Radiodynamic Therapy with Acridine Orange Is an Effective Treatment for Bone Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.1.1. Carcinoma Cells
2.1.2. Osteoclasts (OCs)
2.2. Extracellular Acidification Assay
2.3. Invasion Assay
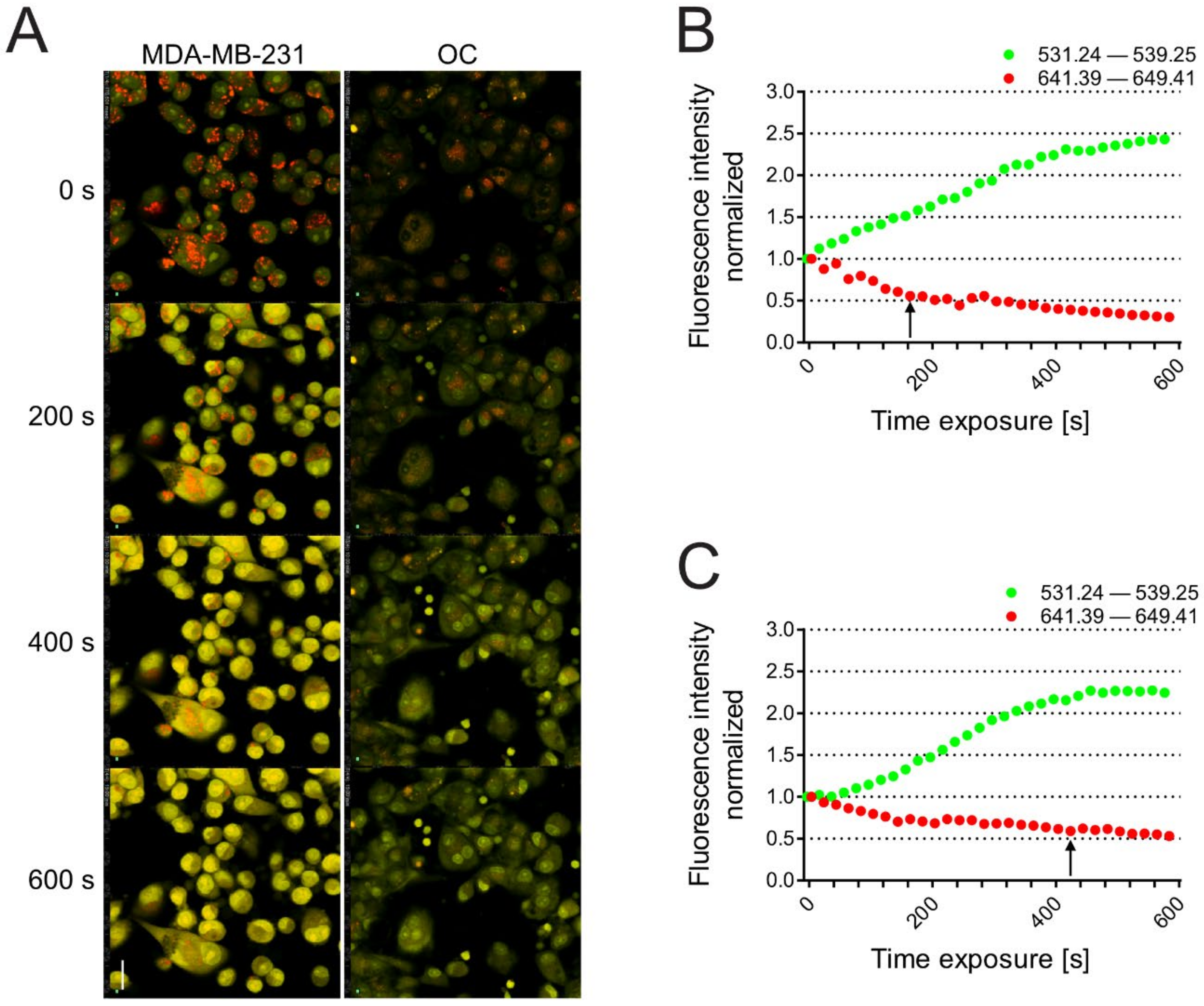
2.4. Acridine Orange Uptake and Confocal Spectral Analysis of the Lysosomal pH
2.5. AO Uptake in 3D Tumor Spheroids
2.6. In Vitro Cell Viability Assays
2.7. In Vivo Study
2.7.1. Omeprazole Systemic Administration
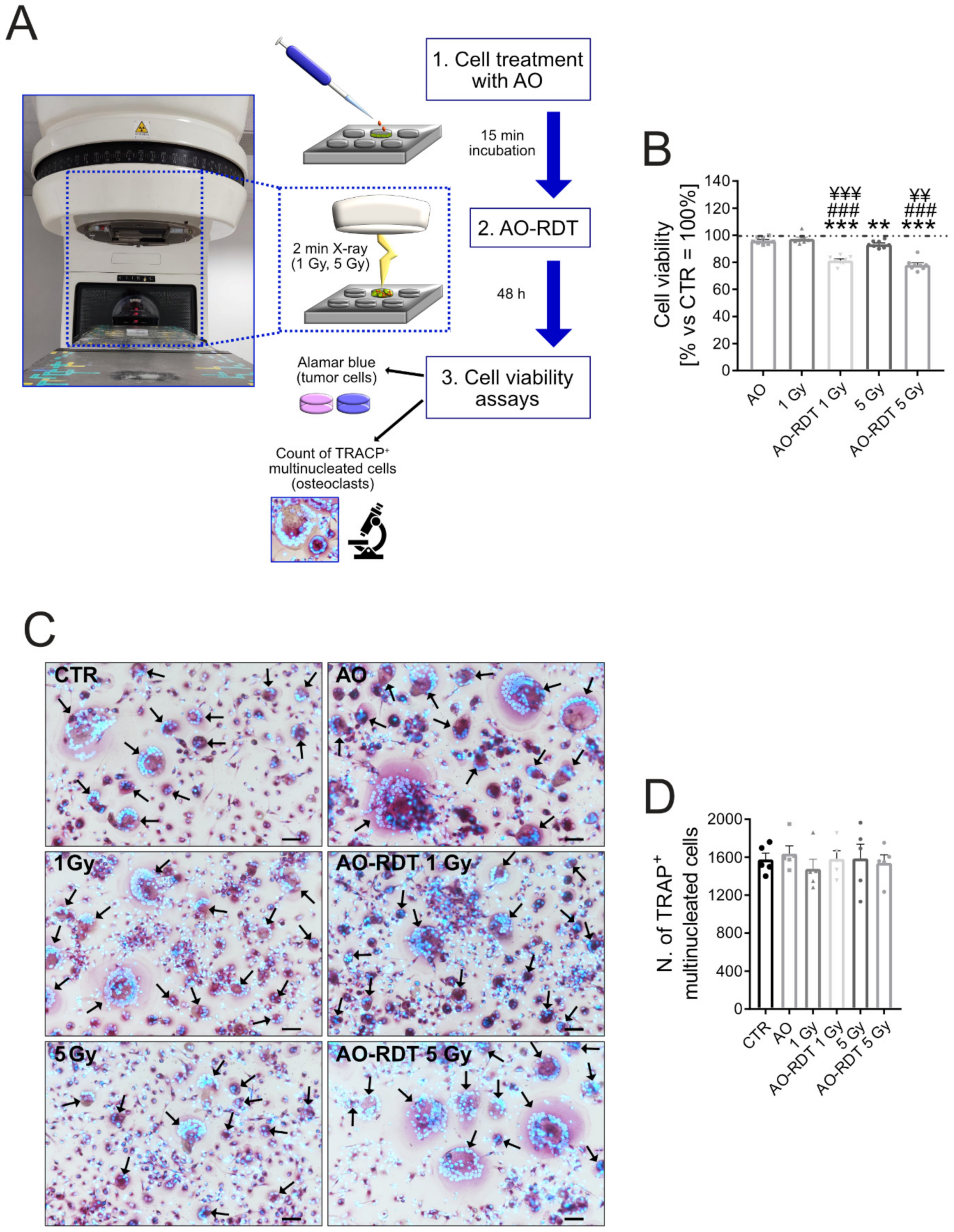
2.7.2. Radiodynamic Therapy with Acridine Orange (AO-RDT)
2.7.3. Histological Analysis
2.7.4. Histomorphometric Analysis
2.8. Statistics
3. Results and Discussion
3.1. Tumor Acidosis in BM Enhances Local Carcinoma Aggressiveness
3.2. In Vivo Targeting of Intratumoral Acidification by V-ATPase Blockage Does Not Reduce Osteolysis
3.3. Breast Carcinoma Cells Are More Sensitive to Lysosomal Death than OCs
3.4. AO-RDT Impairs Tumor-Induced Osteolysis and Cancer Cell Survival
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019, 15, 100205. [Google Scholar] [CrossRef] [PubMed]
- Clines, G.A.; Guise, T.A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 2008, 10, e7. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Avnet, S.; Di Pompo, G.; Lemma, S.; Baldini, N. Cause and effect of microenvironmental acidosis on bone metastases. Cancer Metastasis Rev. 2019, 38, 133–147. [Google Scholar] [CrossRef]
- Martinez-Zaguilan, R.; Seftor, E.A.; Seftor, R.E.; Chu, Y.W.; Gillies, R.J.; Hendrix, M.J. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 1996, 14, 176–186. [Google Scholar] [CrossRef]
- DeClerck, K.; Elble, R.C. The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Front. Biosci. (Landmark Ed.) 2010, 15, 213–225. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Nagata, Y.; Okui, T.; White, F.A. Acidic microenvironment and bone pain in cancer-colonized bone. Bonekey Rep. 2015, 4, 690. [Google Scholar] [CrossRef]
- Shibutani, T.; Heersche, J.N. Effect of medium pH on osteoclast activity and osteoclast formation in cultures of dispersed rabbit osteoclasts. J. Bone Miner. Res. 1993, 8, 331–336. [Google Scholar] [CrossRef]
- Arnett, T.R.; Dempster, D.W. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology 1986, 119, 119–124. [Google Scholar] [CrossRef]
- Granchi, D.; Torreggiani, E.; Massa, A.; Caudarella, R.; Di Pompo, G.; Baldini, N. Potassium citrate prevents increased osteoclastogenesis resulting from acidic conditions: Implication for the treatment of postmenopausal bone loss. PLoS ONE 2017, 12, e0181230. [Google Scholar] [CrossRef]
