Leber Hereditary Optic Neuropathy: Molecular Pathophysiology and Updates on Gene Therapy
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Study Selection
2.2. Quality Assessment
2.3. Data Extraction
3. Results
3.1. Patient Characteristics
3.2. Risk of Bias Assessment
3.3. Trial Design and BCVA Change: Bilateral VA Improvement after Unilateral Injection
3.4. Optical Coherence Tomography (OCT)—Inconsistent Results
3.5. Humphrey Visual Field (HVF)—Inconsistent Results
3.6. Quality of Life (QOL)—Improvement in Several Scales of National Eye Institute Visual Function Questionnaire 25
3.7. Safety Profile and Dose—Generally, No Severe Adverse Events Were Noted
4. Discussion
4.1. Key Findings
4.2. Bilateral Visual Improvement after Unilateral Injection
4.3. Comparing the Intravitreal Gene Therapy vs. Natural History
4.4. Future Perspectives and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riordan-Eva, P.; Sanders, M.D.; Govan, G.G.; Sweeney, M.G.; Da Costa, J.; Harding, A.E. The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain J. Neurol. 1995, 118 Pt 2, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Turnbull, D.M.; Chinnery, P.F. Leber hereditary optic neuropathy. J. Med. Genet. 2002, 39, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Puomila, A.; Hämäläinen, P.; Kivioja, S.; Savontaus, M.L.; Koivumäki, S.; Huoponen, K.; Nikoskelainen, E. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur. J. Hum. Genet. EJHG 2007, 15, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Griffiths, P.G.; Brown, D.T.; Howell, N.; Turnbull, D.M.; Chinnery, P.F. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 2003, 72, 333–339. [Google Scholar] [CrossRef]
- Harding, A.E.; Sweeney, M.G.; Govan, G.G.; Riordan-Eva, P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am. J. Hum. Genet. 1995, 57, 77–86. [Google Scholar]
- Meyerson, C.; Van Stavern, G.; McClelland, C. Leber hereditary optic neuropathy: Current perspectives. Clin. Ophthalmol. 2015, 9, 1165–1176. [Google Scholar] [CrossRef]
- Newman, N.J.; Lott, M.T.; Wallace, D.C. The clinical characteristics of pedigrees of Leber’s hereditary optic neuropathy with the 11,778 mutation. Am. J. Ophthalmol. 1991, 111, 750–762. [Google Scholar] [CrossRef]
- Lam, B.L.; Feuer, W.J.; Schiffman, J.C.; Porciatti, V.; Vandenbroucke, R.; Rosa, P.R.; Gregori, G.; Guy, J. Trial End Points and Natural History in Patients with G11778A Leber Hereditary Optic Neuropathy: Preparation for Gene Therapy Clinical Trial. JAMA Ophthalmol. 2014, 132, 428–436. [Google Scholar] [CrossRef]
- Milenkovic, D.; Blaza, J.N.; Larsson, N.G.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef]
- Vogel, R.O.; Smeitink, J.A.M.; Nijtmans, L.G.J. Human mitochondrial complex I assembly: A dynamic and versatile process. Biochim. Biophys. Acta BBA Bioenerg. 2007, 1767, 1215–1227. [Google Scholar] [CrossRef]
- Wong, A.; Cavelier, L.; Collins-Schramm, H.E.; Seldin, M.F.; McGrogan, M.; Savontaus, M.L.; Cortopassi, G.A. Differentiation-specific effects of LHON mutations introduced into neuronal NT2 cells. Hum. Mol. Genet. 2002, 11, 431–438. [Google Scholar] [CrossRef]
- King, M.P.; Attardi, G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science 1989, 246, 500–503. [Google Scholar] [CrossRef]
- Brown, M.D.; Trounce, I.A.; Jun, A.S.; Allen, J.C.; Wallace, D.C. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11,778, or 14,484 Leber’s hereditary optic neuropathy mitochondrial DNA mutation. J. Biol. Chem. 2000, 275, 39831–39836. [Google Scholar] [CrossRef]
