in relation to comorbidity—An observational study from the Swedish Stroke Register (Riksstroke). Eur. Stroke J. 2020, 5, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.F.; Wang, D.J.; Liou, S.H.; Shieh, S.M. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur. J. Epidemiol. 2000, 16, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Rheum. 2009, 61, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Amaro, S.; Urra, X.; Gómez-Choco, M.; Obach, V.; Cervera, A.; Vargas, M.; Torres, F.; Rios, J.; Planas, A.M.; Chamorro, A. Uric acid levels are relevant in patients with stroke treated with thrombolysis. Stroke 2011, 42, S28–S32. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, J.X.; Sun, X.S.; Lai, R.; Sheng, W.L. High serum uric acid levels are a protective factor against unfavourable neurological functional outcome in patients with ischaemic stroke. J. Int. Med. Res. 2018, 46, 1826–1838. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Li, M.; Hou, W.; Zhang, X.; Hu, L.; Tang, Z. Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclerosis 2014, 232, 265–270. [Google Scholar] [CrossRef]
- Zhong, C.; Zhong, X.; Xu, T.; Xu, T.; Zhang, Y. Sex-specific relationship between serum uric acid and risk of stroke: A dose-response meta-analysis of prospective studies. J. Am. Heart Assoc. 2017, 6, e005042. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the rric acid thresholds predicting an increased total and cardiovascular mortality over 20 Years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef]
- Verdecchia, P.; Schillaci, G.; Reboldi, G.; Santeusanio, F.; Porcellati, C.; Brunetti, P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000, 36, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, J.; Wang, Q.; Wang, C.; Wang, Y.; Gong, T.; Liu, D. J-shaped relationship between serum uric acid levels and the risk of ischemic stroke in high-risk individuals: A hospital-based observational study. Clin. Neurol. Neurosurg. 2020, 195, 105906. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Hisatome, I.; Niwa, K.; Bjornstad, P.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Kanbay, M.; Johnson, R.J.; Lanaspa, M.A. The optimal range of serum uric acid for cardiometabolic diseases: A 5-year Japanese Cohort Study. J. Clin. Med. 2020, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Wu, H.; Peng, W. The relationship between elevated serum uric acid and risk of stroke in adult: An updated and dose-response meta-analysis. Front. Neurol. 2021, 12, 674398. [Google Scholar] [CrossRef]
- Tang, H.; Mo, J.; Chen, Z.; Xu, J.; Wang, A.; Dai, L.; Cheng, A.; Wang, Y. Uric acid contributes to obesity-paradox of the outcome of ischemic stroke. Front. Neurol. 2019, 10, 1279. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Y.; Liu, Y.; Chen, Y.; Wang, B.; Li, C.; Yan, S.; Wang, Y.; Zhao, W. Serum uric acid levels and outcomes after acute ischemic stroke. Mol. Neurobiol. 2016, 53, 1753–1759. [Google Scholar] [CrossRef]
- Chen, L.H.; Zhong, C.; Xu, T.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Ju, Z.; et al. Sex-specific association between uric acid and outcomes after acute ischemic stroke: A prospective study from CATIS Trial. Sci. Rep. 2016, 6, 38351. [Google Scholar] [CrossRef]
- Lei, Z.; Cai, J.; Hong, H.; Wang, Y. Serum uric acid level and outcome of patients with ischemic stroke: A systematic review and meta-analysis. Neurologist 2019, 24, 121–131. [Google Scholar] [CrossRef]
