The Relationship between Stress, Inflammation, and Depression
Abstract
1. Introduction
2. Stress and Inflammation
3. Stress, Inflammation and Depression
4. MDD Is Associated with an Activated Immune System
5. Antidepressants Exert Immunomodulatory Effects
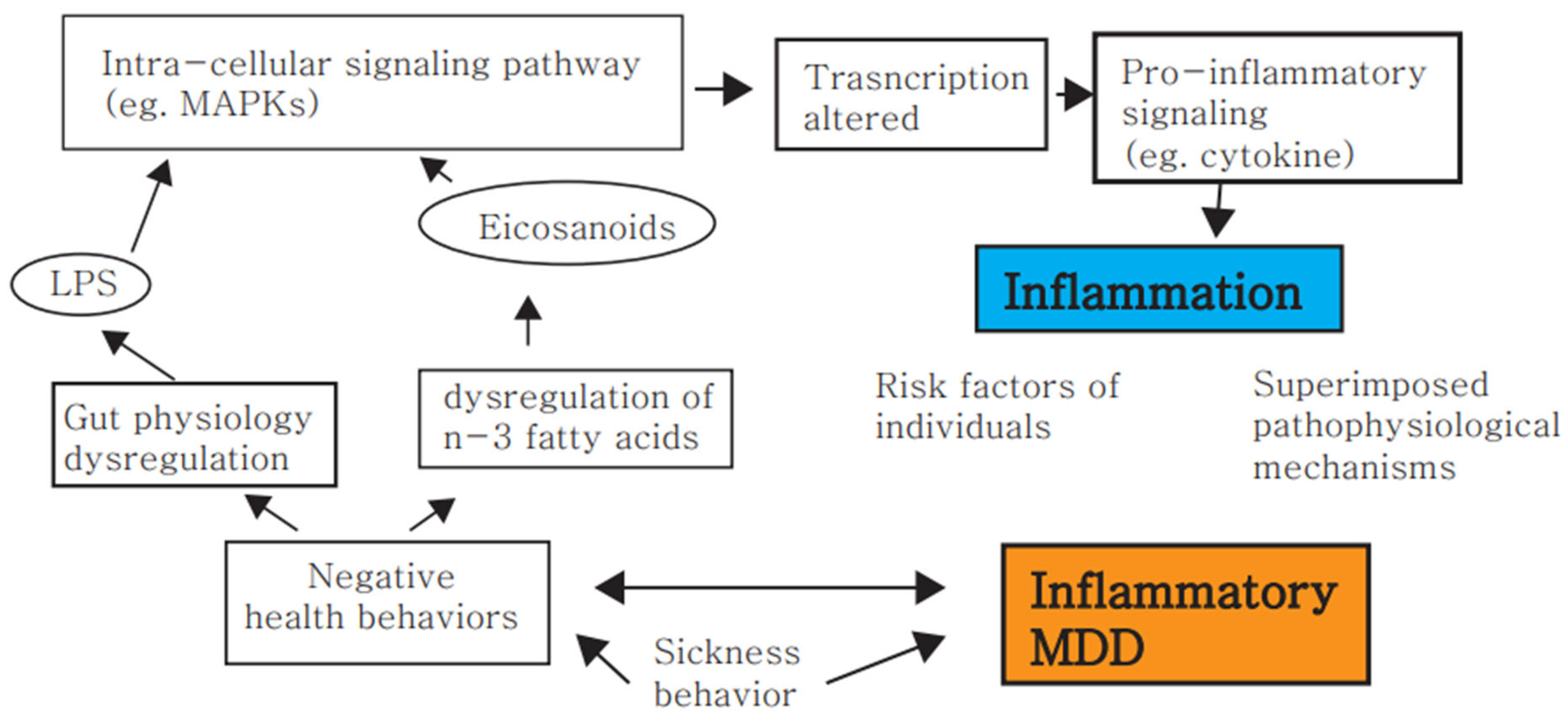
6. Non-Pharmacological Treatment Effects on Inflammatory Depression
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, W.W. Clinical Psychopharmacology for the 21st Century, 3rd ed.; Ho-Chi Publishing Company: Taipei, Taiwan, 2011. [Google Scholar]
- Drazinix, C.M.; Sazbo, S.T. Neurotramitters and Receptors in Psychiatric Disorder (Chapter 2). In The American Psychiatric Association Publishing Textbook of Psychopharmacology; Schatzberg, A.F., Nemeroff, C.B., Eds.; APA Publishing: Washington, DC, USA, 2017. [Google Scholar]
- Jessberger, S.; Kempermann, G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur. J. Neurosci. 2003, 18, 2707–2712. [Google Scholar] [CrossRef]
- Landsbergis, P.A. The changing organization of work and the safety and health of working people: A commentary. J. Occup. Environ Med. 2003, 45, 61–72. [Google Scholar] [CrossRef]
- Molina, P.E. Rethinking Integration of Environmental and Behavioral Stressors; Back to Energy Homeostasis and Function. Function 2022, 3, zqab074. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Cohen, S.; Gianaros, P.J.; Manuck, S.B. A stage model of stress and disease. Perspect. Psychol. Sci. 2016, 11, 456–463. [Google Scholar] [CrossRef]
- Turner, A.I.; Smyth, N.; Hall, S.J.; Torres, S.J.; Hussein, M.; Jayasinghe, S.U.; Ball, K.; Clow, A.J. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology 2020, 114, 104599. [Google Scholar] [CrossRef]
- Rohleder, N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation—The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Viljoen, M.; Lee Thomas neé Negrao, B. Low-grade systemic inflammation and the workplace. Work 2021, 69, 903–915. [Google Scholar] [CrossRef]
- Hantsoo, L.; Kornfield, S.; Anguera, M.C.; Epperson, C.N. Inflammation: A proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biol. Psychiatr. 2019, 85, 97–106. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Hendricks, S.E.; Johnson, D.R.; Wieseler, J.; Burke, W.J. Antidepressants augment natural killer cell activity: In vivo and in vitro. Neuropsychobiology 1999, 39, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial dysfunction, oxidative stress, and neuroinflammation: Intertwined roads to neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef]
- Black, P.H. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav. Immun. 2002, 16, 622–653. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef]
- Liezmann, C.; Klapp, B.; Peters, E. Stress, atopy and allergy: A re-evaluation from a psychoneuroimmunologic persepective. Dermato Endocrinology 2011, 3, 37–40. [Google Scholar] [CrossRef]
- Jiang, C.-L.; Lu, C.-L.; Liu, X.-Y. The molecular basis for bidirectional communication between the immune and neuroendocrine systems. Domest. Anim. Endocrinol. 1998, 15, 363–369. [Google Scholar] [CrossRef]
- Quan, N.; Banks, W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007, 21, 727–735. [Google Scholar] [CrossRef]
- Arambula, S.E.; McCarthy, M.M. Neuroendocrine-immune crosstalk shapes sex-specific brain development. Endocrinology 2020, 161, bqaa055. [Google Scholar] [CrossRef]
- Quatrini, L.; Vivier, E.; Ugolini, S. Neuroendocrine regulation of innate lymphoid cells. Immunol. Rev. 2018, 286, 120–136. [Google Scholar] [CrossRef]
- Weigent, D.A.; Blalock, J.E. Associations between the neuroendocrine and immune systems. J. Leukoc. Biol. 1995, 58, 137–150. [Google Scholar] [CrossRef]
- Weigent, D.A.; Carr, D.; Blalock, J.E. Bidirectional communication between the neuroendocrine and immune systems. Common hormones and hormone receptors. Ann. N. Y. Acad. Sci. 1990, 579, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; D’Agostino, B.; Vatrella, A.; Fratto, D.; Renda, T.; Galderisi, U.; Piegari, E.; Crimi, N.; Rossi, F.; Caputi, M. Effects of TGF-beta and glucocorticoids on map kinase phosphorylation, IL-6/IL-11 secretion and cell proliferation in primary cultures of human lung fibroblasts. J. Cell. Physiol. 2007, 210, 489–497. [Google Scholar]