- Carelli, V.; Ghelli, A.; Bucchi, L.; Montagna, P.; De Negri, A.; Leuzzi, V.; Carducci, C.; Lenaz, G.; Lugaresi, E.; Degli Esposti, M. Biochemical features of mtDNA 14,484 (ND6/M64V) point mutation associated with Leber’s hereditary optic neuropathy. Ann. Neurol. 1999, 45, 320–328. [Google Scholar] [CrossRef]
- Cock, H.R.; Cooper, J.M.; Schapira, A.H. Functional consequences of the 3460-bp mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. J. Neurol. Sci. 1999, 165, 10–17. [Google Scholar] [CrossRef]
- Baracca, A.; Solaini, G.; Sgarbi, G.; Lenaz, G.; Baruzzi, A.; Schapira, A.H.; Martinuzzi, A.; Carelli, V. Severe impairment of complex I-driven adenosine triphosphate synthesis in leber hereditary optic neuropathy cybrids. Arch. Neurol. 2005, 62, 730–736. [Google Scholar] [CrossRef]
- Carelli, V.; Rugolo, M.; Sgarbi, G.; Ghelli, A.; Zanna, C.; Baracca, A.; Lenaz, G.; Napoli, E.; Martinuzzi, A.; Solaini, G. Bioenergetics shapes cellular death pathways in Leber’s hereditary optic neuropathy: A model of mitochondrial neurodegeneration. Biochim. Biophys. Acta 2004, 1658, 172–179. [Google Scholar] [CrossRef]
- Carelli, V.; Napoli, E.; Valentino, L.; Martinuzzi, A. ROS production in cybrids carrying the three primary mutations associated with Leber’s hereditary optic neuropathy. Neurology 2002, 58 (Suppl. 3), A507. [Google Scholar]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, and apoptosis. Am. J. Med. Genet. 2001, 106, 62–70. [Google Scholar] [CrossRef]
- Danielson, S.R.; Wong, A.; Carelli, V.; Martinuzzi, A.; Schapira, A.H.V.; Cortopassi, G.A. Cells Bearing Mutations Causing Leber’s Hereditary Optic Neuropathy Are Sensitized to Fas-induced Apoptosis. J. Biol. Chem. 2002, 277, 5810–5815. [Google Scholar] [CrossRef]
- Zanna, C.; Ghelli, A.; Porcelli, A.M.; Carelli, V.; Martinuzzi, A.; Rugolo, M. Apoptotic cell death of cybrid cells bearing Leber’s hereditary optic neuropathy mutations is caspase independent. Ann. N. Y. Acad. Sci. 2003, 1010, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Kruse, S.E.; Watt, W.C.; Marcinek, D.J.; Kapur, R.P.; Schenkman, K.A.; Palmiter, R.D. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 2008, 7, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.K.; Song, L.; Murray, K.D.; van der List, D.; Sun, C.; Shen, Y.; Xia, Z.; Cortopassi, G.A. Mitochondrial complex I deficiency leads to inflammation and retinal ganglion cell death in the Ndufs4 mouse. Hum. Mol. Genet. 2015, 24, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.K.; Datta, S.; McMackin, M.Z.; Cortopassi, G.A. Rescue of cell death and inflammation of a mouse model of complex 1-mediated vision loss by repurposed drug molecules. Hum. Mol. Genet. 2017, 26, 4929–4936. [Google Scholar] [CrossRef]
- Fan, W.; Waymire, K.G.; Narula, N.; Li, P.; Rocher, C.; Coskun, P.E.; Vannan, M.A.; Narula, J.; Macgregor, G.R.; Wallace, D.C. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science 2008, 319, 958–962. [Google Scholar] [CrossRef]
- Lin, C.S.; Sharpley, M.S.; Fan, W.; Waymire, K.G.; Sadun, A.A.; Carelli, V.; Ross-Cisneros, F.N.; Baciu, P.; Sung, E.; McManus, M.J.; et al. Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc. Natl. Acad. Sci. USA 2012, 109, 20065–20070. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A. Idebenone: A Review in Leber’s Hereditary Optic Neuropathy. Drugs 2016, 76, 805–813. [Google Scholar] [CrossRef]