- Wang, C.; Cui, T.; Wang, L.; Zhu, Q.; Wang, A.; Yuan, Y.; Hao, Z.; Wu, B. Prognostic significance of uric acid change in acute ischemic stroke patients with reperfusion therapy. Eur. J. Neurol. 2021, 28, 1218–1224. [Google Scholar] [CrossRef]
- Gerber, Y.; Tanne, D.; Medalie, J.H.; Goldbourt, U. Serum uric acid and long-term mortality from stroke, coronary heart disease and all causes. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 193–198. [Google Scholar] [CrossRef]
- Hamirani, Y.S.; Pandey, S.; Rivera, J.J.; Ndumele, C.; Budoff, M.J.; Blumenthal, R.S.; Nasir, K. Is uric acid protective or deleterious in acute ischemic stroke? A prospective cohort study. Atherosclerosis 2010, 209, 215–219. [Google Scholar] [CrossRef]
- Kuo, C.F.; See, L.C.; Yu, K.H.; Chou, I.J.; Chiou, M.J.; Luo, S.F. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology 2013, 52, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Li, Y.; Ding, L.; Sheng, L.; Xie, Z.; Wen, C. U-shaped relationship between functional outcome and serum uric acid in ischemic stroke. Cell Physiol. Biochem. 2018, 47, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Lamacchia, O.; Fontana, A.; Pacilli, A.; Copetti, M.; Fariello, S.; Garofolo, M.; Penno, G.; Trischitta, V.; De Cosmo, S.; Cignarelli, M. On the non-linear association between serum uric acid levels and all-cause mortality rate in patients with type 2 diabetes mellitus. Atherosclerosis 2017, 260, 20–26. [Google Scholar] [CrossRef]
- Browne, L.D.; Jaouimaa, F.Z.; Walsh, C.; Perez-Ruiz, F.; Richette, P.; Burke, K.; Stack, A.G. Serum uric acid and mortality thresholds among men and women in the Irish health system: A cohort study. Eur. J. Intern. Med. 2021, 84, 46–55. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, H.Y.; Chou, P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J. Rheumatol. 2000, 27, 1045–1050. [Google Scholar]
- Chen, J.H.; Yeh, W.T.; Chuang, S.Y.; Wu, Y.Y.; Pan, W.H. Gender-specific risk factors for incident gout: A prospective cohort study. Clin. Rheumatol. 2012, 31, 239–245. [Google Scholar] [CrossRef]
- Tariq, M.A.; Shamim, S.A.; Rana, K.F.; Saeed, A.; Malik, B.H. Serum uric acid-risk factor for acute ischemic stroke and poor outcomes. Cureus 2019, 11, e6007. [Google Scholar] [CrossRef]
- Hak, A.E.; Choi, H.K. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the Third National Health and Nutrition Examination Survey. Arthritis Res. Ther. 2008, 10, R116. [Google Scholar] [CrossRef]
- Akizuki, S. Serum uric acid levels among thirty-four thousand people in Japan. Ann. Rheum. Dis. 1982, 41, 272–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.W.; Chen, P.Y.; Lin, S.K. Correlation between immune-inflammatory markers and clinical features in patients with acute ischemic stroke. Acta Neurol. Taiwan 2020, 29, 103–113. [Google Scholar]
- Lin, S.K.; Chen, P.Y.; Chen, G.C.; Hsu, P.J.; Hsiao, C.L.; Yang, F.Y.; Liu, C.Y.; Tsou, A. Association of a high neutrophil-to-lymphocyte ratio with hyperdense artery sign and unfavorable short-term outcomes in patients with acute ischemic stroke. J. Inflamm. Res. 2021, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Toyoda, S.; Sakuma, M.; Hayashi, K.; Ouchi, M.; Fujita, T.; Anzai, N.; Tanaka, A.; Node, K.; Uemura, N. Effects of uric acid on vascular endothelial function from bedside to bench. Hypertens. Res. 2018, 41, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Lyngdoh, T.; Marques-Vidal, P.; Paccaud, F.; Preisig, M.; Waeber, G.; Bochud, M.; Vollenweider, P. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS ONE 2011, 6, e19901. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, L.; Huang, W.; Zhong, X.; Li, L.; Wang, H.; Peng, B.; Mao, M. Meta-analysis of the correlation between serumuric acid level and carotid intima-media thickness. PLoS ONE 2021, 16, e0246416. [Google Scholar] [CrossRef]