- Di Pompo, G.; Errani, C.; Gillies, R.; Mercatali, L.; Ibrahim, T.; Tamanti, J.; Baldini, N.; Avnet, S. Acid-Induced Inflammatory Cytokines in Osteoblasts: A Guided Path to Osteolysis in Bone Metastasis. Front. Cell Dev. Biol. 2021, 9, 678532. [Google Scholar] [CrossRef]
- Di Pompo, G.; Lemma, S.; Canti, L.; Rucci, N.; Ponzetti, M.; Errani, C.; Donati, D.M.; Russell, S.; Gillies, R.; Chano, T.; et al. Intratumoral acidosis fosters cancer-induced bone pain through the activation of the mesenchymal tumor-associated stroma in bone metastasis from breast carcinoma. Oncotarget 2017, 8, 54478–54496. [Google Scholar] [CrossRef]
- Zelenin, A.V. Fluorescence microscopy of lysosomes and related structures in living cells. Nature 1966, 212, 425–426. [Google Scholar] [CrossRef]
- Krolenko, S.A.; Adamyan, S.Y.; Belyaeva, T.N.; Mozhenok, T.P. Acridine orange accumulation in acid organelles of normal and vacuolated frog skeletal muscle fibres. Cell Biol. Int. 2006, 30, 933–939. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Hosogi, S.; Ashihara, E.; Matsubara, T.; Satonaka, H.; Nakamura, T.; Matsumine, A.; Sudo, A.; Uchida, A.; Murata, H.; et al. Translational research of photodynamic therapy with acridine orange which targets cancer acidity. Curr. Pharm. Des. 2012, 18, 1414–1420. [Google Scholar] [CrossRef]
- Brunk, U.T.; Dalen, H.; Roberg, K.; Hellquist, H.B. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 1997, 23, 616–626. [Google Scholar] [CrossRef]
- Byvaltsev, V.A.; Bardonova, L.A.; Onaka, N.R.; Polkin, R.A.; Ochkal, S.V.; Shepelev, V.V.; Aliyev, M.A.; Potapov, A.A. Acridine Orange: A Review of Novel Applications for Surgical Cancer Imaging and Therapy. Front. Oncol. 2019, 9, 925. [Google Scholar] [CrossRef]
- Faris, P.; Shekha, M.; Montagna, D.; Guerra, G.; Moccia, F. Endolysosomal Ca2+ Signalling and Cancer Hallmarks: Two-Pore Channels on the Move, TRPML1 Lags Behind! Cancers 2018, 11, 27. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Geisslinger, F.; Muller, M.; Vollmar, A.M.; Bartel, K. Targeting Lysosomes in Cancer as Promising Strategy to Overcome Chemoresistance—A Mini Review. Front. Oncol. 2020, 10, 1156. [Google Scholar] [CrossRef]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef]
- Chen, R.; Jaattela, M.; Liu, B. Lysosome as a Central Hub for Rewiring PH Homeostasis in Tumors. Cancers 2020, 12, 2437. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Wang, F.; Gomez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Aomori, K.; Suginoshita, T.; Minami, G.; Takeshita, H.; Murata, H.; Hashiguchi, S.; Ashihara, T.; Hirasawa, Y. Total tumor cell elimination with minimum damage to normal tissues in musculoskeletal sarcomas following photodynamic therapy with acridine orange. Oncology 2000, 59, 174–180. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Suginoshita, T.; Minami, G.; Aomori, K.; Takeshita, H.; Murata, H.; Hashiguchi, S.; Ashihara, T.; Hirasawa, Y. Fluorovisualization effect of acridine orange on mouse osteosarcoma. Anticancer Res. 2000, 20, 3019–3024. [Google Scholar]