- Klopstock, T.; Yu-Wai-Man, P.; Dimitriadis, K.; Rouleau, J.; Heck, S.; Bailie, M.; Atawan, A.; Chattopadhyay, S.; Schubert, M.; Garip, A.; et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain J. Neurol. 2011, 134, 2677–2686. [Google Scholar] [CrossRef]
- Amore, G.; Romagnoli, M.; Carbonelli, M.; Barboni, P.; Carelli, V.; La Morgia, C. Therapeutic Options in Hereditary Optic Neuropathies. Drugs 2021, 81, 57–86. [Google Scholar] [CrossRef]
- Bonnet, C.; Augustin, S.; Ellouze, S.; Bénit, P.; Bouaita, A.; Rustin, P.; Sahel, J.A.; Corral-Debrinski, M. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim. Biophys. Acta 2008, 1783, 1707–1717. [Google Scholar] [CrossRef]
- Bonnet, C.; Kaltimbacher, V.; Ellouze, S.; Augustin, S.; Bénit, P.; Forster, V.; Rustin, P.; Sahel, J.A.; Corral-Debrinski, M. Allotopic mRNA localization to the mitochondrial surface rescues respiratory chain defects in fibroblasts harboring mitochondrial DNA mutations affecting complex I or v subunits. Rejuvenation Res. 2007, 10, 127–144. [Google Scholar] [CrossRef]
- Koilkonda, R.D.; Chou, T.H.; Porciatti, V.; Hauswirth, W.W.; Guy, J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch. Ophthalmol. 2010, 128, 876–883. [Google Scholar] [CrossRef]
- Guy, J.; Qi, X.; Pallotti, F.; Schon, E.A.; Manfredi, G.; Carelli, V.; Martinuzzi, A.; Hauswirth, W.W.; Lewin, A.S. Rescue of a mitochondrial deficiency causing Leber Hereditary Optic Neuropathy. Ann. Neurol. 2002, 52, 534–542. [Google Scholar] [CrossRef]
- Qi, X.; Sun, L.; Lewin, A.S.; Hauswirth, W.W.; Guy, J. The Mutant Human ND4 Subunit of Complex I Induces Optic Neuropathy in the Mouse. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1–10. [Google Scholar] [CrossRef]
- Ellouze, S.; Augustin, S.; Bouaita, A.; Bonnet, C.; Simonutti, M.; Forster, V.; Picaud, S.; Sahel, J.A.; Corral-Debrinski, M. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am. J. Hum. Genet. 2008, 83, 373–387. [Google Scholar] [CrossRef]
- Cwerman-Thibault, H.; Augustin, S.; Lechauve, C.; Ayache, J.; Ellouze, S.; Sahel, J.A.; Corral-Debrinski, M. Nuclear expression of mitochondrial ND4 leads to the protein assembling in complex I and prevents optic atrophy and visual loss. Mol. Ther. Methods Clin. Dev. 2015, 2, 15003. [Google Scholar] [CrossRef]
- Vignal, C.; Uretsky, S.; Fitoussi, S.; Galy, A.; Blouin, L.; Girmens, J.F.; Bidot, S.; Thomasson, N.; Bouquet, C.; Valero, S.; et al. Safety of rAAV2/2-ND4 Gene Therapy for Leber Hereditary Optic Neuropathy. Ophthalmology 2018, 125, 945–947. [Google Scholar] [CrossRef]
- Bouquet, C.; Vignal Clermont, C.; Galy, A.; Fitoussi, S.; Blouin, L.; Munk, M.R.; Valero, S.; Meunier, S.; Katz, B.; Sahel, J.A.; et al. Immune Response and Intraocular Inflammation in Patients with Leber Hereditary Optic Neuropathy Treated with Intravitreal Injection of Recombinant Adeno-Associated Virus 2 Carrying the ND4 Gene: A Secondary Analysis of a Phase 1/2 Clinical Trial. JAMA Ophthalmol. 2019, 137, 399–406. [Google Scholar] [CrossRef]
- GenSight. GenSight Biologics Completes Enrollment of GS010 REFLECT Phase III Trial in the Treatment of Leber Hereditary Optic Neuropathy Ahead of Schedule. 2019. Available online: https://www.businesswire.com/news/home/20190711005645/en/GenSight-Biologics-completes-enrollment-of-GS010-REFLECT-Phase-III-trial-in-the-treatment-of-Leber-Hereditary-Optic-Neuropathy-ahead-of-schedule (accessed on 11 July 2019).
