TERT and TET2 Genetic Variants Affect Leukocyte Telomere Length and Clinical Outcome in Coronary Artery Disease Patients—A Possible Link to Clonal Hematopoiesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Methods
2.3. Genotype Analysis
2.4. Leukocyte Telomere Length (LTL) Determination
2.5. Statistical Analysis
3. Results
3.1. Frequencies of the TERT and TET2 Mutations
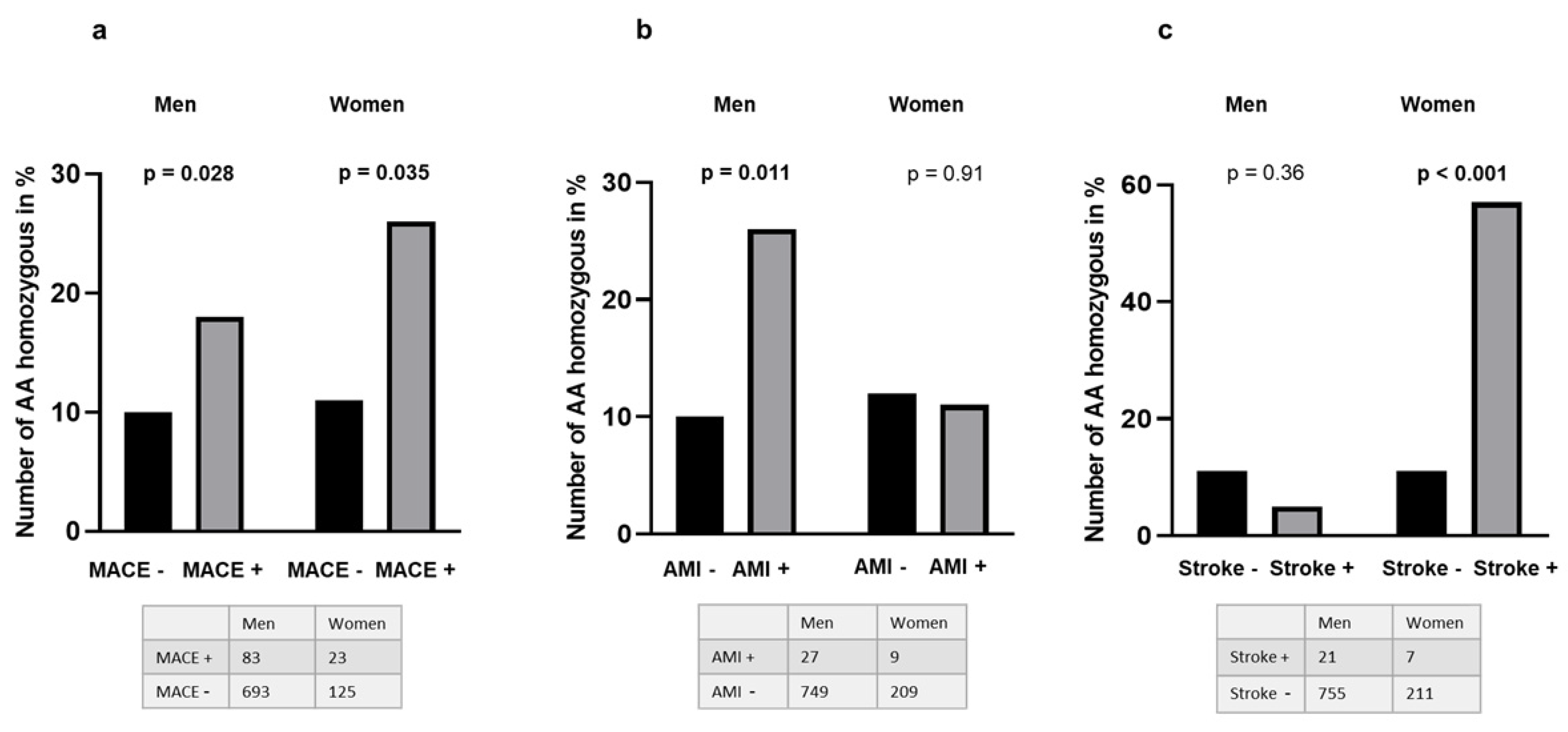
3.2. Presence of the TERT and TET2 Mutations as Related to Clinical Outcome and Comorbidity
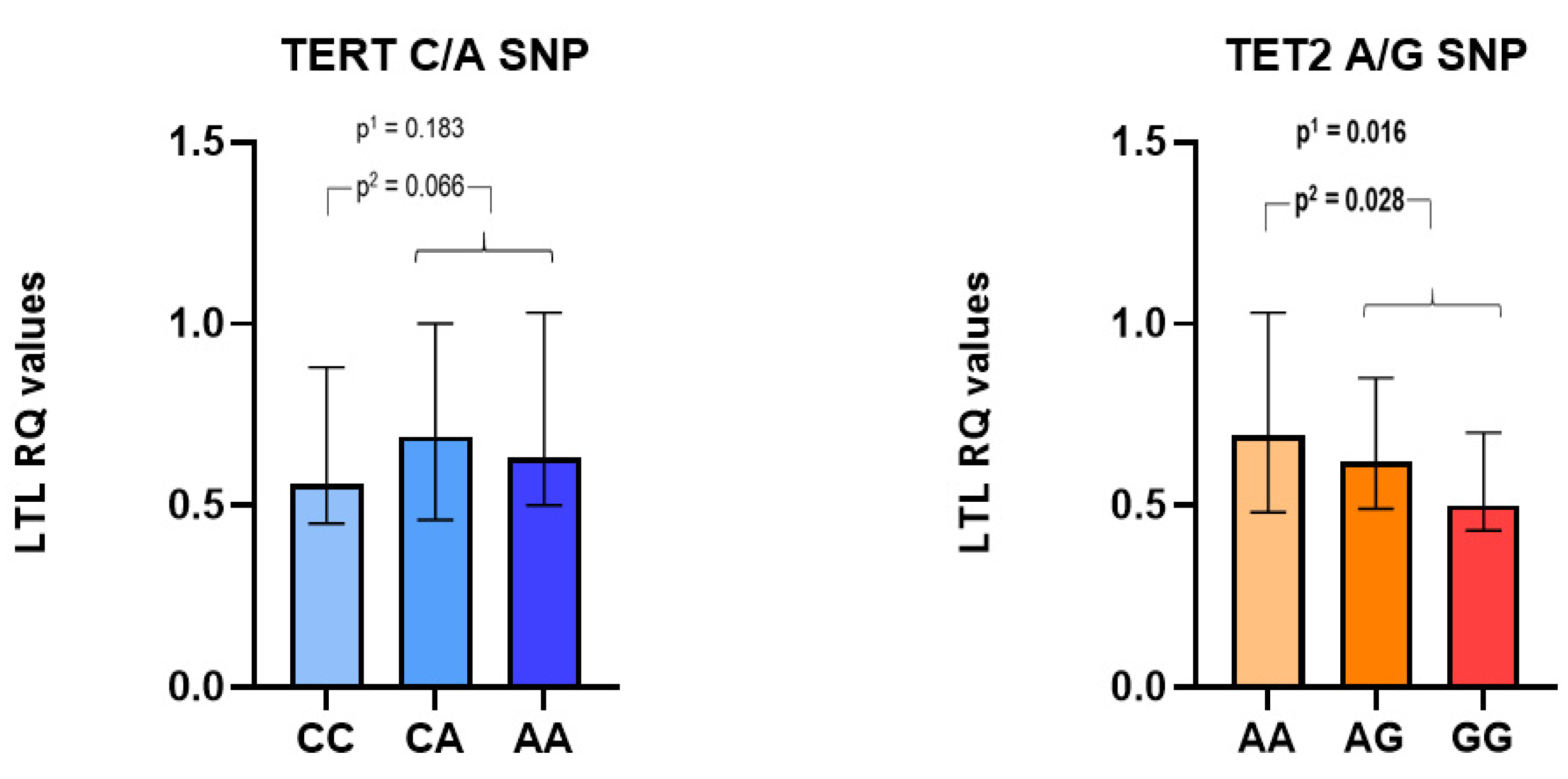
3.3. TERT and TET2 Mutations as Related to Leukocyte Telomere Lengths (LTLs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Bick, A.G.; Weinstock, J.S.; Nandakumar, S.K.; Fulco, C.P.; Bao, E.L.; Zekavat, S.M.; Szeto, M.D.; Liao, X.; Leventhal, M.J.; Nasser, J.; et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 2020, 586, 763–768. [Google Scholar] [CrossRef]
- Brown, D.W.; Lin, S.H.; Loh, P.R.; Chanock, S.J.; Savage, S.A.; Machiela, M.J. Genetically predicted telomere length is associated with clonal somatic copy number alterations in peripheral leukocytes. PLoS Genet. 2020, 16, e1009078. [Google Scholar] [CrossRef]
- Zurek, M.; Altschmied, J.; Kohlgrüber, S.; Ale-Agha, N.; Haendeler, J. Role of Telomerase in the Cardiovascular System. Genes 2016, 7, 29. [Google Scholar] [CrossRef]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- Hannen, R.; Bartsch, J.W. Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis. FEBS Lett. 2018, 592, 2023–2031. [Google Scholar] [CrossRef]
- Opstad, T.B.; Kalstad, A.A.; Holte, K.B.; Berg, T.J.; Solheim, S.; Arnesen, H.; Seljeflot, I. Shorter Leukocyte Telomere Lengths in Healthy Relatives of Patients with Coronary Heart Disease. Rejuv. Res. 2020, 23, 324–332. [Google Scholar] [CrossRef]
- Nakao, T.; Bick, A.G.; Taub, M.A.; Zekavat, S.M.; Uddin, M.M.; Niroula, A.; Carty, C.L.; Lane, J.; Honigberg, M.C.; Weinstock, J.S.; et al. Bidirectional Mendelian randomization supports bidirectional causality between telomere length and clonal hematopoiesis of intermediate potential. medRxiv 2021. [Google Scholar] [CrossRef]