- Martano, M.; Morello, E.; Avnet, S.; Costa, F.; Sammartano, F.; Kusuzaki, K.; Baldini, N. Photodynamic Surgery for Feline Injection-Site Sarcoma. BioMed Res. Int. 2019, 2019, 8275935. [Google Scholar] [CrossRef]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Murata, H.; Marunaka, Y.; Hosogi, S.; Uchida, A.; Sudo, A. Photodynamic therapy with acridine orange in musculoskeletal sarcomas. J. Bone Jt. Surg. Br. 2010, 92, 760–762. [Google Scholar] [CrossRef]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Murata, H.; Nakamura, T.; Uchida, A.; Sudo, A. Clinical outcomes of minimally invasive surgery using acridine orange for musculoskeletal sarcomas around the forearm, compared with conventional limb salvage surgery after wide resection. J. Surg. Oncol. 2010, 102, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.C.; Giacomelli, M.G.; Yoshitake, T.; Vardeh, H.; Faulkner-Jones, B.E.; Connolly, J.L.; Sun, C.K.; Fujimoto, J.G. Rapid virtual hematoxylin and eosin histology of breast tissue specimens using a compact fluorescence nonlinear microscope. Lab. Investig. 2018, 98, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Gareau, D.; Bar, A.; Snaveley, N.; Lee, K.; Chen, N.; Swanson, N.; Simpson, E.; Jacques, S. Tri-modal confocal mosaics detect residual invasive squamous cell carcinoma in Mohs surgical excisions. J. Biomed. Opt. 2012, 17, 066018. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Rajadhyaksha, M.; Nehal, K. Implementation of fluorescence confocal mosaicking microscopy by “early adopter” Mohs surgeons and dermatologists: Recent progress. J. Biomed. Opt. 2017, 22, 024002. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Cortes, A.; Lopez, M.; Wallace, M.; Sabir, S.; Shaw, K.; Mills, G. Ex Vivo Confocal Fluorescence Microscopy for Rapid Evaluation of Tissues in Surgical Pathology Practice. Arch. Pathol. Lab. Med. 2018, 142, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Shintani, K.; Satonaka, H.; Uchida, A. Acridine orange used for photodynamic therapy accumulates in malignant musculoskeletal tumors depending on pH gradient. Anticancer Res. 2006, 26, 187–193. [Google Scholar]