- Yang, X.; Lv, H.; Hidru, T.H.; Wu, J.; Liu, H.; Wang, Y.; Liu, K.; Xia, Y.; Zhou, Y.; Jiang, Y. Relation of serum uric acid to asymptomatic proximal extracranial artery stenosis in a middle-aged Chinese population: A community-based cross-sectional study. BMJ. Open 2018, 8, e020681. [Google Scholar] [CrossRef]
- Yao, T.; Di, A.; Li, J.; Zhang, S.; He, J.; Xu, N.; Xu, D. Association between serum uric acid and intracranial arterial stenosis in a Korean population: A secondary analysis based on a cross-sectional study. Front. Neurol. 2022, 13, 791456. [Google Scholar] [CrossRef]
- Wu, S.; Pan, Y.; Zhang, N.; Jun, W.Y.; Wang, C. Lower serum uric acid level strongly predict short-term poor functional outcome in acute stroke with normoglycaemia: A cohort study in China. BMC. Neurol. 2017, 17, 21. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Wang, J.; Qian, W.; Xiao, H. Low serum uric acid levels in patients with multiple sclerosis and neuromyelitis optica: An updated meta-analysis. Mult. Scler. Relat. Disord. 2016, 9, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Yamashita, A.; Asama, H. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS ONE 2017, 12, e0173731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamorro, Á.; Amaro, S.; Castellanos, M.; Segura, T.; Arenillas, J.; Martí-Fábregas, J.; Gállego, J.; Krupinski, J.; Gomis, M.; Cánovas, D.; et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): A randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014, 13, 453–460. [Google Scholar] [CrossRef]
- Chang, H.Y.; Pan, W.H.; Yeh, W.T.; Tsai, K.S. Hyperuricemia and gout in Taiwan: Results from the Nutritional and Health Survey in Taiwan. J. Rheumatol. 2001, 28, 1640–1646. [Google Scholar] [PubMed]
- Chien, K.L.; Hsu, H.C.; Sung, F.C.; Su, T.C.; Chen, M.F.; Lee, Y.T. Hyperuricemia as a risk factor on cardiovascular events in Taiwan: The Chin-Shan Community Cardiovascular Cohort Study. Atherosclerosis 2005, 183, 147–155. [Google Scholar] [CrossRef]
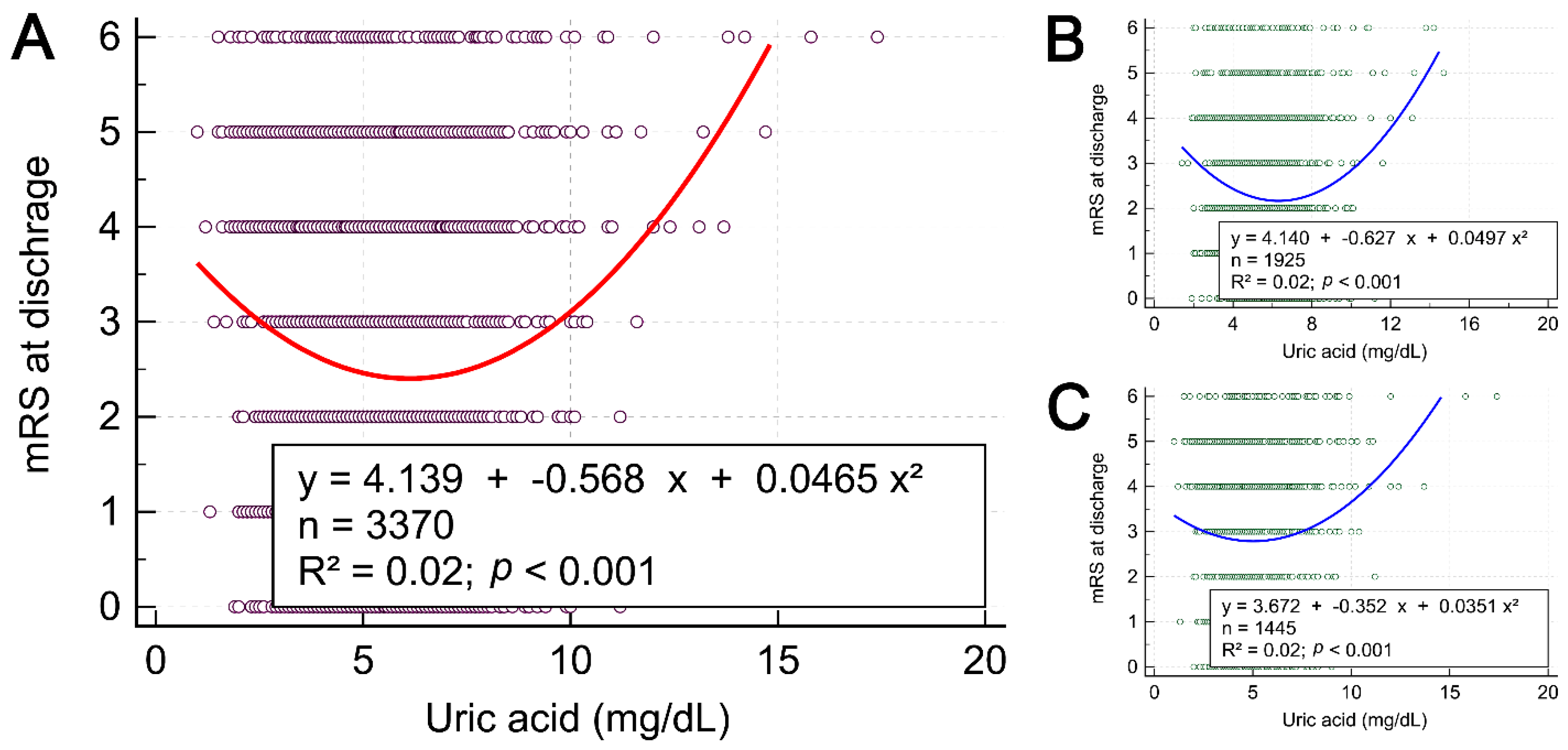
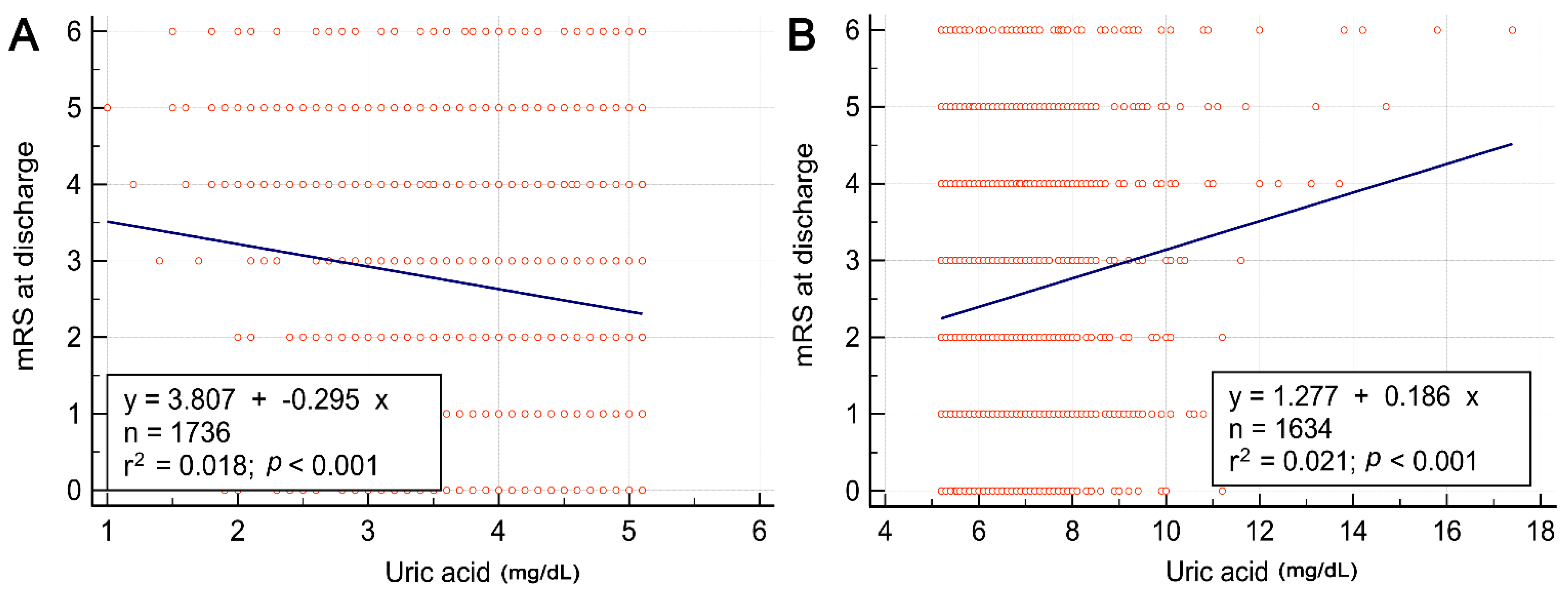
| Characteristics | All Patients (n = 3370) | Gender | ||
|---|---|---|---|---|
| Men (n = 1925) | Women (n = 1445) | p-Value | ||
| Age (years) | 70.6 ± 13.6 | 68.1 ± 13.4 | 74.0 ± 13.0 | <0.001 |
| Body mass index | 25.0 ± 4.2 | 25.3 ± 4.0 | 24.5 ± 0.4 | <0.001 |
| Hemoglobin (g/dL) | 13.6 ± 2.1 | 14.3 ± 1.9 | 12.8 ± 1.9 | <0.001 |
| Platelet (×109/L) | 219 ± 76 | 212 ± 73 | 227 ± 79 | <0.001 |
| White blood cells (×103/mL) | 7.98 ± 2.77 | 8.06 ± 2.71 | 7.87 ± 2.84 | 0.057 |
| Glucose (mg/dL) | 163 ± 79 | 162 ± 76 | 164 ± 83 | 0.409 |
| Creatinine (mg/dL) | 1.3 ± 1.1 | 1.4 ± 1.1 | 1.2 ± 1.1 | <0.001 |
| Cholesterol (mg/dL) | 170 ± 43 | 166 ± 41 | 174 ± 44 | <0.001 |
| LDL-cholesterol (mg/dL) | 108 ± 35 | 107 ± 35 | 109 ± 36 | 0.187 |
| Triglyceride (mg/dL) | 124 ± 95 | 128 ± 105 | 119 ± 79 | 0.007 |
| Uric acid (mg/dL) | 5.3 ± 1.7 | 5.5 ± 1.7 | 4.9 ± 1.7 | <0.001 |
| Hypertension | 2363 (70) | 1329 (69) | 1034 (72) | 0.114 |
| Diabetes mellitus | 1157 (34) | 654 (34) | 203 (35) | 0.613 |
| Dyslipidemia | 718 (21) | 403 (21) | 315 (22) | 0.545 |
