How Effective Is a Late-Onset Antihypertensive Treatment? Studies with Captopril as Monotherapy and in Combination with Nifedipine in Old Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experimental Protocol
2.3. Non-Invasive Blood Pressure Measurement
2.4. Further Analyses on Heart Tissue
2.5. Ribonuclease Protection Assay
2.6. Zymography
2.7. ELISA
2.8. Histological Investigation of the Heart
2.9. Statistical Analysis
3. Results
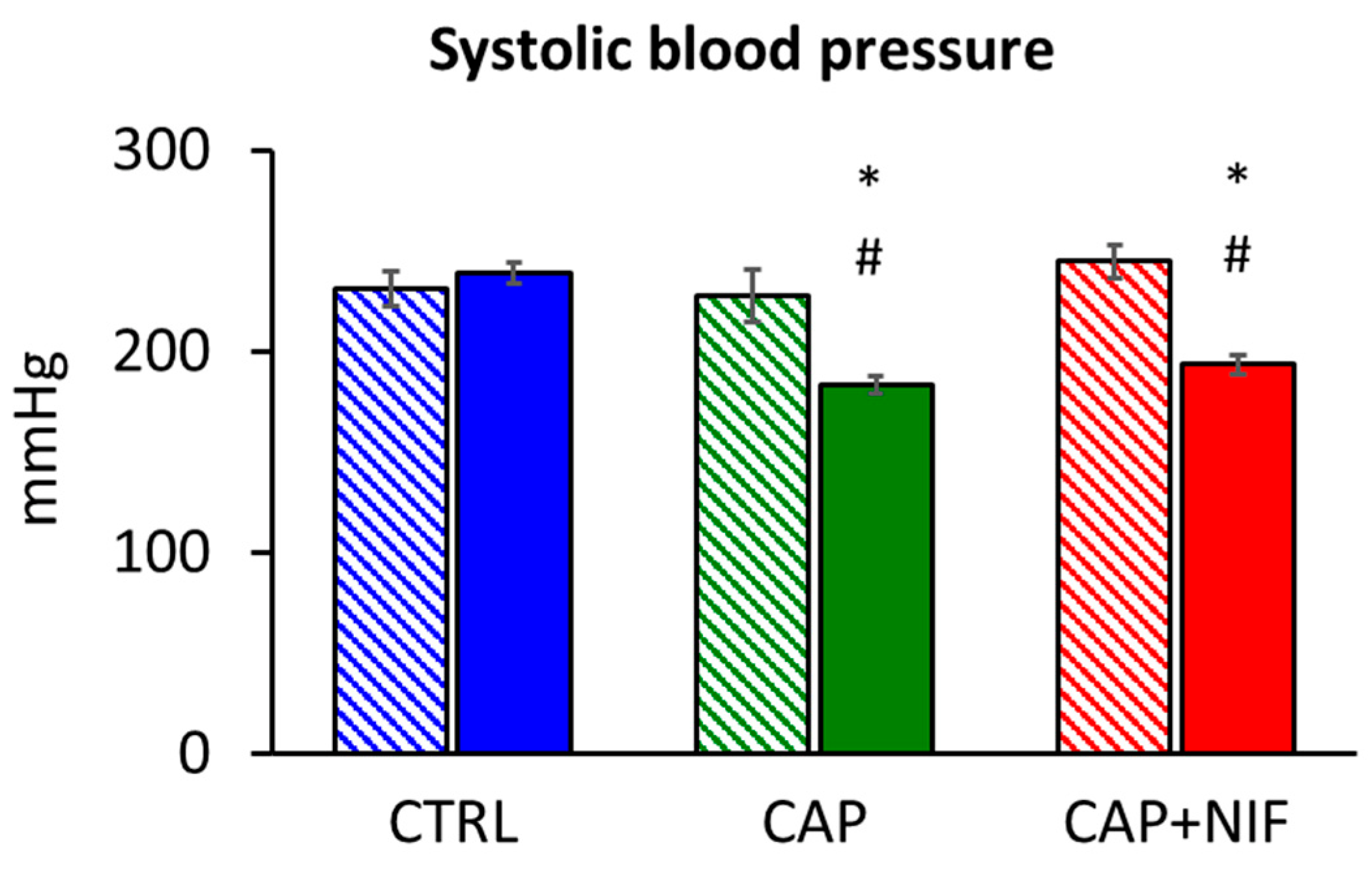
3.1. Systolic Blood Pressure
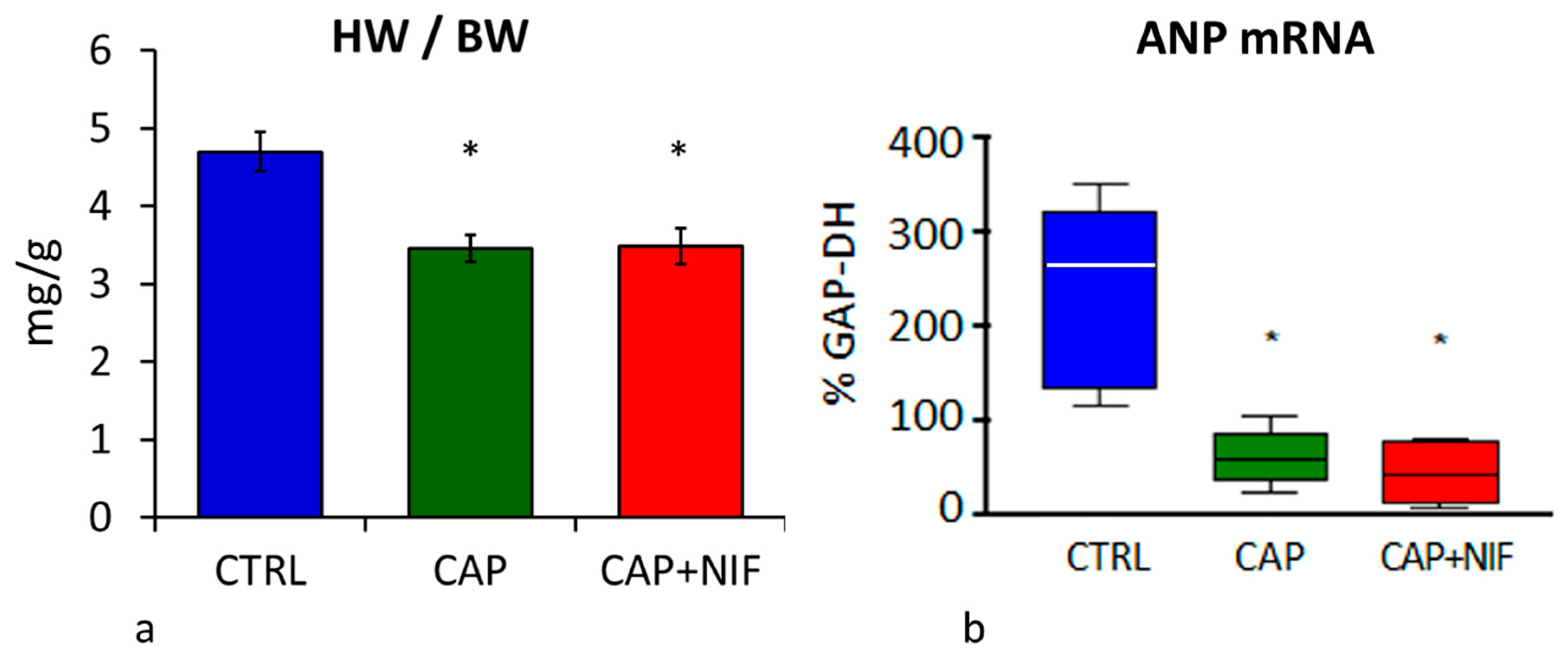
3.2. Cardiac Hypertrophy
3.2.1. Heart Weight
3.2.2. ANP mRNA Expression
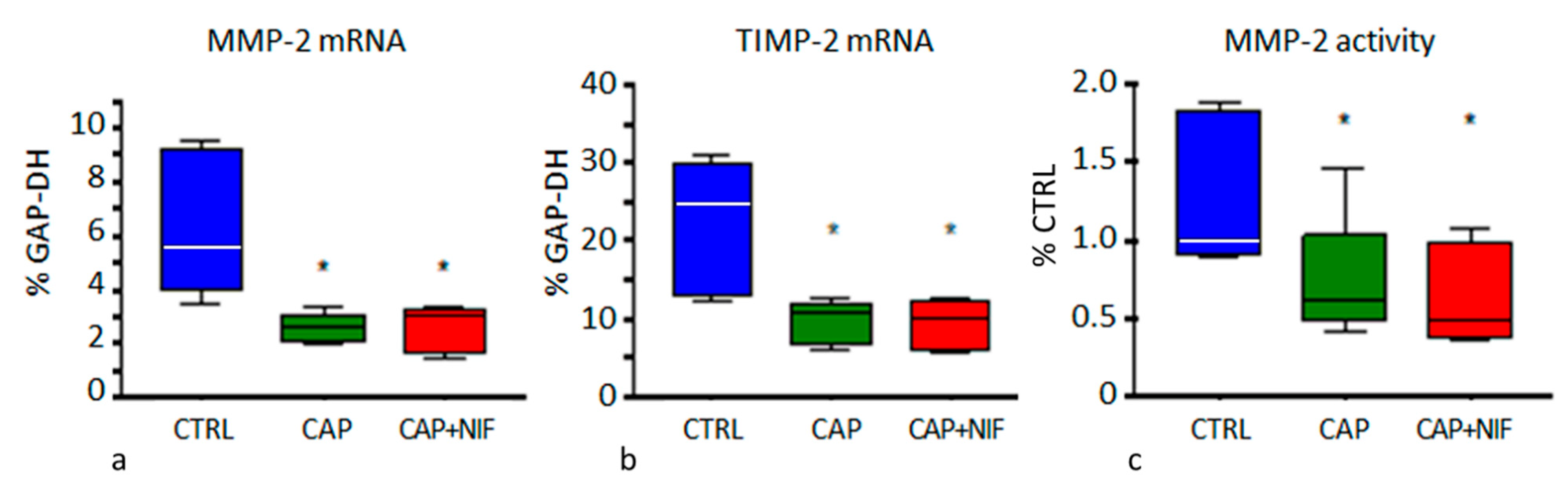
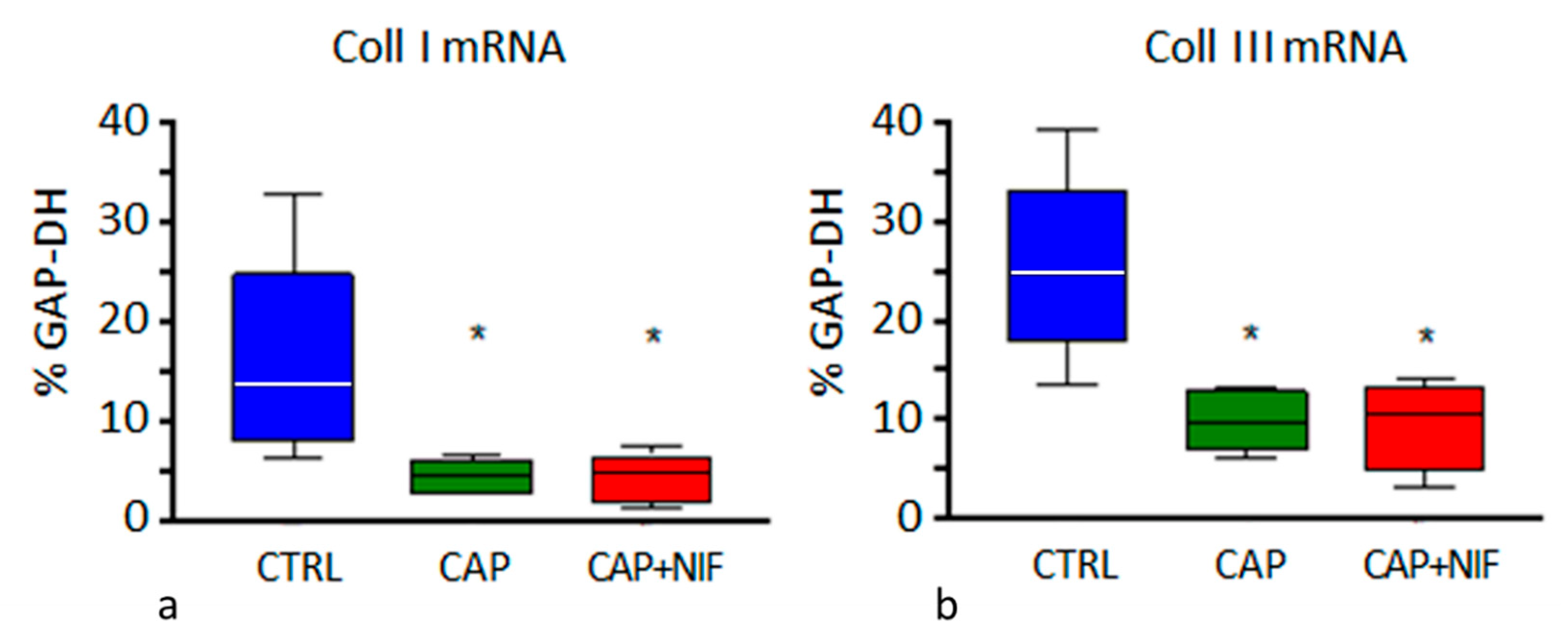
3.3. Cardiac Fibrosis
3.3.1. Biochemical Markers of Cardiac Remodeling
3.3.2. Histological Manifestation of Cardiac Fibrosis
4. Discussion
4.1. Development of Hypertension in SHR in Early and Later Stages of Life
4.2. Effects of Antihypertensive Therapies
4.3. Comparison of Monotherapy CAP with Combination Therapy CAP + NIF
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANP | atrial natriuretic peptide |
| AT | angiotensin |
| BP | blood pressure |
| BW | body weight |
| CAP | captopril (60 mg kg−1 d−1) |
| CAP + NIF | captopril plus nifedipine (60 + 10 mg kg−1 d−1) |
| Coll I | collagen type I |
| Coll III | collagen type III |
| CTRL | untreated hypertensive control rats |
| ECM | extracellular matrix |
| GAP-DH | glyceraldehyde-3-phosphate dehydrogenase |
| HW | heart weight |
| LV | left ventricle, left ventricular |
| MMP-2 | matrix metalloproteinase 2 |
| NIF | nifedipine (10 mg kg−1 d−1) |
| RAAS | renin-angiotensin-aldosterone-system |
| SBP | systolic blood pressure |
| SHR | spontaneously hypertensive rats |
| TGF-β | Transforming Growth Factor-β |
| TIMP-2 | tissue inhibitor of metalloproteinases 2 |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Demirci, B.; McKeown, P.P.; Bayraktutan, U. Blockade of angiotensin II provides additional benefits in hypertension- and ageing-related cardiac and vascular dysfunctions beyond its blood pressure-lowering effects. J. Hypertens. 2005, 23, 2219–2227. [Google Scholar] [CrossRef]