- Bojesen, S.E.; Pooley, K.A.; Johnatty, S.E.; Beesley, J.; Michailidou, K.; Tyrer, J.P.; Edwards, S.L.; Pickett, H.A.; Shen, H.C.; Smart, C.E.; et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat. Genet. 2013, 45, 371–384. [Google Scholar] [CrossRef]
- Bao, E.L.; Nandakumar, S.K.; Liao, X.; Bick, A.G.; Karjalainen, J.; Tabaka, M.; Gan, O.I.; Havulinna, A.S.; Kiiskinen, T.T.J.; Lareau, C.A.; et al. Inherited myeloproliferative neoplasm risk affects haematopoietic stem cells. Nature 2020, 586, 769–775. [Google Scholar] [CrossRef]
- Bussaglia, E.; Anton, R.; Nomdedeu, J.F.; Fuentes-Prior, P. TET2 missense variants in human neoplasia. A proposal of structural and functional classification. Mol. Genet. Genom. Med. 2019, 7, e00772. [Google Scholar] [CrossRef]
- Pirola, C.J.; Scian, R.; Gianotti, T.F.; Dopazo, H.; Rohr, C.; Martino, J.S.; Castaño, G.O.; Sookoian, S. Epigenetic Modifications in the Biology of Nonalcoholic Fatty Liver Disease: The Role of DNA Hydroxymethylation and TET Proteins. Medicine 2015, 94, e1480. [Google Scholar] [CrossRef]
- Sano, S.; Oshima, K.; Wang, Y.; MacLauchlan, S.; Katanasaka, Y.; Sano, M.; Zuriaga, M.A.; Yoshiyama, M.; Goukassian, D.; Cooper, M.A.; et al. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1ꞵNLRP3 Inflammasome. J. Am. Coll. Cardiol. 2018, 71, 875–886. [Google Scholar] [CrossRef]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef]
- Hu, J.; Xu, J.; Tian, T.; Xie, J.; Fan, L.; Zhu, G.; Xia, T.; Chen, X.; Tan, Y.; Chen, X.; et al. TET2 rs2454206, TET2 rs12498609 and ASXL1 rs3746609 single nucleotide polymorphisms in patients with myelodysplastic syndromes. Blood Cells Mol. Dis. 2019, 74, 44–50. [Google Scholar] [CrossRef]
- Pettersen, A.A.; Seljeflot, I.; Abdelnoor, M.; Arnesen, H. High On-Aspirin Platelet Reactivity and Clinical Outcome in Patients with Stable Coronary Artery Disease: Results From ASCET (Aspirin Nonresponsiveness and Clopidogrel Endpoint Trial). J. Am. Heart Assoc. 2012, 1, e000703. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Zink, F.; Stacey, S.N.; Norddahl, G.L.; Frigge, M.L.; Magnusson, O.T.; Jonsdottir, I.; Thorgeirsson, T.E.; Sigurdsson, A.; Gudjonsson, S.A.; Gudmundsson, J.; et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 2017, 130, 742–752. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Collins, K. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol. Cell 2000, 6, 361–371. [Google Scholar] [CrossRef]