- Guy, J.; Feuer, W.J.; Davis, J.L.; Porciatti, V.; Gonzalez, P.J.; Koilkonda, R.D.; Yuan, H.; Hauswirth, W.W.; Lam, B.L. Gene Therapy for Leber Hereditary Optic Neuropathy: Low- and Medium-Dose Visual Results. Ophthalmology 2017, 124, 1621–1634. [Google Scholar] [CrossRef]
- Feuer, W.J.; Schiffman, J.C.; Davis, J.L.; Porciatti, V.; Gonzalez, P.; Koilkonda, R.D.; Yuan, H.; Lalwani, A.; Lam, B.L.; Guy, J. Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results. Ophthalmology 2016, 123, 558–570. [Google Scholar] [CrossRef]
- Lam, B.L.; Feuer, W.J.; Abukhalil, F.; Porciatti, V.; Hauswirth, W.W.; Guy, J. Leber hereditary optic neuropathy gene therapy clinical trial recruitment: Year 1. Arch. Ophthalmol. 2010, 128, 1129–1135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, S.; Ma, S.Q.; Wan, X.; He, H.; Pei, H.; Zhao, M.J.; Chen, C.; Wang, D.W.; Dong, X.Y.; Yuan, J.J.; et al. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy. EBioMedicine 2016, 10, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, H.; Ma, S.Q.; Wang, S.S.; He, H.; Zhao, M.J.; Li, B. Evaluation of Leber’s hereditary optic neuropathy patients prior to a gene therapy clinical trial. Medicine 2016, 95, e5110. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.J.; Zhang, Y.; Wang, L.L.; Cheng, M.S.; Ma, S.Q.; Gao, Q.; Li, B. Visual Field Variability after Gene Therapy for Leber’s Hereditary Optic Neuropathy. Ophthalmic Res. 2018, 60, 176–184. [Google Scholar] [CrossRef]
- Liu, H.L.; Yuan, J.J.; Zhang, Y.; Tian, Z.; Li, X.; Wang, D.; Du, Y.Y.; Song, L.; Li, B. Factors associated with rapid improvement in visual acuity in patients with Leber’s hereditary optic neuropathy after gene therapy. Acta Ophthalmol. 2020, 98, e730–e733. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Yuan, J.; Tian, Z.; Liu, H.; Wang, D.; Li, B. Prognostic factors for visual acuity in patients with Leber’s hereditary optic neuropathy after rAAV2-ND4 gene therapy. Clin. Exp. Ophthalmol. 2019, 47, 774–778. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Newman, N.J.; Carelli, V.; Moster, M.L.; Biousse, V.; Sadun, A.A.; Klopstock, T.; Vignal-Clermont, C.; Sergott, R.C.; Rudolph, G.; et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci. Transl. Med. 2020, 12, eaaz7423. [Google Scholar] [CrossRef]
- Biousse, V.; Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Moster, M.L.; Vignal-Clermont, C.; Klopstock, T.; Sadun, A.A.; Sergott, R.C.; Hage, R.; et al. Long-Term Follow-Up after Unilateral Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy: The RESTORE Study. J. Neuro Ophthalmol. Off. J. N. Am. Neuro Ophthalmol. Soc. 2021, 41, 309–315. [Google Scholar] [CrossRef]
- Moster, M.L.; Sergott, R.C.; Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Bryan, M.S.; Smits, G.; Biousse, V.; Vignal-Clermont, C.; Klopstock, T.; et al. Cross-Sectional Analysis of Baseline Visual Parameters in Subjects Recruited into the RESCUE and REVERSE ND4-LHON Gene Therapy Studies. J. Neuro Ophthalmol. Off. J. N. Am. Neuro Ophthalmol. Soc. 2021, 41, 298–308. [Google Scholar] [CrossRef]
- Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Moster, M.L.; Biousse, V.; Vignal-Clermont, C.; Sergott, R.C.; Klopstock, T.; Sadun, A.A.; Barboni, P.; et al. Efficacy and Safety of Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy Treated within 6 Months of Disease Onset. Ophthalmology 2021, 128, 649–660. [Google Scholar] [CrossRef]
- Wan, X.; Pei, H.; Zhao, M.; Yang, S.; Hu, W.; He, H.; Ma, S.; Zhang, G.; Dong, X.; Chen, C.; et al. Efficacy and Safety of rAAV2-ND4 Treatment for Leber’s Hereditary Optic Neuropathy. Sci. Rep. 2016, 6, 21587. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Liu, H.; Wang, D.; Du, Y.; Tian, Z.; Li, X.; Yang, S.; Pei, H.; Wan, X.; et al. Seven-Year Follow-up of Gene Therapy for Leber’s Hereditary Optic Neuropathy. Ophthalmology 2020, 127, 1125–1127. [Google Scholar] [CrossRef]
- Vignal-Clermont, C.; Girmens, J.F.; Audo, I.; Said, S.M.; Errera, M.H.; Plaine, L.; O’Shaughnessy, D.; Taiel, M.; Sahel, J.A. Safety of Intravitreal Gene Therapy for Treatment of Subjects with Leber Hereditary Optic Neuropathy due to Mutations in the Mitochondrial ND4 Gene: The REVEAL Study. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 201–214. [Google Scholar] [CrossRef]
- Xin, L.; Zhen, T.; Zhang, C.; Bin, L.; Yong, Z. Efficacy evaluation of intravitreal injection of rAAV2-iVD4 gene for Leber hereditary optic neuropathy. Zhonghua Shiyan Yanke Zazhi/Chin. J. Exp. Ophthalmol. 2021, 39, 724–728. [Google Scholar] [CrossRef]
- GenSight. GenSight Biologics Reports Topline Results from REFLECT Phase III Clinical Trial, Confirming LUMEVOQ® Efficacy Including Better Efficacy with Bilateral Treatment. 2021. Available online: https://www.gensight-biologics.com/2021/06/30/gensight-biologics-reports-topline-results-from-reflect-phase-iii-clinical-trial-confirming-lumevoq-efficacy-including-better-efficacy-with-bilateral-treatment/ (accessed on 30 June 2021).