- Zicha, J.; Dobesová, Z.; Kunes, J. Late blood pressure reduction in SHR subjected to transient captopril treatment in youth: Possible mechanisms. Physiol. Res. 2008, 57, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Boluyt, M.O.; Bing, O.H. Matrix gene expression and decompensated heart failure: The aged SHR model. Cardiovasc. Res. 2000, 46, 239–249. [Google Scholar] [CrossRef]
- Frank, K.F.; Bölck, B.; Brixius, K.; Kranias, E.G.; Schwinger, R.H.G. Modulation of SERCA: Implications for the Failing Human Heart. Basic Res. Cardiol. 2002, 97, I72–I78. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.R.; Khurana, S.; Nguyen, P.; Byrne, C.J.; Tai, T.C. Comparative Analysis of Renin-Angiotensin System (RAS)-Related Gene Expression between Hypertensive and Normotensive Rats. Med. Sci. Monit. Basic Res. 2017, 23, 20–24. [Google Scholar] [CrossRef]
- Rocha, W.A.; Lunz, W.; Baldo, M.P.; Pimentel, E.B.; Dantas, E.M.; Rodrigues, S.L.; Mill, J.G. Kinetics of cardiac and vascular remodeling by spontaneously hypertensive rats after discontinuation of long-term captopril treatment. Braz. J. Med. Biol. Res. 2010, 43, 390–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, L.; Ma, A.; Guan, G.; Wang, J.; Wang, Y.; Tian, G. Telmisartan delays myocardial fibrosis in rats with hypertensive left ventricular hypertrophy by TGF- b1/Smad signal pathway. Hypertens. Res. 2014, 37, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Aritomi, S.; Harada, E.; Sugino, K.; Nishimura, M.; Nakamura, T.; Takahara, A. Comparison of the cardioprotective and renoprotective effects of the L/N-type calcium channel blocker cilnidipine in adriamycin-treated spontaneously-hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 344–352. [Google Scholar] [CrossRef]