- Gallelli, L.; Pelaia, G.; Fratto, D.; Muto, V.; Falcone, D.; Vatrella, A.; Curto, L.; Renda, T.; Busceti, M.; Liberto, M. Effects of budesonide on p38 MAPK activation, apoptosis and IL-8 secretion, induced by TNF-α and Haemophilus influenzae in human bronchial epithelial cells. Int. J. Immunopathol. Pharmacol. 2010, 23, 471–479. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Caso, J.R.; Munhoz, C.D.; Sapolsky, R.M. The stressed CNS: When glucocorticoids aggravate inflammation. Neuron 2009, 64, 33–39. [Google Scholar] [CrossRef]
- Hill, A.R.; Spencer-Segal, J.L. Glucocorticoids and the brain after critical illness. Endocrinology 2021, 162, bqaa242. [Google Scholar] [CrossRef]
- Liberman, A.C.; Trias, E.; da Silva Chagas, L.; Trindade, P.; dos Santos Pereira, M.; Refojo, D.; Hedin-Pereira, C.; Serfaty, C.A. Neuroimmune and inflammatory signals in complex disorders of the central nervous system. Neuroimmunomodulation 2018, 25, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; Del Rey, A. Regulating inflammation by glucocorticoids. Nat. Immunol. 2006, 7, 537. [Google Scholar] [CrossRef]
- Duque, E.D.A.; Munhoz, C.D. The pro-inflammatory effects of glucocorticoids in the brain. Front. Endocrinol. 2016, 7, 78. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Munhoz, C.D.; Manley, N.C.; Yen, S.; Sapolsky, R.M. Glucocorticoids increase excitotoxic injury and inflammation in the hippocampus of adult male rats. Neuroendocrinology 2014, 100, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem. Int. 2008, 52, 40–51. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, L.L.; Suliman, S.; Bröcker, E.; Kilian, S.; Stalder, T.; Kirschbaum, C.; Seedat, S. The association between hair cortisol levels, inflammation and cognitive functioning in females. Psychoneuroendocrinology 2022, 136, 105619. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti-and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Oppong, E.; Cato, A.C. Effects of glucocorticoids in the immune system. Adv. Exp. Med. Biol. 2015, 872, 217–233. [Google Scholar]
- Frank, M.G.; Thompson, B.M.; Watkins, L.R.; Maier, S.F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 2012, 26, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Busillo, J.M.; Azzam, K.M.; Cidlowski, J.A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef]
- Gülke, E.; Gelderblom, M.; Magnus, T. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418774254. [Google Scholar] [CrossRef]
- Alley, D.E.; Seeman, T.E.; Kim, J.K.; Karlamangla, A.; Hu, P.; Crimmins, E.M. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav. Immun. 2006, 20, 498–504. [Google Scholar] [CrossRef]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef]
- Steptoe, A.; Hamer, M.; Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Sze, J.; Marin, T.; Arevalo, J.M.; Doll, R.; Ma, R.; Cole, S.W. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiatr. 2008, 64, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Chrousos, G.P. Stress system–organization, physiology and immunoregulation. Neuroimmunomodulation 2006, 13, 257–267. [Google Scholar] [CrossRef]
- Papargyri, P.; Zapanti, E.; Salakos, N.; Papargyris, L.; Bargiota, A.; Mastorakos, G. Links between HPA axis and adipokines: Clinical implications in paradigms of stress-related disorders. Expert Rev. Endocrinol. Metab. 2018, 13, 317–332. [Google Scholar] [CrossRef]
- Borghetti, P.; Saleri, R.; Mocchegiani, E.; Corradi, A.; Martelli, P. Infection, immunity and the neuroendocrine response. Vet. Immunol. Immunopathol. 2009, 130, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; John Wiley & Sons: New York, NY, USA, 1988; pp. 629–649. [Google Scholar]
- Lee, S.W. A Copernican approach to brain advancement: The paradigm of allostatic orchestration. Front. Hum. Neurosci. 2019, 13, 129. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Millar, B.A.; Perez, S.; Carter, J.; Wood, C.; ThyagaRajan, S.; Molinaro, C.; Lubahn, C.; Lorton, D. Sympathetic modulation of immunity: Relevance to disease. Cell Immunol. 2008, 252, 27–56. [Google Scholar] [CrossRef]
- Zhou, J.-R.; Xu, Z.; Jiang, C.-L. Neuropeptide Y promotes TGF-β1 production in RAW264. 7 cells by activating PI3K pathway via Y1 receptor. Neurosci. Bull. 2008, 24, 155–159. [Google Scholar] [CrossRef]