| Heart disease | 1085 (32) | 573 (30) | 512 (35) | <0.001 |
| Prior stroke | 769 (23) | 469 (24) | 310 (21) | 0.047 |
| Current smoker | 717 (21) | 662 (34) | 65 (4) | <0.001 |
| Alcohol consumption | 227 (7) | 220 (11) | 7 (0.5) | <0.001 |
| History of cancer | 220 (7) | 106 (6) | 114 (8) | 0.006 |
| Uremia | 67 (2) | 34 (2) | 33 (2) | 0.319 |
| Hyperuricemia | 400 (12) | 191 (10) | 209 (14) | <0.001 |
| In-hospital complications | 373 (11) | 173 (9) | 200 (14) | <0.001 |
| Length of stay (days) | 14.1 ± 12.6 | 13.4 ± 12.6 | 15.0 ± 12.6 | <0.001 |
| NIHSS score on admission | 6.9 ± 7.2 | 6.0 ± 6.5 | 8.1 ± 7.8 | <0.001 |
| NIHSS score at discharge | 6.5 ± 9.4 | 5.8 ± 8.8 | 7.7 ± 10.1 | <0.001 |
| Barthel index score at discharge | 67 ± 64 | 72 ± 34 | 59 ± 37 | <0.001 |
| mRS score at discharge | 2.6 ± 1.7 | 2.3 ± 1.7 | 2.9 ± 1.8 | <0.001 |
| mRS >2 at discharge | 1658 (49) | 818 (42) | 840 (58) | <0.001 |
| Death at discharge | 133 (3.9) | 68 (3.5) | 65 (4.5) | 0.180 |
| Characteristics | All Patients | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men (n = 1925) | Women (n = 1445) | ||||||||
| No (n = 1712) | Yes (n = 1658) | p-Value | No (n = 1107) | Yes (n = 818) | p-Value | No (n = 605) | Yes (n = 840) | p-Value | |
| Age (years) | 66.2 ± 12.9 | 75.2 ± 12.8 | <0.001 | 64.8 ± 12.5 | 72.5 ± 13.5 | <0.001 | 68.6 ± 13.1 | 77.9 ± 11.5 | <0.001 |
| Admission NIHSS score | 3.1 ± 3.1 | 10.9 ± 7.9 | <0.001 | 3.0 ± 2.9 | 10.1 ± 7.6 | <0.001 | 3.1 ± 3.4 | 11.7 ± 8.2 | <0.001 |
| Hemoglobin (g/dL) | 14.0 ± 1.9 | 13.2 ± 2.1 | <0.001 | 14.5 ± 1.8 | 13.8 ± 2.1 | <0.001 | 13.0 ± 1.8 | 12.7 ± 1.9 | <0.001 |
| Platelet (×109/L) | 222 ± 70 | 215 ± 82 | 0.003 | 215 ± 65 | 208 ± 83 | 0.030 | 235 ± 77 | 221 ± 80 | <0.001 |
| White blood cells (×103/mL) | 7.78 ± 2.50 | 8.19 ± 3.03 | <0.001 | 7.9 ± 2.5 | 8.2 ± 2.9 | 0.096 | 7.43 ± 2.40 | 8.19 ± 3.07 | <0.001 |
| Glucose (mg/dL) | 159 ± 76 | 166 ± 82 | 0.012 | 162 ± 75 | 162 ± 77 | 0.904 | 155 ± 77 | 170 ± 86 | <0.001 |
| Creatinine (mg/dL) | 1.2 ± 1.0 | 1.4 ± 1.3 | <0.001 | 1.3 ± 0.9 | 1.5 ± 1.3 | 0.003 | 1.0 ± 1.0 | 1.3 ± 1.2 | <0.001 |
| Cholesterol (mg/dL) | 172 ± 42 | 167 ± 44 | <0.001 | 168 ± 39 | 163 ± 44 | 0.005 | 179 ± 45 | 171 ± 43 | <0.001 |
| Triglyceride (mg/dL) | 133 ± 91 | 115 ± 98 | <0.001 | 137 ± 97 | 115 ± 114 | <0.001 | 126 ± 78 | 114 ± 80 | 0.006 |
| Uric acid (mg/dL) | 5.3 ± 1.5 | 5.3 ± 1.9 | 0.286 | 5.6 ± 1.5 | 5.5 ± 1.8 | 0.241 | 4.9 ± 1.5 | 5.0 ± 1.9 | 0.051 |
| Female gender | 605 (35) | 840 (51) | <0.001 | - | - | - | - | - | - |
| Hypertension | 1162 (68) | 1201 (72) | 0.004 | 754 (68) | 575 (70) | 0.306 | 408 (67) | 626 (75) | 0.003 |
| Diabetes mellitus | 537 (31) | 620 (37) | <0.001 | 354 (32) | 300 (37) | 0.032 | 183 (30) | 320 (38) | 0.002 |
| Dyslipidemia | 416 (24) | 302 (18) | <0.001 | 256 (23) | 147 (18) | 0.006 | 160 (26) | 155 (18) | <0.001 |
| Heart disease | 428 (25) | 657 (40) | <0.001 | 271 (24) | 302 (37) | <0.001 | 157 (26) | 355 (42) | <0.001 |
| Prior stroke | 298 (17) | 481 (29) | <0.001 | 211 (19) | 258 (32) | <0.001 | 87 (14) | 223 (27) | <0.001 |
| Current smoker | 465 (27) | 262 (16) | <0.001 | 427 (39) | 235 (29) | <0.001 | 38 (6) | 27 (3) | 0.006 |
| Alcohol consumption | 139 (8) | 88 (5) | 0.001 | 135 (12) | 85 (10) | 0.246 | 4 (0.7) | 3 (0.4) | 0.461 |
| History of cancer | 91 (5) | 129 (8) | 0.004 | 55 (5) | 51 (6) | 0.266 | 36 (6) | 78 (9) | 0.023 |
| Uremia | 21 (1) | 46 (3) | 0.001 | 11 (1) | 23 (3) | 0.004 | 10 (2) | 23 (3) | 0.212 |