- European Society of Cardiology/European Society of Hypertension (ESC/ESH). 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, M.; Leenen, F.H. Combination therapy as first-line treatment of arterial hypertension. Can. J. Cardiol. 2002, 18, 1317–1327. [Google Scholar]
- Paulis, L.; Lísková, S.; Pintérová, M.; Dobesová, Z.; Kunes, J.; Zicha, J. Nifedipine-sensitive noradrenergic vasoconstriction is enhanced in spontaneously hypertensive rats: The influence of chronic captopril treatment. Acta Physiol. 2007, 191, 255–266. [Google Scholar] [CrossRef]
- Hale, T.M.; Robertson, S.J.; Burns, K.D.; deBlois, D. Short-term ACE inhibition confers long-term protection against target organ damage. Hypertens. Res. 2012, 35, 604–610. [Google Scholar] [CrossRef][Green Version]
- Hawlitschek, C.; Brendel, J.; Gabriel, P.; Schierle, K.; Salameh, A.; Zimmer, H.G.; Rassler, B. Antihypertensive and cardioprotective effects of different monotherapies and combination therapies in young spontaneously hypertensive rats—A pilot study. Saudi J. Biol. Sci. 2022, 29, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Hojná, S.; Kadlecová, M.; Dobesová, Z.; Valousková, V.; Zicha, J.; Kunes, J. The participation of brain NO synthase in blood pressure control of adult spontaneously hypertensive rats. Mol. Cell. Biochem. 2007, 297, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Council of Europe. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS No 123) Strasbourg 18.III. (1986). Text Amended according to the Provisions of the Protocol (ETS No. 170) as of Its Entry into Force on 2 December 2005. Available online: https://rm.coe.int/168007a67b (accessed on 27 June 2022).
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Matsubara, L.; Weber, K.T. Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin. Biochem. 1993, 26, 191–198. [Google Scholar] [CrossRef]
- Briest, W.; Hölzl, A.; Raßler, B.; Deten, A.; Leicht, M.; Baba, H.A.; Zimmer, H.G. Cardiac remodeling after long-term norepinephrine treatment in rats. Cardiovasc. Res. 2001, 52, 265–273. [Google Scholar] [CrossRef]