- Huang, J.-L.; Zhang, Y.-L.; Wang, C.-C.; Zhou, J.-R.; Ma, Q.; Wang, X.; Shen, X.-H.; Jiang, C.-L. Enhanced phosphorylation of MAPKs by NE promotes TNF-α production by macrophage through α adrenergic receptor. Inflammation 2012, 35, 527–534. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Lorton, D. Sympathetic nerve hyperactivity in the spleen: Causal for nonpathogenic-driven chronic immune-mediated inflammatory diseases (IMIDs)? Int. J. Mol. Sci. 2018, 19, 1188. [Google Scholar] [CrossRef]
- Brinkman, D.J.; Ten Hove, A.S.; Vervoordeldonk, M.J.; Luyer, M.D.; de Jonge, W.J. Neuroimmune interactions in the gut and their significance for intestinal immunity. Cells 2019, 8, 670. [Google Scholar] [CrossRef]
- Song, C.; Kenis, G.; van Gastel, A.; Bosmans, E.; Lin, A.; de Jong, R.; Neels, H.; Scharpé, S.; Janca, A.; Yasukawa, K. Influence of psychological stress on immune-inflammatory variables in normal humans. Part II. Altered serum concentrations of natural anti-inflammatory agents and soluble membrane antigens of monocytes and T lymphocytes. Psychiatr. Res. 1999, 85, 293–303. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatr. 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef] [PubMed]
- Draganov, M.; Arranz, M.J.; Salazar, J.; de Diego-Adeliño, J.; Gallego-Fabrega, C.; Jubero, M.; Carceller-Sindreu, M.; Portella, M.J. Association study of polymorphisms within inflammatory genes and methylation status in treatment response in major depression. Eur. Psychiatr. 2019, 60, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Opel, N.; Cearns, M.; Clark, S.; Toben, C.; Grotegerd, D.; Heindel, W.; Kugel, H.; Teuber, A.; Minnerup, H.; Berger, K. Large-scale evidence for an association between low-grade peripheral inflammation and brain structural alterations in major depression in the BiDirect study. J. Psychiatr. Neurosci. 2019, 44, 423–431. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation and depression but where does the inflammation come from? Curr. Opin. Psychiatr. 2019, 32, 422–428. [Google Scholar] [CrossRef]
- Rhie, S.J.; Jung, E.-Y.; Shim, I. The role of neuroinflammation on pathogenesis of affective disorders. J. Exerc. Rehabil. 2020, 16, 2. [Google Scholar] [CrossRef]
- García-Bueno, B.; Caso, J.R.; Leza, J.C. Stress as a neuroinflammatory condition in brain: Damaging and protective mechanisms. Neurosci. Biobehav. Rev. 2008, 32, 1136–1151. [Google Scholar] [CrossRef]
- Gu, M.; Mei, X.-L.; Zhao, Y.-N. Sepsis and cerebral dysfunction: BBB damage, neuroinflammation, oxidative stress, apoptosis and autophagy as key mediators and the potential therapeutic approaches. Neurotox. Res. 2021, 39, 489–503. [Google Scholar] [CrossRef]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative stress and neuroinflammation potentiate each other to promote progression of dopamine neurodegeneration. Oxid. Med. Cell Longev. 2020, 6137521. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+ T cells in neurodegenerative diseases. Front. Cell Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, C.; Garcia-Bueno, B.; Madrigal, J.; Lepsch, L.; Scavone, C.; Leza, J. Stress-induced neuroinflammation: Mechanisms and new pharmacological targets. Braz. J. Med. Biol. Res. 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Gárate, I.; Garcia-Bueno, B.; Madrigal, J.L.M.; Caso, J.R.; Alou, L.; Gomez-Lus, M.L.; Micó, J.A.; Leza, J.C. Stress-induced neuroinflammation: Role of the Toll-like receptor-4 pathway. Biol. Psychiatr. 2013, 73, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatr. Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef]
- Johnson, J.; Campisi, J.; Sharkey, C.; Kennedy, S.; Nickerson, M.; Greenwood, B.; Fleshner, M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef]
- Johnson, J.D.; Barnard, D.F.; Kulp, A.C.; Mehta, D.M. Neuroendocrine regulation of brain cytokines after psychological stress. J. Endocr. Soc. 2019, 3, 1302–1320. [Google Scholar] [CrossRef]
- Barnard, D.F. The Regulation of Brain Pro-Inflammatory Cytokines: Implications for Stress and Depression. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2020. [Google Scholar]