- Hashiguchi, S.; Kusuzaki, K.; Murata, H.; Takeshita, H.; Hashiba, M.; Nishimura, T.; Ashihara, T.; Hirasawa, Y. Acridine orange excited by low-dose radiation has a strong cytocidal effect on mouse osteosarcoma. Oncology 2002, 62, 85–93. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Minami, G.; Takeshita, H.; Murata, H.; Hashiguchi, S.; Nozaki, T.; Ashihara, T.; Hirasawa, Y. Photodynamic inactivation with acridine orange on a multidrug-resistant mouse osteosarcoma cell line. Jpn. J. Cancer Res. 2000, 91, 439–445. [Google Scholar] [CrossRef]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Murata, H.; Satonaka, H.; Shintani, K.; Nakamura, T.; Hosoi, H.; Iehara, T.; Sugimoto, T.; et al. A new therapeutic modality involving acridine orange excitation by photon energy used during reduction surgery for rhabdomyosarcomas. Oncol. Rep. 2009, 21, 89–94. [Google Scholar]
- Lacombe, J.; Karsenty, G.; Ferron, M. Regulation of lysosome biogenesis and functions in osteoclasts. Cell Cycle 2013, 12, 2744–2752. [Google Scholar] [CrossRef]
- Avnet, S.; Cenni, E.; Granchi, D.; Perut, F.; Amato, I.; Battistelli, L.; Brandi, M.L.; Giunti, A.; Baldini, N. Isolation and characterization of a new cell line from a renal carcinoma bone metastasis. Anticancer Res. 2004, 24, 1705–1711. [Google Scholar]
- Avnet, S.; Di Pompo, G.; Lemma, S.; Salerno, M.; Perut, F.; Bonuccelli, G.; Granchi, D.; Zini, N.; Baldini, N. V-ATPase is a candidate therapeutic target for Ewing sarcoma. Biochim. Biophys. Acta 2013, 1832, 1105–1116. [Google Scholar] [CrossRef][Green Version]
- Ciapetti, G.; Di Pompo, G.; Avnet, S.; Martini, D.; Diez-Escudero, A.; Montufar, E.B.; Ginebra, M.P.; Baldini, N. Osteoclast differentiation from human blood precursors on biomimetic calcium-phosphate substrates. Acta Biomater. 2017, 50, 102–113. [Google Scholar] [CrossRef]
- Fotia, C.; Avnet, S.; Kusuzaki, K.; Roncuzzi, L.; Baldini, N. Acridine Orange is an Effective Anti-Cancer Drug that Affects Mitochondrial Function in Osteosarcoma Cells. Curr. Pharm. Des. 2015, 21, 4088–4094. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Roth, A.G.; Petersen, N.H.; Mahalka, A.K.; Olsen, O.D.; Moilanen, I.; Zylicz, A.; Knudsen, J.; Sandhoff, K.; Arenz, C.; et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010, 463, 549–553. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gawlinski, E.T.; Gmitro, A.F.; Kaylor, B.; Gillies, R.J. Acid-mediated tumor invasion: A multidisciplinary study. Cancer Res. 2006, 66, 5216–5223. [Google Scholar] [CrossRef]
- Kolosenko, I.; Avnet, S.; Baldini, N.; Viklund, J.; De Milito, A. Therapeutic implications of tumor interstitial acidification. Semin. Cancer Biol. 2017, 43, 119–133. [Google Scholar] [CrossRef]