- Zimmer, J.; Hawlitschek, C.; Rabald, S.; Hagendorff, A.; Zimmer, H.G.; Rassler, B. Effects of late-onset and long-term captopril and nifedipine treatment in aged spontaneously hypertensive rats: Echocardiographic studies. Hypertens. Res. 2015, 38, 716–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anishchenko, A.M.; Aliev, O.I.; Sidekhmenova, A.V.; Shamanaev, A.Y.; Plotnikov, M.B. Dynamics of Blood Pressure Elevation and Endothelial Dysfunction in SHR Rats During the Development of Arterial Hypertension. Bull. Exp. Biol. Med. 2015, 159, 591–593. [Google Scholar] [CrossRef]
- Bencze, M.; Behuliak, M.; Vavrínová, A.; Zicha, J. Altered contractile responses of arteries from spontaneously hypertensive rat: The role of endogenous mediators and membrane depolarization. Life Sci. 2016, 166, 46–53. [Google Scholar] [CrossRef]
- Waleska, C.D.; Marcelo, E.S. Animal models for the study of arterial hypertension. J. Biosci. 2011, 36, 731–737. [Google Scholar]
- Kokubo, M.; Uemura, A.; Matsubara, T.; Murohara, T. Noninvasive evaluation of the time course of change in cardiac function in spontaneously hypertensive rats by echocardiography. Hypertens. Res. 2005, 28, 601–609. [Google Scholar] [CrossRef][Green Version]
- Van Empel, V.P.; De Windt, L.J. Myocyte hypertrophy and apoptosis: A balancing act. Cardiovasc. Res. 2004, 63, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, G.L.; Vitullo, J.C.; Gerrity, R.G. Morphometric analysis of cardiac hypertrophy during development maturation and senescence in spontaneously hypertensive rats. Circ. Res. 1987, 60, 487–494. [Google Scholar] [CrossRef]
- Day, M.L.; Schwartz, D.; Wiegand, R.C.; Stockman, P.T.; Brunnert, S.R.; Tolunay, H.E.; Currie, M.G.; Standaert, D.G.; Needleman, P. Ventricular Atriopeptin. Unmasking of Messenger RNA and Peptide Synthesis by Hypertrophy or Dexamethasone. Hypertension 1987, 9, 485–491. [Google Scholar] [CrossRef]
- Du, X.J. Divergence of hypertrophic growth and fetal gene profile: The influence of β-blockers. Br. J. Pharmacol. 2007, 152, 169–171. [Google Scholar] [CrossRef]