- Hinds, D.A.; Barnholt, K.E.; Mesa, R.A.; Kiefer, A.K.; Do, C.B.; Eriksson, N.; Mountain, J.L.; Francke, U.; Tung, J.Y.; Nguyen, H.M.; et al. Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood 2016, 128, 1121–1128. [Google Scholar] [CrossRef]
- Buscarlet, M.; Provost, S.; Zada, Y.F.; Barhdadi, A.; Bourgoin, V.; Lépine, G.; Mollica, L.; Szuber, N.; Dubé, M.-P.; Busque, L. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 2017, 130, 753–762. [Google Scholar] [CrossRef]
- Hamed, N.A.; Elhalawani, N.A.; Kassem, H.S.; Ayad, M.W.; Dammag, E.A. The Prognostic Significance of TET2 Single Nucleotide Polymorphism in Egyptian Chronic Myeloid Leukemia. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020004. [Google Scholar]
- Villivalam, S.D.; Kim, J.; Kang, S. DNMT3a and TET2 in adipocyte insulin sensitivity. Oncotarget 2018, 9, 35289–35290. [Google Scholar] [CrossRef]
- Sookoian, S.; Rosselli, M.S.; Gemma, C.; Burgueño, A.L.; Fernández Gianotti, T.; Castaño, G.O.; Pirola, C.J. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: Impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology 2010, 52, 1992–2000. [Google Scholar] [CrossRef]
- Fuster, J.J.; Zuriaga, M.A.; Zorita, V.; MacLauchlan, S.; Polackal, M.N.; Viana-Huete, V.; Ferrer-Pérez, A.; Matesanz, N.; Herrero-Cervera, A.; Sano, S.; et al. TET2-Loss-of-Function-Driven Clonal Hematopoiesis Exacerbates Experimental Insulin Resistance in Aging and Obesity. Cell Rep. 2020, 33, 108326. [Google Scholar] [CrossRef]
- Yang, J.; Guo, R.; Wang, H.; Ye, X.; Zhou, Z.; Dan, J.; Wang, H.; Gong, P.; Deng, W.; Yin, Y.; et al. Tet Enzymes Regulate Telomere Maintenance and Chromosomal Stability of Mouse ESCs. Cell Rep. 2016, 15, 1809–1821. [Google Scholar] [CrossRef]
- Hu, H.; Li, B.; Duan, S. The Alteration of Subtelomeric DNA Methylation in Aging-Related Diseases. Front. Genet. 2018, 9, 697. [Google Scholar] [CrossRef]