- Foertsch, S.; Reber, S.O. The role of physical trauma in social stress-induced immune activation. Neurosci. Biobehav. Rev. 2020, 113, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.D.; Godbout, J.P.; Sheridan, J.F. Repeated social defeat, neuroinflammation, and behavior: Monocytes carry the signal. Neuropsychopharmacology 2017, 42, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Fornaguera-Trías, J.; Sheridan, J.F. Stress-induced microglia activation and monocyte trafficking to the brain underlie the development of anxiety and depression. Inflamm. Assoc. Depress. Evid. Mech. Implic. 2016, 31, 155–172. [Google Scholar]
- Mendiola, A.S.; Ryu, J.K.; Bardehle, S.; Meyer-Franke, A.; Ang, K.K.-H.; Wilson, C.; Baeten, K.M.; Hanspers, K.; Merlini, M.; Thomas, S. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 2020, 21, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Weber, M.D.; Daut, R.A.; Kitt, M.M.; Frank, M.G.; Watkins, L.R.; Maier, S.F. Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology 2016, 66, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.D.; Frank, M.G.; Tracey, K.J.; Watkins, L.R.; Maier, S.F. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: A priming stimulus of microglia and the NLRP3 inflammasome. J. Neurosci. 2015, 35, 316–324. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Delpech, J.-C. Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2017, 79, 40–48. [Google Scholar] [CrossRef]
- Park, S.-C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019, 377, 95–106. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Park, S.-C. An alternative approach to future diagnostic standards for major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2021, 105, 110133. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.-C. Neural Circuitry–Neurogenesis Coupling Model of Depression. Int. J. Mol. Sci. 2021, 22, 2468. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.-C. Machine Learning-Based Definition of Symptom Clusters and Selection of Antidepressants for Depressive Syndrome. Diagnostics 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.B.; Park, S.-C. The Entorhinal Cortex and Adult Neurogenesis in Major Depression. Int. J. Mol. Sci. 2021, 22, 11725. [Google Scholar] [CrossRef] [PubMed]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Martone, G. The inflammation hypothesis and mental illness. J. Clin. Psychiatr. Neurosci. 2019, 2, 3–12. [Google Scholar]
- Dolsen, M.R.; Prather, A.A.; Lamers, F.; Penninx, B.W. Suicidal ideation and suicide attempts: Associations with sleep duration, insomnia, and inflammation. Psychol. Med. 2021, 51, 2094–2103. [Google Scholar] [CrossRef]
- Brahadeeswaran, S.; Sivagurunathan, N.; Calivarathan, L. Inflammasome Signaling in the Aging Brain and Age-Related Neurodegenerative Diseases. Mol. Neurobiol. 2022, 59, 2288–2304. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Raison, C.L.; Musselman, D.L.; Lawson, D.H.; Nemeroff, C.B.; Miller, A.H. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am. J. Psychiatr. 2003, 160, 1342–1345. [Google Scholar] [CrossRef]
- Davies, K.A.; Cooper, E.; Voon, V.; Tibble, J.; Cercignani, M.; Harrison, N.A. Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms. Mol. Psychiatr. 2021, 26, 5150–5160. [Google Scholar] [CrossRef]
- Su, K.-P.; Lai, H.-C.; Peng, C.-Y.; Su, W.-P.; Chang, J.P.-C.; Pariante, C.M. Interferon-alpha-induced depression: Comparisons between early-and late-onset subgroups and with patients with major depressive disorder. Brain Behav. Immun. 2019, 80, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Dooley, L.N.; Kuhlman, K.R.; Robles, T.F.; Eisenberger, N.I.; Craske, M.G.; Bower, J.E. The role of inflammation in core features of depression: Insights from paradigms using exogenously-induced inflammation. Neurosci. Biobehav. Rev. 2018, 94, 219–237. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, H.; Baloch, Z. Pathogenetic and therapeutic applications of tumor necrosis factor-α (TNF-α) in major depressive disorder: A systematic review. Int. J. Mol. Sci. 2016, 17, 733. [Google Scholar] [CrossRef]