- Hall, M.C.; Young, D.A.; Waters, J.G.; Rowan, A.D.; Chantry, A.; Edwards, D.R.; Clark, I.M. The Comparative Role of Activator Protein 1 and Smad Factors in the Regulation of Timp-1 and MMP-1 Gene Expression by Transforming Growth Factor-β1. J. Biol. Chem. 2003, 278, 10304–10313. [Google Scholar] [CrossRef]
- Wu, J.; Jackson-Weaver, O.; Xu, J. The TGFβ superfamily in cardiac dysfunction. Acta Biochim. Biophys. Sin. 2018, 50, 323–335. [Google Scholar] [CrossRef]
- Perrucci, G.L.; Barbagallo, V.A.; Corlianò, M.; Tosi, D.; Santoro, R.; Nigro, P.; Poggio, P.; Bulfamante, G.; Lombardi, F.; Pompilio, G. Integrin ανβ5 in vitro inhibition limits pro-fibrotic response in cardiac fibroblasts of spontaneously hypertensive rats. J. Transl. Med. 2018, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Li, H.; Zeng, C.; Chen, W.; Wei, L.; Liu, Y.; Qi, X. Endogenous CCN5 Participates in Angiotensin II/TGF-β1 Networking of Cardiac Fibrosis in High Angiotensin II-Induced Hypertensive Heart Failure. Front. Pharmacol. 2020, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.H.; Brooks, W.W.; Hayes, J.A.; Sen, S.; Robinson, K.G.; Bing, O.H. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 1995, 91, 161–170. [Google Scholar] [CrossRef]
- Kost, C.K., Jr.; Li, P.; Jackson, E.K. Blood Pressure after Captopril Withdrawal from Spontaneously Hypertensive Rats. Hypertension 1995, 25, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Harrap, S.B.; Van der Merwe, W.M.; Griffin, S.A.; Macpherson, F.; Lever, A.F. Brief angiotensin converting enzyme inhibitor treatment in young spontaneously hypertensive rats reduces blood pressure long-term. Hypertension 1990, 16, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ohishi, M.; Yamamoto, K.; Tatara, Y.; Shiota, A.; Hayashi, N.; Komai, N.; Yanagitani, Y.; Rakugi, H.; Ogihara, T. Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 792–799. [Google Scholar] [CrossRef]