- Yang, S.; He, H.; Zhu, Y.; Wan, X.; Zhou, L.F.; Wang, J.; Wang, W.F.; Liu, L.; Li, B. Chemical and material communication between the optic nerves in rats. Clin. Exp. Ophthalmol. 2015, 43, 742–748. [Google Scholar] [CrossRef]
- Sabel, B.A.; Flammer, J.; Merabet, L.B. Residual vision activation and the brain-eye-vascular triad: Dysregulation, plasticity and restoration in low vision and blindness—A review. Restor. Neurol. Neurosci. 2018, 36, 767–791. [Google Scholar] [CrossRef]
- Ariazi, J.; Benowitz, A.; De Biasi, V.; Den Boer, M.L.; Cherqui, S.; Cui, H.; Douillet, N.; Eugenin, E.A.; Favre, D.; Goodman, S.; et al. Tunneling Nanotubes and Gap Junctions-Their Role in Long-Range Intercellular Communication during Development, Health, and Disease Conditions. Front. Mol. Neurosci. 2017, 10, 333. [Google Scholar] [CrossRef]
- Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Biousse, V.; Moster, M.L.; Vignal-Clermont, C.; Sergott, R.C.; Klopstock, T.; Sadun, A.A.; Girmens, J.F.; et al. Intravitreal Gene Therapy vs. Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A ND4 Mutation: Systematic Review and Indirect Comparison. Front. Neurol. 2021, 12, 662838. [Google Scholar] [CrossRef]