| Hyperuricemia | 166 (10) | 234 (14) | <0.001 | 101 (9) | 90 (11) | 0.189 | 65 (11) | 144 (17) | <0.001 |
| Characteristics | All patients | Gender | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men (n = 1925) | Women (n = 1445) | ||||||||
| No (n = 3237) | Yes (n = 133) | p-Value | No (n = 1857) | Yes (n = 68) | p-Value | No (n = 1380) | Yes (n = 65) | p-Value | |
| Age (years) | 70.3 ± 13.5 | 78.1 ± 13.5 | <0.001 | 67.8 ± 13.4 | 74.8 ± 14.7 | <0.001 | 73.7 ± 12.9 | 81.4 ± 11.3 | <0.001 |
| Admission NIHSS score | 6.4 ± 6.6 | 19.4 ± 9.1 | <0.001 | 5.6 ± 5.8 | 18.6 ± 9.8 | <0.001 | 7.5 ± 7.3 | 20.2 ± 8.2 | <0.001 |
| Hemoglobin (g/dL) | 13.7 ± 2.0 | 12.9 ± 2.4 | <0.001 | 14.2 ± 1.9 | 13.4 ± 2.4 | <0.001 | 12.8 ± 1.9 | 12.5 ± 2.2 | 0.139 |
| Platelet (×109/L) | 218 ± 73 | 221 ± 123 | 0.697 | 212 ± 69 | 230 ± 150 | 0.049 | 227 ± 78 | 212 ± 87 | 0.127 |
| White blood cells (×103/mL) | 7.91 ± 2.70 | 9.59 ± 3.69 | <0.001 | 8.00 ± 2.66 | 9.64 ± 3.56 | <0.001 | 7.79 ± 2.76 | 9.53 ± 3.85 | <0.001 |
| Glucose (mg/dL) | 162 ± 79 | 182 ± 83 | 0.004 | 161 ± 76 | 172 ± 67 | 0.248 | 163 ± 82 | 192 ± 96 | 0.005 |
| Creatinine (mg/dL) | 1.3 ± 1.1 | 1.7 ± 1.3 | <0.001 | 1.4 ± 1.1 | 1.6 ± 1.0 | 0.127 | 1.2 ± 1.1 | 1.7 ± 1.6 | <0.001 |
| Cholesterol (mg/dL) | 190 ± 42 | 164 ± 54 | 0.266 | 166 ± 40 | 165 ± 60 | 0.871 | 175 ± 44 | 164 ± 47 | 0.051 |
| Triglyceride (mg/dL) | 125 ± 94 | 110 ± 107 | 0.092 | 129 ± 106 | 102 ± 67 | 0.048 | 119 ± 76 | 118 ± 135 | 0.922 |
| Uric acid (mg/dL) | 5.3 ± 1.7 | 5.8 ± 2.7 | <0.001 | 5.5 ± 1.6 | 5.6 ± 2.5 | 0.574 | 4.9 ± 1.7 | 5.9 ± 2.9 | <0.001 |
| Female gender | 1380 (43) | 65 (49) | 0.180 | - | - | - | - | - | - |
| Hypertension | 2276 (70) | 87 (65) | 0.246 | 1286 (69) | 43 (63) | 0.288 | 990 (72) | 44 (68) | 0.483 |
| Diabetes mellitus | 1106 (34) | 51 (38) | 0.351 | 634 (34) | 20 (29) | 0.514 | 472 (34) | 31 (48) | 0.032 |
| Dyslipidemia | 698 (22) | 20 (15) | 0.083 | 393 (21) | 10 (15) | 0.227 | 305 (22) | 10 (15) | 0.222 |
| Heart disease | 1003 (31) | 82 (62) | <0.001 | 537 (29) | 36 (53) | <0.001 | 466 (34) | 46 (71) | <0.001 |
| Prior stroke | 755 (23) | 24 (18) | 0.173 | 455 (25) | 14 (21) | 0.565 | 300 (22) | 10 (15) | 0.279 |
| Current smoker | 706 (22) | 21 (16) | 0.107 | 642 (35) | 20 (29) | 0.436 | 64 (5) | 1 (2) | 0.361 |
| Alcohol consumption | 222 (7) | 5 (4) | 0.214 | 215 (12) | 5 (7) | 0.337 | 7 (0.5) | 0 (0) | 0.999 |
| History of cancer | 204 (6) | 16 (12) | 0.018 | 100 (5) | 6 (9) | 0.269 | 104 (8) | 10 (15) | 0.032 |
| Uremia | 60 (2) | 7 (5) | 0.015 | 31 (2) | 3 (4) | 0.116 | 29 (2) | 4 (6) | 0.057 |
| Hyperuricemia | 366 (11) | 34 (26) | <0.001 | 179 (10) | 12 (18) | 0.038 | 187 (14) | 22 (34) | <0.001 |
| TOAST Classification | Uric Acid | Hyperuricemia | mRS Score | mRS Score > 2 | Death |
|---|---|---|---|---|---|
| Small artery occlusion (n = 1485) | 5.2 ± 1.5 | 134 (9) | 1.9 ± 1.4 | 497 (33) | 2 (0.1) |
| Large artery atherosclerosis (n = 1091) | 5.3 ± 1.8 | 140 (13) | 3.1 ± 1.8 | 681 (62) | 69 (6.3) |
| Cardioembolism (n = 618) | 5.5 ± 1.9 | 111 (18) | 3.2 ± 1.8 | 400 (65) | 52 (8.4) |
| Other determined etiology (n = 68) | 4.8 ± 1.6 | 4 (6) | 2.5 ± 1.9 | 29 (43) | 6 (7.4) |
| Undetermined etiology (n = 108) | 5.2 ± 1.7 | 11 (10) | 2.5 ± 1.9 | 51 (47) | 5 (4.6) |
| p-Value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Uric Acid Level a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | All Patients (n = 3370) | Men (n = 1925) | Women (n = 1945) | ||||||