- Susic, D.; Varagic, J.; Frohlich, E.D. Pharmacologic agents on cardiovascular mass coronary dynamics and collagen in aged spontaneously hypertensive rats. J. Hypertens. 1999, 17, 1209–1215. [Google Scholar] [CrossRef]
- Mukherjee, D.; Sen, S. Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats. Circ. Res. 1990, 67, 1474–1480. [Google Scholar] [CrossRef]
- Brooks, W.W.; Bing, A.H.L.; Robinson, K.G.; Slawsky, M.T.; Chaletsky, D.M.; Conrad, C.H. Effect of angiotensin-converting enzyme inhibition on myocardial fibrosis and function in hypertrophied and failing myocardium from the spontaneously hypertensive rat. Circulation 1997, 96, 4002–4010. [Google Scholar] [CrossRef]
- Qi, G.; Jia, L.; Li, Y.; Bian, Y.; Cheng, J.; Li, H.; Xiao, C.; Du, J. Angiotensin II infusion- induced inflammation monocytic fibroblast precursor infiltration and cardiac fibrosis are pressure dependent. Cardiovasc. Toxicol. 2011, 11, 157–167. [Google Scholar] [CrossRef]
- Ma, F.; Li, Y.; Jia, L.; Han, Y.; Cheng, J.; Li, H.; Qi, Y.; Du, J. Macrophage-Stimulated Cardiac Fibroblast Production of IL-6 Is Essential for TGF β/Smad Activation and Cardiac Fibrosis Induced by Angiotensin II. PLoS ONE 2012, 7, e35144. [Google Scholar] [CrossRef] [PubMed]
- Araujo, I.G.; Trindade, D.C.; Mecawi, A.S.; Sonoda-Cortes, R.; Werneck-de-Castro, J.P.S.; Costa-e-Sousa, R.H.; Reis, L.C.; Olivares, E.L. Inhibition of brain renin- Angiotensin system improves diastolic cardiac function following myocardial infarction in rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Ziegelhöffer-Mihalovicova, B.; Arnold, N.; Marx, G.; Tannapfel, A.; Zimmer, H.G.; Rassler, B. Effects of salt loading and various therapies on cardiac hypertrophy and fibrosis in young spontaneously hypertensive rats. Life Sci. 2006, 79, 838–846. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Young SHR (10 Weeks) | Old SHR (82 Weeks) | p Value |
|---|---|---|---|
| final SBP | 202 ± 6 | 239 ± 5 | 0.002 |
| HW/BW | 3.50 ± 0.07 | 4.70 ± 0.25 | 0.003 |