- Lloyd, M.C.; Cunningham, J.J.; Bui, M.M.; Gillies, R.J.; Brown, J.S.; Gatenby, R.A. Darwinian Dynamics of Intratumoral Heterogeneity: Not Solely Random Mutations but Also Variable Environmental Selection Forces. Cancer Res. 2016, 76, 3136–3144. [Google Scholar] [CrossRef]
- Rozhin, J.; Sameni, M.; Ziegler, G.; Sloane, B.F. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994, 54, 6517–6525. [Google Scholar]
- Kato, Y.; Lambert, C.A.; Colige, A.C.; Mineur, P.; Noel, A.; Frankenne, F.; Foidart, J.M.; Baba, M.; Hata, R.; Miyazaki, K.; et al. Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J. Biol. Chem. 2005, 280, 10938–10944. [Google Scholar] [CrossRef] [PubMed]
- Sennoune, S.R.; Bakunts, K.; Martinez, G.M.; Chua-Tuan, J.L.; Kebir, Y.; Attaya, M.N.; Martinez-Zaguilan, R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: Distribution and functional activity. Am. J. Physiol. Cell Physiol. 2004, 286, C1443–C1452. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zaguilan, R.; Raghunand, N.; Lynch, R.M.; Bellamy, W.; Martinez, G.M.; Rojas, B.; Smith, D.; Dalton, W.S.; Gillies, R.J. pH and drug resistance. I. Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines. Biochem. Pharmacol. 1999, 57, 1037–1046. [Google Scholar] [CrossRef]
- Raghunand, N.; Altbach, M.I.; van Sluis, R.; Baggett, B.; Taylor, C.W.; Bhujwalla, Z.M.; Gillies, R.J. Plasmalemmal pH-gradients in drug-sensitive and drug-resistant MCF-7 human breast carcinoma xenografts measured by 31P magnetic resonance spectroscopy. Biochem. Pharmacol. 1999, 57, 309–312. [Google Scholar] [CrossRef]
- De Milito, A.; Marino, M.L.; Fais, S. A rationale for the use of proton pump inhibitors as antineoplastic agents. Curr. Pharm. Des. 2012, 18, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Torigoe, T.; Ishiguchi, H.; Uramoto, H.; Yoshida, Y.; Tanabe, M.; Ise, T.; Murakami, T.; Yoshida, T.; Nomoto, M.; et al. Cellular pH regulators: Potentially promising molecular targets for cancer chemotherapy. Cancer Treat. Rev. 2003, 29, 541–549. [Google Scholar] [CrossRef]
- Luciani, F.; Spada, M.; De Milito, A.; Molinari, A.; Rivoltini, L.; Montinaro, A.; Marra, M.; Lugini, L.; Logozzi, M.; Lozupone, F.; et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J. Natl. Cancer Inst. 2004, 96, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- Ouar, Z.; Bens, M.; Vignes, C.; Paulais, M.; Pringel, C.; Fleury, J.; Cluzeaud, F.; Lacave, R.; Vandewalle, A. Inhibitors of vacuolar H+-ATPase impair the preferential accumulation of daunomycin in lysosomes and reverse the resistance to anthracyclines in drug-resistant renal epithelial cells. Biochem. J. 2003, 370, 185–193. [Google Scholar] [CrossRef]
- Perut, F.; Avnet, S.; Fotia, C.; Baglio, S.R.; Salerno, M.; Hosogi, S.; Kusuzaki, K.; Baldini, N. V-ATPase as an effective therapeutic target for sarcomas. Exp. Cell Res. 2014, 320, 21–32. [Google Scholar] [CrossRef]