- Hernández-Hernández, O.T.; Martínez-Mota, L.; Herrera-Pérez, J.J.; Jiménez-Rubio, G. Role of estradiol in the expression of genes involved in serotonin neurotransmission: Implications for female depression. Curr. Neuropharmacol. 2019, 17, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Baudry, A.; Pietri, M.; Launay, J.-M.; Kellermann, O.; Schneider, B. Multifaceted regulations of the serotonin transporter: Impact on antidepressant response. Front. Neurosci. 2019, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Smith, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef]
- Norman, G.; Karelina, K.; Zhang, N.; Walton, J.; Morris, J.; Devries, A. Stress and IL-1β contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatr. 2010, 15, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Han, Q.-Q.; Gong, W.-Q.; Pan, D.-H.; Wang, L.-Z.; Hu, W.; Yang, M.; Li, B.; Yu, J.; Liu, Q. Microglial activation mediates chronic mild stress-induced depressive-and anxiety-like behavior in adult rats. J. Neuroinflamm. 2018, 15, 198–209. [Google Scholar] [CrossRef]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune influencers in action: Metabolites and enzymes of the Tryptophan-Kynurenine metabolic pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Raison, C.; Borisov, A.; Woolwine, B.; Massung, B.; Vogt, G.; Miller, A. Interferon-α effects on diurnal hypothalamic–pituitary–adrenal axis activity: Relationship with proinflammatory cytokines and behavior. Mol. Psychiatr. 2010, 15, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Bekhbat, M.; Treadway, M.T.; Felger, J.C. Inflammation as a Pathophysiologic Pathway to Anhedonia: Mechanisms and Therapeutic Implications; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Bauer, M.E.; Teixeira, A.L. Inflammation in psychiatric disorders: What comes first? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-L.; Liu, Y.-N.; Liu, L.; Wang, X.; Jiang, C.-L.; Wang, Y.-X. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J. Neuroinflamm. 2012, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J.; Huang, Y.; Wynne, A.; Hanke, M.; Himler, J.; Bailey, M.T.; Sheridan, J.F.; Godbout, J.P. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflamm. 2008, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Inflammasomes. Curr. Biol. 2020, 30, R689–R694. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Peng, Y.L.; Liu, Y.Z.; Wu, T.Y.; Shen, X.L.; Zhou, J.R.; Sun, D.Y.; Huang, A.J.; Wang, X. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci. Ther. 2014, 20, 119–124. [Google Scholar] [CrossRef]
- Zhang, L.; Previn, R.; Lu, L.; Liao, R.-F.; Jin, Y.; Wang, R.-K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res. Bull. 2018, 142, 352–359. [Google Scholar] [CrossRef]
- Wong, M.-L.; Inserra, A.; Lewis, M.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S. Inflammasome signaling affects anxiety-and depressive-like behavior and gut microbiome composition. Mol. Psychiatr. 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Inserra, A.; Mastronardi, C.A.; Rogers, G.; Licinio, J.; Wong, M.-L. Neuroimmunomodulation in major depressive disorder: Focus on caspase 1, inducible nitric oxide synthase, and interferon-gamma. Mol. Neurobiol. 2019, 56, 4288–4305. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota–brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001, 24, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Stone, M.; Peters, J.; Davies, M.; Khunti, K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef]
- Laake, J.-P.S.; Stahl, D.; Amiel, S.A.; Petrak, F.; Sherwood, R.A.; Pickup, J.C.; Ismail, K. The association between depressive symptoms and systemic inflammation in people with type 2 diabetes: Findings from the South London Diabetes Study. Diabetes Care 2014, 37, 2186–2192. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, T.; Yin, R.; Zhang, Q.; Shen, B. Prevalence of depression and anxiety in systemic lupus erythematosus: A systematic review and meta-analysis. BMC Psychiatr. 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Van Exel, E.; Jacobs, J.; Korswagen, L.; Voskuyl, A.; Stek, M.; Dekker, J.; Bultink, I. Depression in systemic lupus erythematosus, dependent on or independent of severity of disease. Lupus 2013, 22, 1462–1469. [Google Scholar] [CrossRef]
- Xie, X.; Wu, D.; Chen, H. Prevalence and risk factors of anxiety and depression in patients with systemic lupus erythematosus in Southwest China. Rheumatol. Int. 2016, 36, 1705–1710. [Google Scholar] [CrossRef]