| ANP mRNA | 11.0 [7.5/20.3] | 264 [154/293] | 0.008 |
| TGF-β1 mRNA | 1.37 [1.34/1.54] | 2.68 [2.10/2.74] | 0.004 |
| TGF-β2 mRNA | 0.16 [0.14/0.18] | 0.56 [0.55/0.72] | 0.004 |
| TGF-β3 mRNA | 0.64 [0.54/0.67] | 1.02 [0.93/1.12] | 0.02 |
| MMP-2 mRNA | 3.92 [3.57/4.69] | 5.58 [4.43/8.98] | 0.222 |
| TIMP-2 mRNA | 8.01 [6.95/10.2] | 24.9 [13.7/28.8] | 0.008 |
| Coll I mRNA | 16.3 [9.6/16.6] | 13.8 [9.7/16.7] | 1.0 |
| Coll III mRNA | 20.9 [20.1/22.4] | 24.8 [22.4/26.8] | 0.548 |
| Degree of fibrosis | 1.47 ± 0.12 | 2.30 ± 0.11 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawlitschek, C.; Brendel, J.; Gabriel, P.; Schierle, K.; Salameh, A.; Zimmer, H.-G.; Rassler, B. How Effective Is a Late-Onset Antihypertensive Treatment? Studies with Captopril as Monotherapy and in Combination with Nifedipine in Old Spontaneously Hypertensive Rats. Biomedicines 2022, 10, 1964. https://doi.org/10.3390/biomedicines10081964
Hawlitschek C, Brendel J, Gabriel P, Schierle K, Salameh A, Zimmer H-G, Rassler B. How Effective Is a Late-Onset Antihypertensive Treatment? Studies with Captopril as Monotherapy and in Combination with Nifedipine in Old Spontaneously Hypertensive Rats. Biomedicines. 2022; 10(8):1964. https://doi.org/10.3390/biomedicines10081964
Chicago/Turabian StyleHawlitschek, Christina, Julia Brendel, Philipp Gabriel, Katrin Schierle, Aida Salameh, Heinz-Gerd Zimmer, and Beate Rassler. 2022. "How Effective Is a Late-Onset Antihypertensive Treatment? Studies with Captopril as Monotherapy and in Combination with Nifedipine in Old Spontaneously Hypertensive Rats" Biomedicines 10, no. 8: 1964. https://doi.org/10.3390/biomedicines10081964
APA StyleHawlitschek, C., Brendel, J., Gabriel, P., Schierle, K., Salameh, A., Zimmer, H.-G., & Rassler, B. (2022). How Effective Is a Late-Onset Antihypertensive Treatment? Studies with Captopril as Monotherapy and in Combination with Nifedipine in Old Spontaneously Hypertensive Rats. Biomedicines, 10(8), 1964. https://doi.org/10.3390/biomedicines10081964