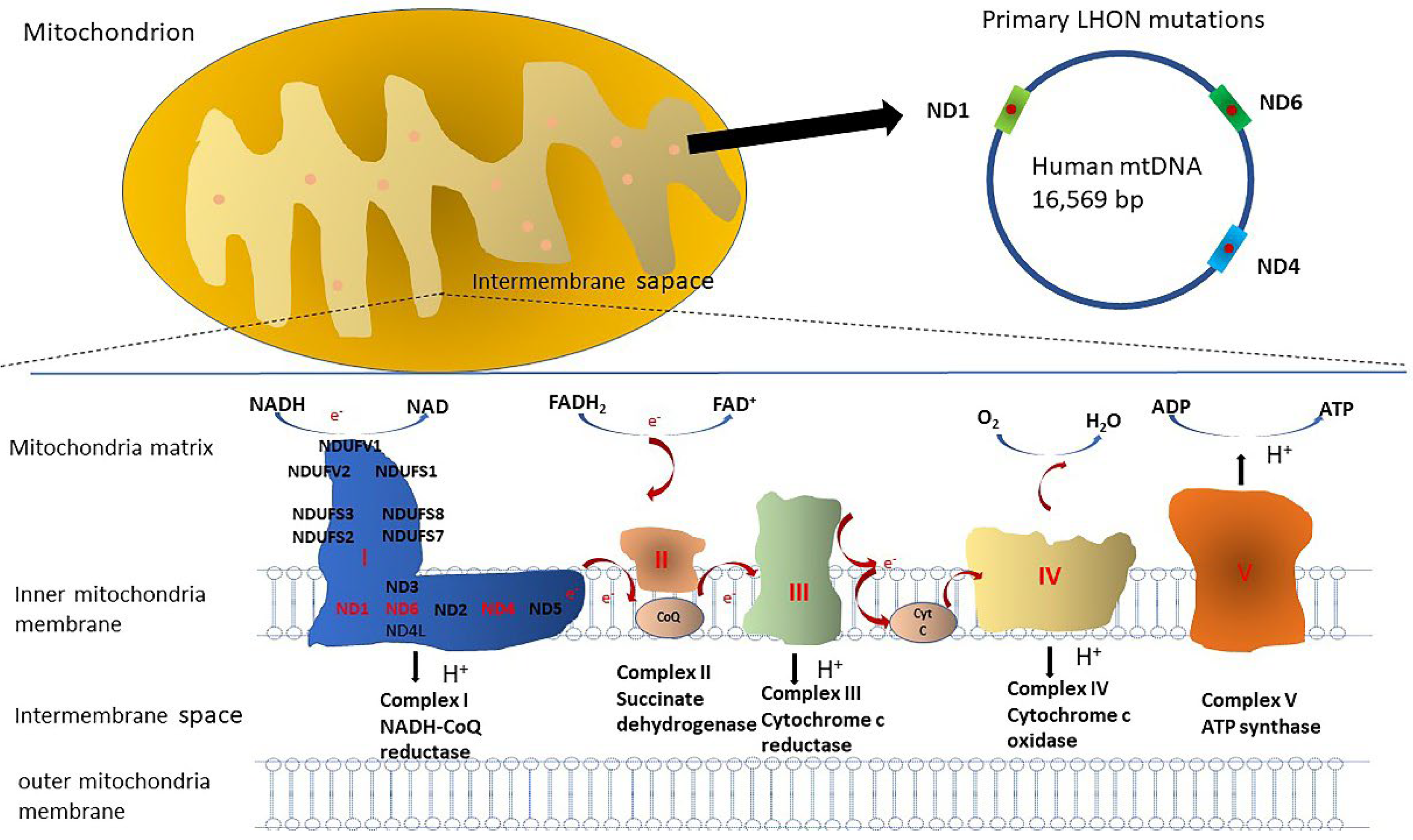
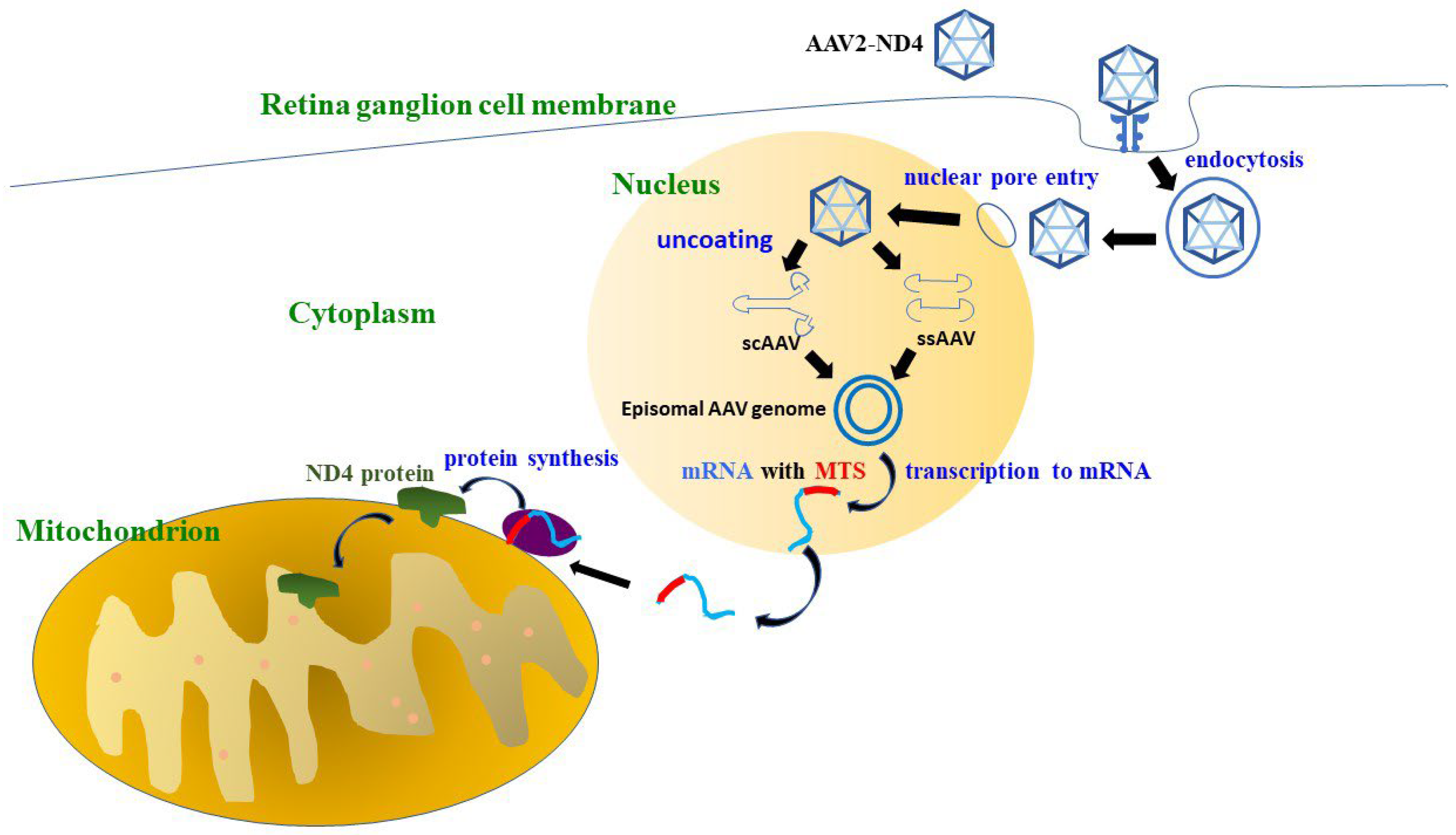
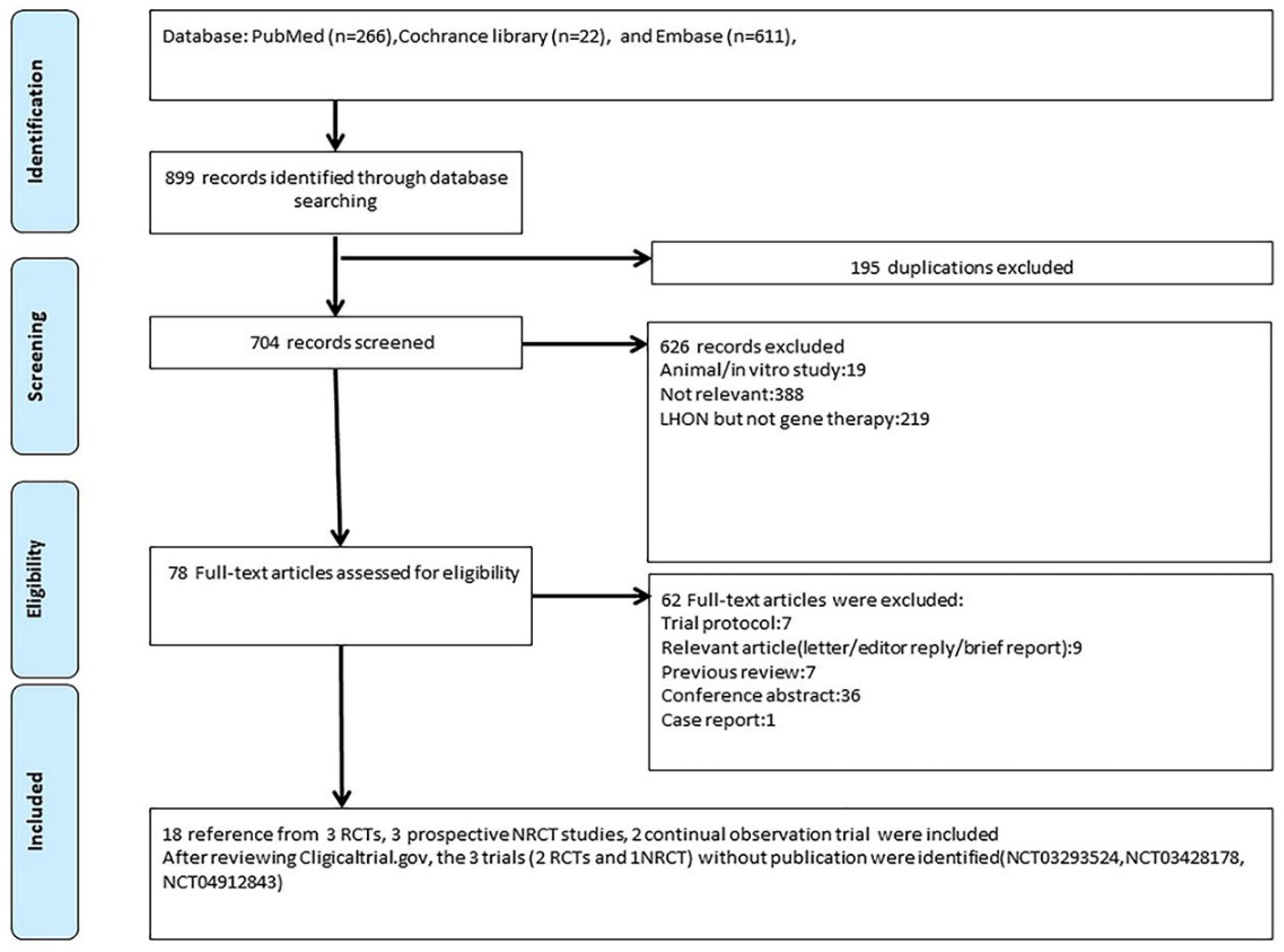
| Trial ID | Study Phase | Year | Follow-Up Time | Patients Number | Age (Mean) | Gender (M/F) | Randomized or Not | Trial Design | Location | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT01267422 | 1 | 2011 | 36 months | 9 | 19.22 | 7/2 | Non-randomized | Dose-escalation Single-arm | Wuhan, China | BCVA//HVF/OCT/safety profile/VEP |
| NCT02064569 | 1/2 | 2014 | 12 months | 15 | 47.9 | 13/2 | Randomized | Dose-escalation Four arms | Paris, France, | Safety profile |
| NCT02161380 | 1 | 2014 | 6 months | 5 | 43.00 | 4/1 | Non-randomized | Dose-escalation Three arms Bilateral chronic group Bilateral acute group Unilateral acute group | Miami, Florida, | BCVA/HVF/neutralizing antibodies and quantitative PCR/OCT/pattern ERG/safety profile |
| NCT02652767 (Rescue) | 3 | 2016 | 24 months | 38 | 36.8 | 31/7 | Randomized | Two arms: Treated eye Sham-treated eye | United States/France/Germany /Italy/United Kingdom | BCVA/contrast sensitivity/HVF/OCT/QOL/safety profile |