| Coefficient | R2 | p-Value | Coefficient | R2 | p-Value | Coefficient | R2 | p-Value | |
| Age | 0.019 | <0.001 | 0.889 | −0.349 | 0.002 | 0.060 | 1.111 | 0.022 | <0.001 |
| Body mass index | 0.408 | 0.027 | <0.001 | 0.345 | 0.019 | <0.001 | 0.429 | 0.027 | <0.001 |
| Hemoglobin | 0.107 | 0.008 | <0.001 | 0.087 | 0.005 | 0.001 | −0.021 | <0.001 | 0.469 |
| White blood cells | 0.151 | 0.009 | <0.001 | 0.160 | 0.010 | <0.001 | 0.129 | 0.006 | 0.003 |
| Glucose | −1.967 | 0.002 | 0.016 | −3.879 | 0.007 | <0.001 | 0.469 | <0.001 | 0.716 |
| Creatinine | 0.124 | 0.035 | <0.001 | 0.100 | 0.022 | <0.001 | 0.138 | 0.045 | <0.001 |
| Cholesterol | 2.063 | 0.007 | <0.001 | 3.977 | 0.025 | <0.001 | 0.752 | <0.001 | 0.273 |
| Triglyceride | 8.17 | 0.022 | <0.001 | 7.157 | 0.013 | <0.001 | 8.932 | 0.038 | <0.001 |
| Admission NIHSS score | 0.131 | 0.001 | 0.069 | 0.278 | 0.005 | 0.002 | 0.191 | 0.002 | 0.107 |
| Discharge NIHSS score | 0.239 | 0.002 | 0.012 | 0.206 | 0.002 | 0.089 | 0.528 | 0.008 | <0.001 |
| Discharge BI score | −0.024 | <0.001 | 0.947 | −0.179 | <0.001 | 0.702 | −1.229 | 0.003 | 0.030 |
| Discharge mRS score | −0.0003 | <0.001 | 0.988 | −0.011 | <0.001 | 0.653 | 0.074 | 0.005 | 0.005 |
| Hyperuricemia (n = 400) b | |||||||||
| Variables | All 3370 Patients | Men (n = 1925) | Women (n = 1945) | ||||||
| Yes | No | p Value | Yes | No | p Value | Yes | No | p Value | |
| Hypertension (n = 2363) | 316 (13) | 84 (8) | <0.001 | 140 (10) | 51 (9) | 0.188 | 176 (17) | 33 (8) | <0.001 |
| Diabetes mellitus (n = 1157) | 152 (13) | 248 (11) | 0.102 | 59 (9) | 132 (10) | 0.376 | 93 (18) | 116 (12) | 0.002 |
| Dyslipidemia (n = 718) | 101 (14) | 299 (11) | 0.044 | 55 (14) | 136 (9) | 0.007 | 46 (15) | 163 (14) | 0.928 |
| Heart disease (n = 1085) | 173 (16) | 227 (10) | <0.001 | 71 (12) | 120 (9) | 0.019 | 102 (20) | 107 (11) | <0.001 |
| Prior stroke (n = 769) | 90 (12) | 310 (12) | 0.871 | 43 (9) | 148 (10) | 0.594 | 47 (15) | 162 (14) | 0.716 |
| Current smoker (n = 717) | 73 (10) | 327 (12) | 0.119 | 67 (10) | 124 (10) | 0.873 | 6 (9) | 203 (15) | 0.279 |
| Alcohol consumption (n = 227) | 20 (9) | 380 (12) | 0.166 | 20 (9) | 171 (10) | 0.721 | 0 (0) | 209 (15) | 0.603 |
| History of cancer (n = 220) | 20 (9) | 380 (12) | 0.235 | 8 (8) | 183 (10) | 0.504 | 12 (11) | 197 (15) | 0.266 |
| Uremia (n = 67) | 12 (18) | 388 (12) | 0.127 | 6 918) | 185 (10) | 0.141 | 6 (18) | 203 (14) | 0.462 |
| Complications (n = 373) | 64 (17) | 336 (11) | 0.002 | 22 (13) | 169 (10) | 0.229 | 42 (21) | 167 (13) | 0.007 |
| Discharge mRS > 2 (n = 1658) | 234 (14) | 166 (10) | <0.001 | 90 (11) | 101 (9) | 0.189 | 144 (17) | 65 (11) | <0.001 |
| Death (n = 133) | 34 (26) | 366 (11) | <0.001 | 12 (18) | 179 (10) | 0.038 | 22 (34) | 187 (14) | <0.001 |
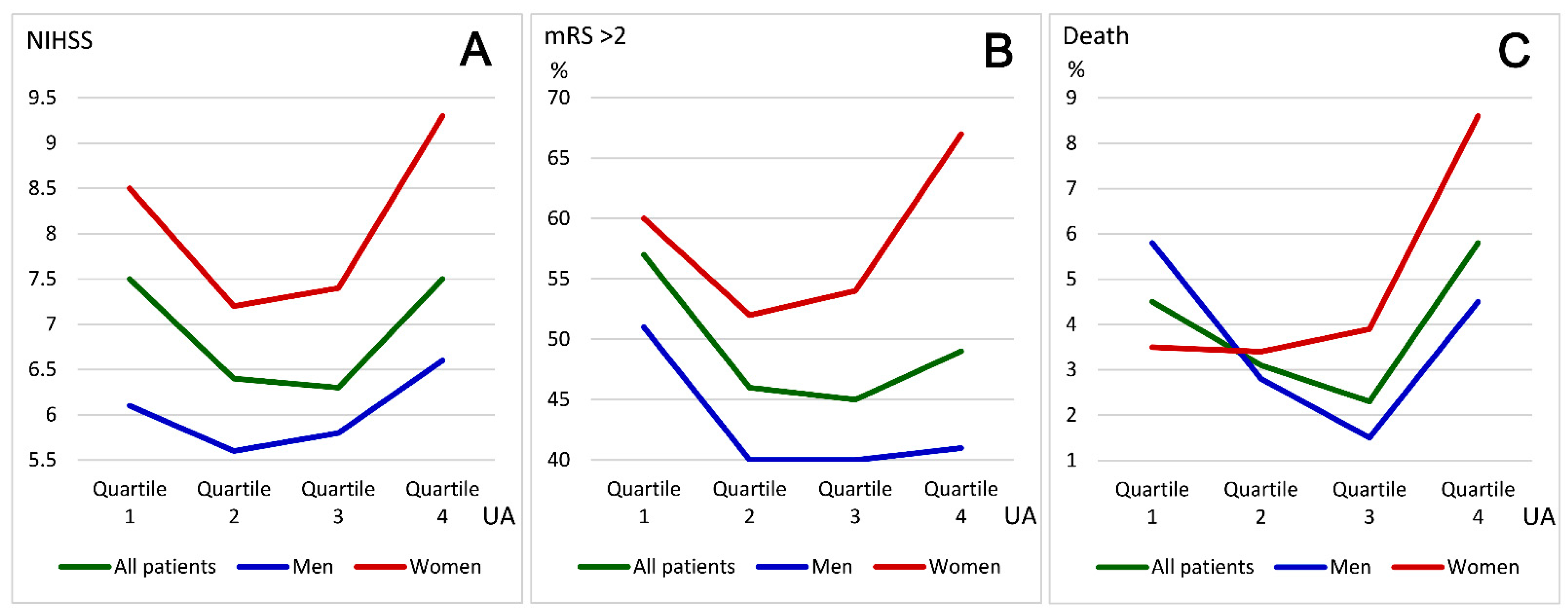
| Quartile 1 (<4.1) a | Quartile 2 (4.1–5.1) a | Quartile 3 (5.2–6.2) a | Quartile 4 (>6.2) a | p-Value | |
|---|---|---|---|---|---|
| All patients (n = 3370) | |||||
| Number of patients | 889 | 847 | 811 | 823 | |
| Age (years) | 71.0 ± 13.6 | 70.3 ± 13.5 | 70.4 ± 13.3 | 70.8 ± 14.1 | 0.619 |
| Female gender | 510 (57) | 386 (46) | 280 (35) | 269 (33) | <0.001 |
| NIHSS score on admission | 7.5 ± 7.2 | 6.4 ± 6.9 | 6.3 ± 6.6 | 7.5 ± 7.9 | <0.001 |
| mRS score >2 at discharge | 510 (57) | 386 (46) | 280 (45) | 403 (49) | <0.001 |
| Death at discharge | 40 (4.5) | 26 (3.1) | 19 (2.3) | 48 (5.8) | 0.001 |
| Male patients (n = 1925) | |||||
| Number of patients | 379 | 461 | 531 | 554 | |