- Schmeding, A.; Schneider, M. Fatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2013, 27, 363–375. [Google Scholar] [CrossRef]
- Mak, A.; Tang, C.; Ho, R.C.-M. Serum tumour necrosis factor-alpha is associated with poor health-related quality of life and depressive symptoms in patients with systemic lupus erythematosus. Lupus 2013, 22, 254–261. [Google Scholar] [CrossRef]
- Quan, W.; An, J.; Li, G.; Qian, G.; Jin, M.; Feng, C.; Li, S.; Li, X.; Xu, Y.; Hu, X. Th cytokine profile in childhood-onset systemic lupus erythematosus. BMC Pediatr. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Guo, H.-R.; Lu, M.-C.; Livneh, H.; Lai, N.-S.; Tsai, T.-Y. Increased risk of depression in patients with rheumatoid arthritis: A seven-year population-based cohort study. Clinics 2015, 70, 91–96. [Google Scholar] [CrossRef]
- Matcham, F.; Rayner, L.; Steer, S.; Hotopf, M. The prevalence of depression in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatology 2013, 52, 2136–2148. [Google Scholar] [CrossRef]
- Stebbings, S.; Treharne, G.J. Fatigue in rheumatic disease: An overview. Int. J. Clin. Rheumatol. 2010, 5, 487–502. [Google Scholar] [CrossRef]
- Kojima, M.; Kojima, T.; Suzuki, S.; Oguchi, T.; Oba, M.; Tsuchiya, H.; Sugiura, F.; Kanayama, Y.; Furukawa, T.A.; Tokudome, S. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Care Res. 2009, 61, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.G.; Danneskiold-Samsøe, B.; Stockmarr, A.; Bartels, E. Correlations between fatigue and disease duration, disease activity, and pain in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2016, 45, 255–261. [Google Scholar] [CrossRef]
- Almeida, C.; Choy, E.H.; Hewlett, S.; Kirwan, J.R.; Cramp, F.; Chalder, T.; Pollock, J.; Christensen, R. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst. Rev. 2016, 2016, CD008334. [Google Scholar] [CrossRef]
- Yende, S.; D’Angelo, G.; Kellum, J.A.; Weissfeld, L.; Fine, J.; Welch, R.D.; Kong, L.; Carter, M.; Angus, D.C. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 1242–1247. [Google Scholar] [CrossRef]
- Shukla, P.; Rao, G.M.; Pandey, G.; Sharma, S.; Mittapelly, N.; Shegokar, R.; Mishra, P.R. Therapeutic interventions in sepsis: Current and anticipated pharmacological agents. Br. J. Pharmacol. 2014, 171, 5011–5031. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing recovery from sepsis: A review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Davydow, D.S.; Hough, C.L.; Langa, K.M.; Iwashyna, T.J. Symptoms of depression in survivors of severe sepsis: A prospective cohort study of older Americans. Am. J. Geriatr. Psychiatr. 2013, 21, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.M.; Connor, T.J.; Harkin, A. Stress-related immune markers in depression: Implications for treatment. Int. J. Neuropsychopharmacol. 2016, 19, pyw001. [Google Scholar] [CrossRef] [PubMed]
- Ojard, C.; Donnelly, J.P.; Safford, M.M.; Griffin, R. Psychosocial stress as a risk factor for sepsis: A population-based cohort study. Psychosom. Med. 2015, 77, 93. [Google Scholar] [CrossRef]
- Anderson, S.T.; Commins, S.; Moynagh, P.N.; Coogan, A.N. Lipopolysaccharide-induced sepsis induces long-lasting affective changes in the mouse. Brain Behav. Immun. 2015, 43, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Cassol-Jr, O.J.; Comim, C.M.; Petronilho, F.; Constantino, L.S.; Streck, E.L.; Quevedo, J.; Dal-Pizzol, F. Low dose dexamethasone reverses depressive-like parameters and memory impairment in rats submitted to sepsis. Neurosci. Lett. 2010, 473, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.; Su, Y.; Su, K.; Chen, P. Recurrence of depressive disorders after interferon-induced depression. Transl. Psychiatr. 2017, 7, e1026. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, S.; Zhang, Z.; Zhang, Y.; Wu, Y.; An, J.; Lin, J.; Yuan, Z.; Shen, L.; Si, T. Identification of key genes and the pathophysiology associated with major depressive disorder patients based on integrated bioinformatics analysis. Front. Psychiatr. 2020, 11, 192. [Google Scholar] [CrossRef]
- Ohgi, Y.; Futamura, T.; Kikuchi, T.; Hashimoto, K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2013, 103, 853–859. [Google Scholar] [CrossRef]