| With Endpoints (n = 106) | Without Endpoint (n = 895) | p | |
|---|---|---|---|
| Age (years, mean (range)) | 63 (41–80) | 62 (36–81) | 0.499 |
| Men/women n (%) | 83/23 (78/22) | 700/195 (78/22) | 0.983 |
| Type 2 diabetes Mellitus n (%) | 24 (23) | 176 (20) | 0.469 |
| Previous myocardial infarction n (%) | 57 (54) | 380 (43) | 0.026 |
| Metabolic syndrome (%) | 25 (24) | 219 (25) | 0.836 |
| Previous stroke n (%) | 6 (6) | 21 (2.3) | 0.047 |
| Hypertension n (%) | 63 (59) | 493 (55) | 0.394 |
| SBP mm/Hg | 140 (125, 150) | 140 (125, 150) | 0.831 |
| DBP mm/Hg | 80 (75, 90) | 80 (75, 90) | 0.616 |
| Current smokers n (%) | 23 (22) | 180 (20) | 0.666 |
| BMI (kg/m2) a | 27.4 (4.0) | 27.7 (9.4) | 0.742 |
| Total cholesterol (mmol/L) | 4.5 (1.0) | 4.6 (1.0) | 0.877 |
| HDL cholesterol (mmol/L) | 1.3 (0.4) | 1.3 (0.4) | 0.898 |
| LDL cholesterol (mmol/L) | 2.5 (0.8) | 2.5 (0.8) | 0.758 |
| Triglycerides (mmol/L) a | 1.5 (0.9) | 1.6 (1.1) | 0.887 |
| Fasting glucose (mmol/L) | 6.1 (1.7) | 6.0 (1.9) | 0.914 |
| HbA1c (%) | 6.05 (0.87) | 5.97 (0.91) | 0.42 |
| Medication (%) | |||
| Statins | 98 | 99 | 0.524 |
| β-lockers | 74 | 76 | 0.867 |
| Nitrates | 27 | 21 | 0.145 |
| ACE inhibitors | 31 | 26 | 0.32 |
| ARB | 26 | 24 | 0.711 |
| CCB | 27 | 25 | 0.656 |
| Diuretics | 26 | 22 | 0.417 |
| TERT Genotypes | TET2 p.Ile1762Val | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | CA | AA | VAF | p | AA | AG | GG | VAF | p | |
| CAD patients | 461 | 421 | 112 | 0.324 | 434 | 434 | 127 | 0.346 | ||
| Men (n = 777) | 346 | 345 | 85 | 0.333 | 0.033 | 342 | 339 | 96 | 0.342 | 0.74 |
| Women (n = 218) | 115 | 76 | 27 | 0.299 | 92 | 95 | 31 | 0.360 | ||
| Clinical Status | n a | TERT Genotypes | TET2 Genotypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CA | AA | VAF | p | AA | AG | GG | VAF | p | |||
| Composite endpoint | Yes | 106 | 41 | 44 | 21 | 0.406 | 0.010 | 49 | 46 | 11 | 0.321 | 0.70 |
| No | 889 | 420 | 377 | 91 | 0.315 | 385 | 388 | 116 | 0.348 | |||
| Diabetes type 2 | Yes | 198 | 93 | 85 | 20 | 0.315 | 0.85 | 198 | 74 | 82 | 0.417 | <0.001 |