- De Milito, A.; Iessi, E.; Logozzi, M.; Lozupone, F.; Spada, M.; Marino, M.L.; Federici, C.; Perdicchio, M.; Matarrese, P.; Lugini, L.; et al. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007, 67, 5408–5417. [Google Scholar] [CrossRef]
- Canitano, A.; Iessi, E.; Spugnini, E.P.; Federici, C.; Fais, S. Proton pump inhibitors induce a caspase-independent antitumor effect against human multiple myeloma. Cancer Lett. 2016, 376, 278–283. [Google Scholar] [CrossRef]
- Prause, M.; Seeliger, C.; Unger, M.; Rosado Balmayor, E.; van Griensven, M.; Haug, A.T. Pantoprazole decreases cell viability and function of human osteoclasts in vitro. Mediat. Inflamm. 2015, 2015, 413097. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Henriksen, K.; Sorensen, M.G.; Gram, J.; Schaller, S.; Dziegiel, M.H.; Heegaard, A.M.; Christophersen, P.; Martin, T.J.; Christiansen, C.; et al. Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am. J. Pathol. 2005, 166, 467–476. [Google Scholar] [CrossRef]
- Nishi, T.; Forgac, M. The vacuolar (H+)-ATPases—Nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002, 3, 94–103. [Google Scholar] [CrossRef]
- Sun-Wada, G.H.; Wada, Y.; Futai, M. Vacuolar H+ pumping ATPases in luminal acidic organelles and extracellular compartments: Common rotational mechanism and diverse physiological roles. J. Bioenerg. Biomembr. 2003, 35, 347–358. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Reis, S.; Teixeira, S.; Lopes, S.; Fernandes, M.H. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J. 2013, 280, 5052–5064. [Google Scholar] [CrossRef]
- Visentin, L.; Dodds, R.A.; Valente, M.; Misiano, P.; Bradbeer, J.N.; Oneta, S.; Liang, X.; Gowen, M.; Farina, C. A selective inhibitor of the osteoclastic V-H+-ATPase prevents bone loss in both thyroparathyroidectomized and ovariectomized rats. J. Clin. Investig. 2000, 106, 309–318. [Google Scholar] [CrossRef]
- Tuukkanen, J.; Vaananen, H.K. Omeprazole, a specific inhibitor of H+-K+-ATPase, inhibits bone resorption in vitro. Calcif. Tissue Int. 1986, 38, 123–125. [Google Scholar] [CrossRef]
- Yang, Y.X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef]
- Ito, T.; Jensen, R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr. Gastroenterol. Rep. 2010, 12, 448–457. [Google Scholar] [CrossRef]