- Ramirez, K.; Shea, D.T.; McKim, D.B.; Reader, B.F.; Sheridan, J.F. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav. Immun. 2015, 46, 212–220. [Google Scholar] [CrossRef][Green Version]
- Qiu, W.; Wu, M.; Liu, S.; Chen, B.; Pan, C.; Yang, M.; Wang, K.-J. Suppressive immunoregulatory effects of three antidepressants via inhibition of the nuclear factor-κB activation assessed using primary macrophages of carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2017, 322, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Munzer, A.; Sack, U.; Mergl, R.; Schönherr, J.; Petersein, C.; Bartsch, S.; Kirkby, K.C.; Bauer, K.; Himmerich, H. Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins 2013, 5, 2227–2240. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2018, 80, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yeh, Y.-W.; Kuo, S.-C.; Liang, C.-S.; Ho, P.-S.; Huang, C.-C.; Yen, C.-H.; Shyu, J.-F.; Lu, R.-B.; Huang, S.-Y. Differences in immunomodulatory properties between venlafaxine and paroxetine in patients with major depressive disorder. Psychoneuroendocrinology 2018, 87, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Mohr, D.C.; Goodkin, D.E.; Islar, J.; Hauser, S.L.; Genain, C.P. Treatment of depression is associated with suppression of nonspecific and antigen-specific TH1 responses in multiple sclerosis. Arch. Neurol. 2001, 58, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Machado-Vieira, R.; Zarate, C.A.; Valiengo, L.; Vieira, E.L.; Benseñor, I.M.; Lotufo, P.A.; Gattaz, W.F.; Teixeira, A.L. Cytokines plasma levels during antidepressant treatment with sertraline and transcranial direct current stimulation (tDCS): Results from a factorial, randomized, controlled trial. Psychopharmacology 2014, 231, 1315–1323. [Google Scholar] [CrossRef]
- Rethorst, C.D.; Toups, M.S.; Greer, T.L.; Nakonezny, P.A.; Carmody, T.J.; Grannemann, B.D.; Huebinger, R.M.; Barber, R.C.; Trivedi, M.H. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol. Psychiatr. 2013, 18, 1119–1124. [Google Scholar] [CrossRef]
- Yrondi, A.; Sporer, M.; Peran, P.; Schmitt, L.; Arbus, C.; Sauvaget, A. Electroconvulsive therapy, depression, the immune system and inflammation: A systematic review. Brain Stimul. 2018, 11, 29–51. [Google Scholar] [CrossRef]
- Freire, T.F.V.; da Rocha, N.S.; de Almeida Fleck, M.P. The association of electroconvulsive therapy to pharmacological treatment and its influence on cytokines. J. Psychiatr. Res. 2017, 92, 205–211. [Google Scholar] [CrossRef]
- Kronfol, Z.; Nair, M.P.; Weinberg, V.; Young, E.A.; Aziz, M. Acute effects of electroconvulsive therapy on lymphocyte natural killer cell activity in patients with major depression. J. Affect Disord. 2002, 71, 211–215. [Google Scholar] [CrossRef]
- Kiraly, D.; Horn, S.; Van Dam, N.; Costi, S.; Schwartz, J.; Kim-Schulze, S.; Patel, M.; Hodes, G.E.; Russo, S.; Merad, M. Altered peripheral immune profiles in treatment-resistant depression: Response to ketamine and prediction of treatment outcome. Translational Psychiatr. 2017, 7, e1065. [Google Scholar] [CrossRef] [PubMed]
- Myung, W.; Lim, S.-W.; Woo, H.I.; Park, J.H.; Shim, S.; Lee, S.-Y.; Kim, D.K. Serum cytokine levels in major depressive disorder and its role in antidepressant response. Psychiatr. Investig. 2016, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Tansey, K.E.; Dew, T.; Maier, W.; Mors, O.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatr. 2014, 171, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Hori, H.; Ikenouchi-Sugita, A.; Umene-Nakano, W.; Ueda, N.; Nakamura, J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI-or SNRI-refractory depression. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2009, 33, 722–726. [Google Scholar] [CrossRef]
- Eller, T.; Vasar, V.; Shlik, J.; Maron, E. Pro-inflammatory cytokines and treatment response to escitaloprsam in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2008, 32, 445–450. [Google Scholar] [CrossRef]
- Benedetti, F.; Lucca, A.; Brambilla, F.; Colombo, C.; Smeraldi, E. Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2002, 26, 1167–1170. [Google Scholar] [CrossRef]
- Sorri, A.; Järventausta, K.; Kampman, O.; Lehtimäki, K.; Björkqvist, M.; Tuohimaa, K.; Hämäläinen, M.; Moilanen, E.; Leinonen, E. Low tumor necrosis factor-α levels predict symptom reduction during electroconvulsive therapy in major depressive disorder. Brain Behav. 2018, 8, e00933. [Google Scholar] [CrossRef]