| NCT02652780 (Reverse) | 3 | 2016 | 24 months | 37 | 34.2 | 29/8 | Randomized | Two arms: Treated eye Sham-treated eye | United States/France/Germany /Italy/United Kingdom | BCVA/contrast sensitivity/HVF/OCT/QOL/safety profile |
| NCT03153293 | 2/3 | 2017 | 12 months | 149 | 19 | 131/18 | Non-randomized | Two arms: Rapid response arm Slow response arm | Wuhan, Hubei, | BCVA/HVF/OCT |
| NCT03293524 (REFLECT) | 3 | 2018 | 12 months | 98 | N/A | N/A | Randomized | Two arms: Bilateral treatment arm Unilateral treatment arm | United States/Belgium/France/Italy/Spain/Taiwan/United Kingdom | BCVA/contrast sensitivity/HVF/OCT/QOL/responder analysis/safety profile |
| NCT03406104 | 3 | 2016 | 60 months | 61 | 35.1 | 48/13 | Follow-up study of RESCUE and REVERSE | Two arms: Treated eye Sham-treated eye | United States/France/Germany /Italy/United Kingdom | BCVA/QOL |
| NCT03428178 | N/A | 2018 | 12 months | 120 | N/A | N/A | Non-randomized | Five arms according to different periods of disease onset | Wuhan, China | BCVA/HVF/OCT/VEP/liver and kidney function |
| NCT04912843 | 1/2/3 | 2021 | 13 months | 102 | N/A | N/A | Randomized | Part one: dose finding Part two: Treatment group Sham injection group | Beijing, China | BCVA/contrast sensitivity/cell immunogenicity/fluids immunogenicity/HVF/QOL safety profile/vector biodistribution |