| No | 797 | 368 | 336 | 92 | 0.327 | 360 | 352 | 85 | 0.328 | |||
| Metabolic syndrome | Yes | 242 | 114 | 105 | 23 | 0.312 | 0.60 | 97 | 99 | 47 | 0.397 | 0.002 |
| No | 751 | 347 | 315 | 89 | 0.329 | 337 | 334 | 80 | 0.330 | |||
| Previous MI | Yes | 433 | 209 | 173 | 51 | 0.318 | 0.40 | 190 | 193 | 51 | 0.341 | 0.69 |
| No | 561 | 252 | 248 | 61 | 0.330 | 244 | 241 | 76 | 0.350 | |||
| Previous Stroke | Yes | 26 | 16 | 7 | 3 | 0.250 | 0.25 | 8 | 12 | 6 | 0.462 | 0.196 |
| No | 967 | 445 | 413 | 109 | 0.327 | 425 | 422 | 121 | 0.343 | |||
| Hypertension | Yes | 554 | 242 | 245 | 67 | 0.342 | 0.153 | 226 | 249 | 80 | 0.369 | 0.063 |
| No | 440 | 219 | 176 | 45 | 0.302 | 208 | 185 | 47 | 0.317 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opstad, T.B.; Solheim, S.; Pettersen, A.-Å.R.; Kalstad, A.A.; Arnesen, H.; Seljeflot, I. TERT and TET2 Genetic Variants Affect Leukocyte Telomere Length and Clinical Outcome in Coronary Artery Disease Patients—A Possible Link to Clonal Hematopoiesis. Biomedicines 2022, 10, 2027. https://doi.org/10.3390/biomedicines10082027
Opstad TB, Solheim S, Pettersen A-ÅR, Kalstad AA, Arnesen H, Seljeflot I. TERT and TET2 Genetic Variants Affect Leukocyte Telomere Length and Clinical Outcome in Coronary Artery Disease Patients—A Possible Link to Clonal Hematopoiesis. Biomedicines. 2022; 10(8):2027. https://doi.org/10.3390/biomedicines10082027
Chicago/Turabian StyleOpstad, Trine B., Svein Solheim, Alf-Åge R. Pettersen, Are A. Kalstad, Harald Arnesen, and Ingebjørg Seljeflot. 2022. "TERT and TET2 Genetic Variants Affect Leukocyte Telomere Length and Clinical Outcome in Coronary Artery Disease Patients—A Possible Link to Clonal Hematopoiesis" Biomedicines 10, no. 8: 2027. https://doi.org/10.3390/biomedicines10082027
APA StyleOpstad, T. B., Solheim, S., Pettersen, A.-Å. R., Kalstad, A. A., Arnesen, H., & Seljeflot, I. (2022). TERT and TET2 Genetic Variants Affect Leukocyte Telomere Length and Clinical Outcome in Coronary Artery Disease Patients—A Possible Link to Clonal Hematopoiesis. Biomedicines, 10(8), 2027. https://doi.org/10.3390/biomedicines10082027