- Gagnemo-Persson, R.; Samuelsson, A.; Hakanson, R.; Persson, P. Chicken parathyroid hormone gene expression in response to gastrin, omeprazole, ergocalciferol, and restricted food intake. Calcif. Tissue Int. 1997, 61, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.L.; Syversen, U.; Zhao, C.M.; Chen, D.; Waldum, H.L. Long-term omeprazole treatment suppresses body weight gain and bone mineralization in young male rats. Scand. J. Gastroenterol. 2001, 36, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Mizunashi, K.; Furukawa, Y.; Katano, K.; Abe, K. Effect of omeprazole, an inhibitor of H+, K+-ATPase, on bone resorption in humans. Calcif. Tissue Int. 1993, 53, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.J.; Chun, H.J.; Keum, B.; Seo, Y.S.; Kim, Y.S.; Jeen, Y.T.; Lee, H.S.; Um, S.H.; Kim, C.D.; Ryu, H.S.; et al. Effect of omeprazole on the expression of transcription factors in osteoclasts and osteoblasts. Int. J. Mol. Med. 2010, 26, 877–883. [Google Scholar] [CrossRef]
- Glunde, K.; Guggino, S.E.; Solaiyappan, M.; Pathak, A.P.; Ichikawa, Y.; Bhujwalla, Z.M. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 2003, 5, 533–545. [Google Scholar] [CrossRef]
- Piao, S.; Amaravadi, R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016, 1371, 45–54. [Google Scholar] [CrossRef]
- Serrano-Puebla, A.; Boya, P. Lysosomal membrane permeabilization as a cell death mechanism in cancer cells. Biochem. Soc. Trans. 2018, 46, 207–215. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; Satonaka, H.; Wakabayashi, T.; Matsumine, A.; Uchida, A. Review. Acridine orange could be an innovative anticancer agent under photon energy. In Vivo 2007, 21, 205–214. [Google Scholar]
- Nakamura, T.; Kusuzaki, K.; Matsubara, T.; Matsumine, A.; Murata, H.; Uchida, A. A new limb salvage surgery in cases of high-grade soft tissue sarcoma using photodynamic surgery, followed by photo- and radiodynamic therapy with acridine orange. J. Surg. Oncol. 2008, 97, 523–528. [Google Scholar] [CrossRef]
- Nakamura, T.; Kusuzaki, K.; Matsubara, T.; Murata, H.; Hagi, T.; Asanuma, K.; Sudo, A. Long-term clinical outcome in patients with high-grade soft tissue sarcoma who were treated with surgical adjuvant therapy using acridine orange after intra-lesional or marginal resection. Photodiagn. Photodyn. Ther. 2018, 23, 165–170. [Google Scholar] [CrossRef]
- Tsuchie, H.; Emori, M.; Miyakoshi, N.; Nagasawa, H.; Okada, K.; Murahashi, Y.; Mizushima, E.; Shimizu, J.; Yamashita, T.; Shimada, Y. Impact of Acridine Orange in Patients With Soft Tissue Sarcoma Treated With Marginal Resection. Anticancer Res. 2019, 39, 6365–6372. [Google Scholar] [CrossRef]
- Paglin, S.; Hollister, T.; Delohery, T.; Hackett, N.; McMahill, M.; Sphicas, E.; Domingo, D.; Yahalom, J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001, 61, 439–444. [Google Scholar]
- Lovejoy, D.B.; Jansson, P.J.; Brunk, U.T.; Wong, J.; Ponka, P.; Richardson, D.R. Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 2011, 71, 5871–5880. [Google Scholar] [CrossRef]