| EUDRACT N° 2013-001405-90. | 1/2 | 2014 | 60 months | 15 | 47.9 | 13/2 | Follow-up study of NCT02064569 | Fours arms with a fifth cohort (expansion) | Paris, France | BCVA/contrast sensitivity/HVF/OCT/pattern ERG/QOL/VEP/safety profile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, S.-C.; Cheng, H.-C.; Wang, A.-G. Leber Hereditary Optic Neuropathy: Molecular Pathophysiology and Updates on Gene Therapy. Biomedicines 2022, 10, 1930. https://doi.org/10.3390/biomedicines10081930
Chi S-C, Cheng H-C, Wang A-G. Leber Hereditary Optic Neuropathy: Molecular Pathophysiology and Updates on Gene Therapy. Biomedicines. 2022; 10(8):1930. https://doi.org/10.3390/biomedicines10081930
Chicago/Turabian StyleChi, Sheng-Chu, Hui-Chen Cheng, and An-Guor Wang. 2022. "Leber Hereditary Optic Neuropathy: Molecular Pathophysiology and Updates on Gene Therapy" Biomedicines 10, no. 8: 1930. https://doi.org/10.3390/biomedicines10081930
APA StyleChi, S.-C., Cheng, H.-C., & Wang, A.-G. (2022). Leber Hereditary Optic Neuropathy: Molecular Pathophysiology and Updates on Gene Therapy. Biomedicines, 10(8), 1930. https://doi.org/10.3390/biomedicines10081930