- Yang, T.-T.; Wang, L.; Deng, X.-Y.; Yu, G. Pharmacological treatments for fatigue in patients with multiple sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 380, 256–261. [Google Scholar] [CrossRef]
- Rogóż, Z.; Kubera, M.; Rogóż, K.; Basta-Kaim, A.; Budziszewska, B. Effect of co-administration of fluoxetine and amantadine on immunoendocrine parameters in rats subjected to a forced swimming test. Pharmacol. Rep. 2009, 61, 1050–1060. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Huang, S.-Y.; Su, K.-P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatr. 2010, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Voyias, P.D.; Sallis, H.M.; Dawson, S.; Ness, A.R.; Churchill, R.; Perry, R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst. Rev. 2021, 11, CD004692. [Google Scholar] [PubMed]
- Hallahan, B.; Ryan, T.; Hibbeln, J.R.; Murray, I.T.; Glynn, S.; Ramsden, C.E.; SanGiovanni, J.P.; Davis, J.M. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br. J. Psychiatr. 2016, 209, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-D.; Feng, J.-S.; Yang, Z.; Huang, Q.-T.; Lin, J.-D.; Yang, B.; Su, K.-P.; Pan, J.-Y. High-dose omega-3 polyunsaturated fatty acid supplementation might be more superior than low-dose for major depressive disorder in early therapy period: A network meta-analysis. BMC Psychiatr. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Ren, H.; Huang, Z.; Peng, Y.; He, B.; Yao, X.; Yuan, T.-F.; Su, H. Fish oil prevents lipopolysaccharide-induced depressive-like behavior by inhibiting neuroinflammation. Mol. Neurobiol. 2017, 54, 7327–7334. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-P.; Lai, H.-C.; Yang, H.-T.; Su, W.-P.; Peng, C.-Y.; Chang, J.P.-C.; Chang, H.-C.; Pariante, C.M. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol. Psychiatr. 2014, 76, 559–566. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Morres, I.D.; Hatzigeorgiadis, A.; Stathi, A.; Comoutos, N.; Arpin-Cribbie, C.; Krommidas, C.; Theodorakis, Y. Aerobic exercise for adult patients with major depressive disorder in mental health services: A systematic review and meta-analysis. Depress. Anxiety 2019, 36, 39–53. [Google Scholar] [CrossRef]
- Wipfli, B.M.; Rethorst, C.D.; Landers, D.M. The anxiolytic effects of exercise: A meta-analysis of randomized trials and dose–response analysis. J. Sport Exerc. Psychol. 2008, 30, 392–410. [Google Scholar] [CrossRef]
- Phillips, C.; Fahimi, A. Immune and neuroprotective effects of physical activity on the brain in depression. Front. Neurosci. 2018, 12, 498. [Google Scholar] [CrossRef]
- Carrea-Gonzalez, M.D.P.; Canton-Habas, V.; Rich-Ruiz, M. Age, depression and dementia: The inflammatory process. Adv. Clin. Exp. Med. 2022, 31, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Recent advances in the study of the comorbidity of depressive and anxiety disorders. Adv. Clin. Exp. Med. 2022, 31, 355–358. [Google Scholar] [CrossRef]
- Tanaka, M.; Spekker, E.; Szabó, Á.; Polyák, H.; Vécsei, L. Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J. Neural Transm. 2022, 129, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Macedo ECordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Sanjay Park, M.; Lee, H.J. Roles of Fatty Acids in Microglial Polarization: Evidence from In Vitro and In Vivo Studies on Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 7300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.-B.; Lee, J.-H.; Park, S.-C. The Relationship between Stress, Inflammation, and Depression. Biomedicines 2022, 10, 1929. https://doi.org/10.3390/biomedicines10081929
Kim I-B, Lee J-H, Park S-C. The Relationship between Stress, Inflammation, and Depression. Biomedicines. 2022; 10(8):1929. https://doi.org/10.3390/biomedicines10081929
Chicago/Turabian StyleKim, Il-Bin, Jae-Hon Lee, and Seon-Cheol Park. 2022. "The Relationship between Stress, Inflammation, and Depression" Biomedicines 10, no. 8: 1929. https://doi.org/10.3390/biomedicines10081929
APA StyleKim, I.-B., Lee, J.-H., & Park, S.-C. (2022). The Relationship between Stress, Inflammation, and Depression. Biomedicines, 10(8), 1929. https://doi.org/10.3390/biomedicines10081929