| Age (years) | 69.3 ± 13.9 | 68.0 ± 12.9 | 68.0 ± 13.2 | 67.4 ± 13.9 | 0.208 |
| NIHSS score on admission | 6.1 ± 6.1 | 5.6 ± 6.2 | 5.8 ± 6.0 | 6.6 ± 7.3 | 0.080 |
| mRS score >2 at discharge | 195 (51) | 185 (40) | 211 (40) | 227 (41) | 0.001 |
| Death at discharge | 22 (5.8) | 13 (2.8) | 8 (1.5) | 25 (4.5) | 0.002 |
| Female patients (n = 1445) | |||||
| Number of patients | 510 | 386 | 280 | 269 | |
| Age (years) | 72.3 ± 13.2 | 73.0 ± 13.7 | 74.9 ± 12.1 | 77.8 ± 11.6 | <0.001 |
| NIHSS score on admission | 8.5 ± 7.7 | 7.2 ± 7.4 | 7.4 ± 7.6 | 9.3 ± 8.7 | 0.002 |
| mRS score >2 at discharge | 308 (60) | 202 (52) | 150 (54) | 180 (67) | <0.001 |
| Death at discharge | 18 (3.5) | 13 (3.4) | 11 (3.9) | 23 (8.6) | 0.005 |
| Characteristics | All Patients (n = 3370) | Male Patients (n = 1925) | Female Patients (n = 1445) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) b | Model I | Model II | OR (95% CI) | Model I | OR (95% CI) b | Model I | Model II | |
| p Value | p Value | p Value | p Value | p Value | ||||
| Age | 1.051 (1.042–1.061) | <0.001 | <0.001 | 1.045 (1.033–1.057) | <0.001 | 1.055 (1.040–1.070) | <0.001 | <0.001 |
| Admission NIHSS score | 1.422 (1.377–1.467) | <0.001 | <0.001 | 1.434 (1.377–1.494) | <0.001 | 1.409 (1.342–1.479) | <0.001 | <0.001 |
| Female gender | 1.200 (0.955–1.508) | 0.110 | 0.117 | - | - | - | - | - |
| Hemoglobin | 0.952(0.899–1.008) | 0.147 | 0.094 | 0.926 (0.861–0.996) | 0.038 | 0.992 (0.908–1.084) | 0.862 | 0.815 |
| Platelet | 0.999 (0.998–1.001) | 0.424 | 0.436 | 1.000 (0.998–1.002) | 0.736 | 0.999 (0.997–1.001) | 0.450 | 0.450 |
| White blood cells | 1.058 (1.016–1.102) | 0.005 | 0.007 | - | - | 1.131 (1.060–1.207) | <0.001 | <0.001 |
| Glucose | 1.002 (1.001–1.004) | 0.012 | 0.005 | - | - | 1.003 (1.001–1.005) | 0.043 | 0.035 |
| Creatinine | 0.978 (0.862–1.110) | 0.929 | 0.732 | 0.900 (0.771–1.049) | 0.178 | 1.074 (0.927–1.243) | 0.342 | 0.432 |
| Cholesterol | 1.003 (1.001–1.006) | 0.014 | 0.019 | 1.004 (1.000–1.008) | 0.016 | 1.003 (0.999–1.007) | 0.136 | 0.131 |
| Triglyceride | 1.000 (0.999–1.001) | 0.509 | 0.662 | 1.001 (0.999–1.002) | 0.337 | 1.000 (0.997–1.002) | 0.661 | 0.527 |
| Hypertension | 1.024 (0.825–1.270) | 0.657 | 0.832 | - | - | 1.135 (0.802–1.607) | 0.474 | 0.592 |
| Diabetes mellitus | 1.147 (0.904–1.455) | 0.309 | 0.259 | 1.389 (1.075–1.796) | 0.012 | 1.019 (0.692–1.501) | 0.925 | 0.848 |
| Dyslipidemia | 0.837 (0.653–1.072) | 0.218 | 0.159 | 0.952 (0.691–1.312) | 0.763 | 0.781 (0.533–1.144) | 0.204 | 0.166 |
| Heart disease | 0.888 (0.711–1.110) | 0.428 | 0.298 | 0.997(0.753–1.320) | 0.981 | 0.848 (0.596–1.208) | 0.361 | 0.290 |
| Prior stroke | 1.574 (1.254–1.977) | <0.001 | <0.001 | 1.683 (1.276–2.220) | <0.001 | 1.507 (1.033–2.197) | 0.033 | 0.032 |
| Current smoker | 1.023 (0.787–1.328) | 0.838 | 0.867 | 1.050 (0.810–1.361) | 0.713 | 0.629 (0.293–1.355) | 0.236 | 0.212 |
| Alcohol consumption | 0.926 (0.618–1.388) | 0.688 | 0.711 | - | - | - | - | - |
| History of cancer | 1.655 (1.139–2.405) | 0.011 | 0.008 | - | - | 1.920 (1.102–3.344) | 0.021 | 0.017 |
| Uremia | 1.492 (0.557–3.994) | 0.670 | 0.426 | 2.525 (0.730–8.727) | 0.143 | - | - | - |
| Quartile 1 of uric acid a | 1.359 (1.068–1.730) | 0.013 | - | 1.561 (1.144–2.130) | 0.005 | 1.282 (0.882–1.863) | 0.192 | - |
| Quartile 4 of uric acid a | 0.972 (0.766–1.233) | 0.815 | - | 0.915 (0.682–1.228) | 0.554 | 1.019 (0.695–1.493) | 0.925 | - |
| Hyperuricemia | 1.054 (0.764–1.452) | - | 0.749 | - | - | 1.115 (0.702–1.772) | - | 0.644 |