- Petersen, N.H.; Olsen, O.D.; Groth-Pedersen, L.; Ellegaard, A.M.; Bilgin, M.; Redmer, S.; Ostenfeld, M.S.; Ulanet, D.; Dovmark, T.H.; Lonborg, A.; et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 2013, 24, 379–393. [Google Scholar] [CrossRef]
- Clement, S.; Anwer, A.G.; Pires, L.; Campbell, J.; Wilson, B.C.; Goldys, E.M. Radiodynamic Therapy Using TAT Peptide-Targeted Verteporfin-Encapsulated PLGA Nanoparticles. Int. J. Mol. Sci. 2021, 22, 6425. [Google Scholar] [CrossRef]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef]
- Pogue, B.W.; Wilson, B.C. Optical and X-ray technology synergies enabling diagnostic and therapeutic applications in medicine. J. Biomed. Opt. 2018, 23, 121610. [Google Scholar] [CrossRef]
- Bernier, J.; Hall, E.J.; Giaccia, A. Radiation oncology: A century of achievements. Nat. Rev. Cancer 2004, 4, 737–747. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; Miyazaki, S.; Okamura, A.; Seto, M.; Matsumine, A.; Hosoi, H.; Sugimoto, T.; Uchida, A. Clinical trial of photodynamic therapy using acridine orange with/without low dose radiation as new limb salvage modality in musculoskeletal sarcomas. Anticancer Res. 2005, 25, 1225–1235. [Google Scholar]




| Effect | Sum of Squares | Degrees of Freedom | F-Value | p | Interpretation |
|---|---|---|---|---|---|
| Acridine orange | 0.35 | 1 | 1.09 | 0.3037 | |
| Irradiation | 0.02 | 1 | 0.07 | 0.7975 | |
| Interaction | 2.11 | 1 | 6.69 | 0.0146 | Interaction |
| Residuals | 9.79 | 31 | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pompo, G.; Kusuzaki, K.; Ponzetti, M.; Leone, V.F.; Baldini, N.; Avnet, S. Radiodynamic Therapy with Acridine Orange Is an Effective Treatment for Bone Metastases. Biomedicines 2022, 10, 1904. https://doi.org/10.3390/biomedicines10081904
Di Pompo G, Kusuzaki K, Ponzetti M, Leone VF, Baldini N, Avnet S. Radiodynamic Therapy with Acridine Orange Is an Effective Treatment for Bone Metastases. Biomedicines. 2022; 10(8):1904. https://doi.org/10.3390/biomedicines10081904
Chicago/Turabian StyleDi Pompo, Gemma, Katsuyuki Kusuzaki, Marco Ponzetti, Vito Ferdinando Leone, Nicola Baldini, and Sofia Avnet. 2022. "Radiodynamic Therapy with Acridine Orange Is an Effective Treatment for Bone Metastases" Biomedicines 10, no. 8: 1904. https://doi.org/10.3390/biomedicines10081904
APA StyleDi Pompo, G., Kusuzaki, K., Ponzetti, M., Leone, V. F., Baldini, N., & Avnet, S. (2022). Radiodynamic Therapy with Acridine Orange Is an Effective Treatment for Bone Metastases. Biomedicines, 10(8), 1904. https://doi.org/10.3390/biomedicines10081904