| Characteristics | All Patients (n = 3370) | Male Patients (n = 1925) | Female Patients (n = 1445) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) b | Model I | Model II | Model III | OR (95% CI) b | Model I | Model II | OR (95% CI) b | Model I | Model II | Model III | |
| p Value | p Value | p Value | p Value | p Value | p Value | p Value | p Value | ||||
| Age | 1.019 (1.001–1.036) | 0.025 | 0.036 | 0.030 | 1.023 (1.000–1.046) | 0.047 | 0.053 | 1.022 (0.994–1.052) | 0.095 | 0.126 | 0.134 |
| Admission NIHSS score | 1.134 (1.111–1.157) | <0.001 | <0.001 | <0.001 | 1.166 (1.131–1.202) | <0.001 | <0.001 | 1.124 (1.090–1.159) | <0.001 | <0.001 | <0.001 |
| Hemoglobin | 0.952 (0.862–1.053) | 0.360 | 0.339 | 0.291 | 0.887 (0.772–1.020) | 0.092 | 0.062 | - | - | - | - |
| Platelet | - | - | - | - | 1.001 (0.998–1.004) | 0.568 | 0.431 | - | - | - | - |
| White blood cells | 1.110 (1.049–1.176) | <0.001 | <0.001 | <0.001 | 1.137 (1.041–1.243) | 0.005 | 0.005 | 1.085 (1.002–1.174) | 0.033 | 0.044 | 0.044 |
| Glucose | 1.002 (1.000–1.004) | 0.078 | 0.084 | 0.061 | - | - | - | 1.001 (0.998–1.004) | 0.466 | 0.569 | 0.541 |
| Creatinine | 1.064 (0.883–1.283) | 0.472 | 0.515 | 0.586 | - | - | - | 1.212 (1.033–1.422) | 0.019 | 0.018 | 0.029 |
| Triglyceride | - | - | - | - | 1.001 (0.997–1.004) | 0.682 | 0.920 | - | - | - | - |
| Diabetes mellitus | - | - | - | - | - | - | - | 1.477 (0.795–2.743) | 0.201 | 0.217 | 0.210 |
| Heart disease | 1.844 (1.205–2.820) | 0.004 | 0.005 | 0.004 | 1.265 (0.688–2.328) | 0.449 | 0.542 | 2.394 (1.253–4.578) | 0.007 | 0.008 | 0.008 |
| History of cancer | 2.100 (1.114–3.958) | 0.033 | 0.022 | 0.022 | - | - | - | 2.136 (0.934–4.885) | 0.070 | 0.072 | 0.067 |
| Uremia | 1.410 (0.376–5.295) | 0.669 | 0.611 | 0.571 | - | - | - | - | - | - | - |
| Quartile 1 of uric acid a | 1.474 (0.895–2.427) | 0.127 | - | - | 3.109 (1.518–6.367) | 0.002 | - | 0.692 (0.328–1.458) | 0.333 | - | - |
| Quartile 4 of uric acid a | 1.498 (0.942–2.381) | 0.088 | - | - | 1.509 (0.750–3.036) | 0.249 | - | 1.215 (0.640–2.304) | 0.552 | - | - |
| Hyperuricemia | 1.518 (0.935–2.463) | - | 0.091 | - | 1.215 (0.554–2.665) | - | 0.627 | 1.905 (1.009–3.596) | - | 0.047 | - |
| Uric acid | 1.111 (0.973–1.169) | - | - | 0.171 | - | - | - | 1.151 (1.014–1.307) | - | - | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-Y.; Hsiao, C.-L.; Chen, P.-Y.; Tsou, A.; Tzeng, I.-S.; Lin, S.-K. J-Shaped Relationship of Serum Uric Acid with Unfavorable Short-Term Outcomes among Patients with Acute Ischemic Stroke. Biomedicines 2022, 10, 2185. https://doi.org/10.3390/biomedicines10092185
Liu C-Y, Hsiao C-L, Chen P-Y, Tsou A, Tzeng I-S, Lin S-K. J-Shaped Relationship of Serum Uric Acid with Unfavorable Short-Term Outcomes among Patients with Acute Ischemic Stroke. Biomedicines. 2022; 10(9):2185. https://doi.org/10.3390/biomedicines10092185
Chicago/Turabian StyleLiu, Chih-Yang, Cheng-Lun Hsiao, Pei-Ya Chen, Adam Tsou, I-Shiang Tzeng, and Shinn-Kuang Lin. 2022. "J-Shaped Relationship of Serum Uric Acid with Unfavorable Short-Term Outcomes among Patients with Acute Ischemic Stroke" Biomedicines 10, no. 9: 2185. https://doi.org/10.3390/biomedicines10092185
APA StyleLiu, C.-Y., Hsiao, C.-L., Chen, P.-Y., Tsou, A., Tzeng, I.-S., & Lin, S.-K. (2022). J-Shaped Relationship of Serum Uric Acid with Unfavorable Short-Term Outcomes among Patients with Acute Ischemic Stroke. Biomedicines, 10(9), 2185. https://doi.org/10.3390/biomedicines10092185






