Discovery of Pharmaceutical Composition for Prevention and Treatment in Patient-Derived Metastatic Medullary Thyroid Carcinoma Model
Abstract
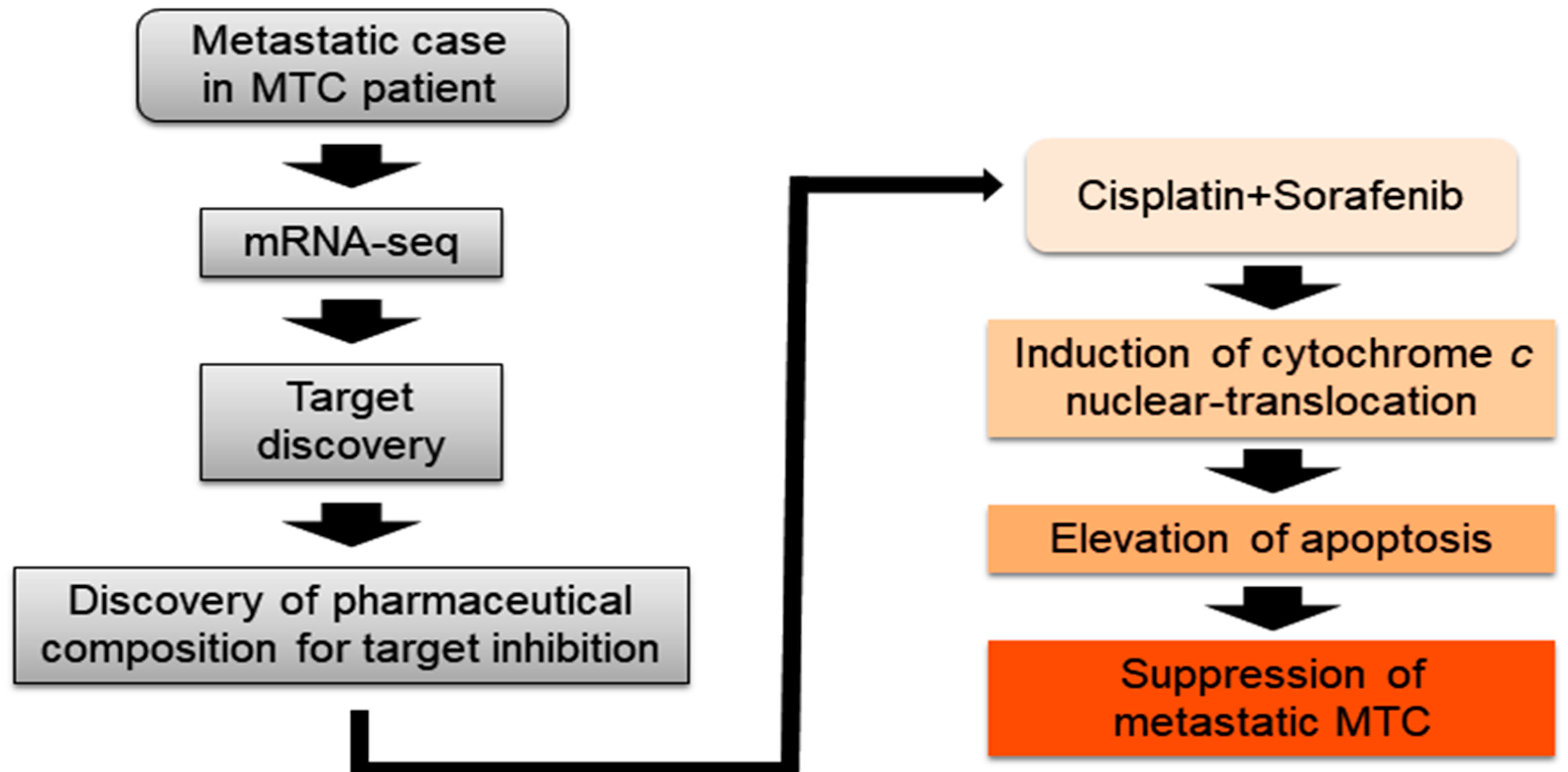
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Patients
2.3. Patient Tissue Specimens and Clinical Course
2.4. Tumor Cell Isolation and Primary Culture
2.5. mRNA-Seq Data
2.6. Statistical Analysis of Gene Expression Level
2.7. Hierarchical Clustering
2.8. Protein–Protein Interaction
2.9. Cell Culture
2.10. Cell Viability Assay
2.11. Cell Cycle Analysis Using Flow Cytometry
2.12. Immunofluorescence Analysis and Confocal Imaging
2.13. Cellular Fractionation
2.14. Immunoblot Analysis
2.15. Human MTC Cell Xenograft
2.16. Statistical Analysis
3. Results
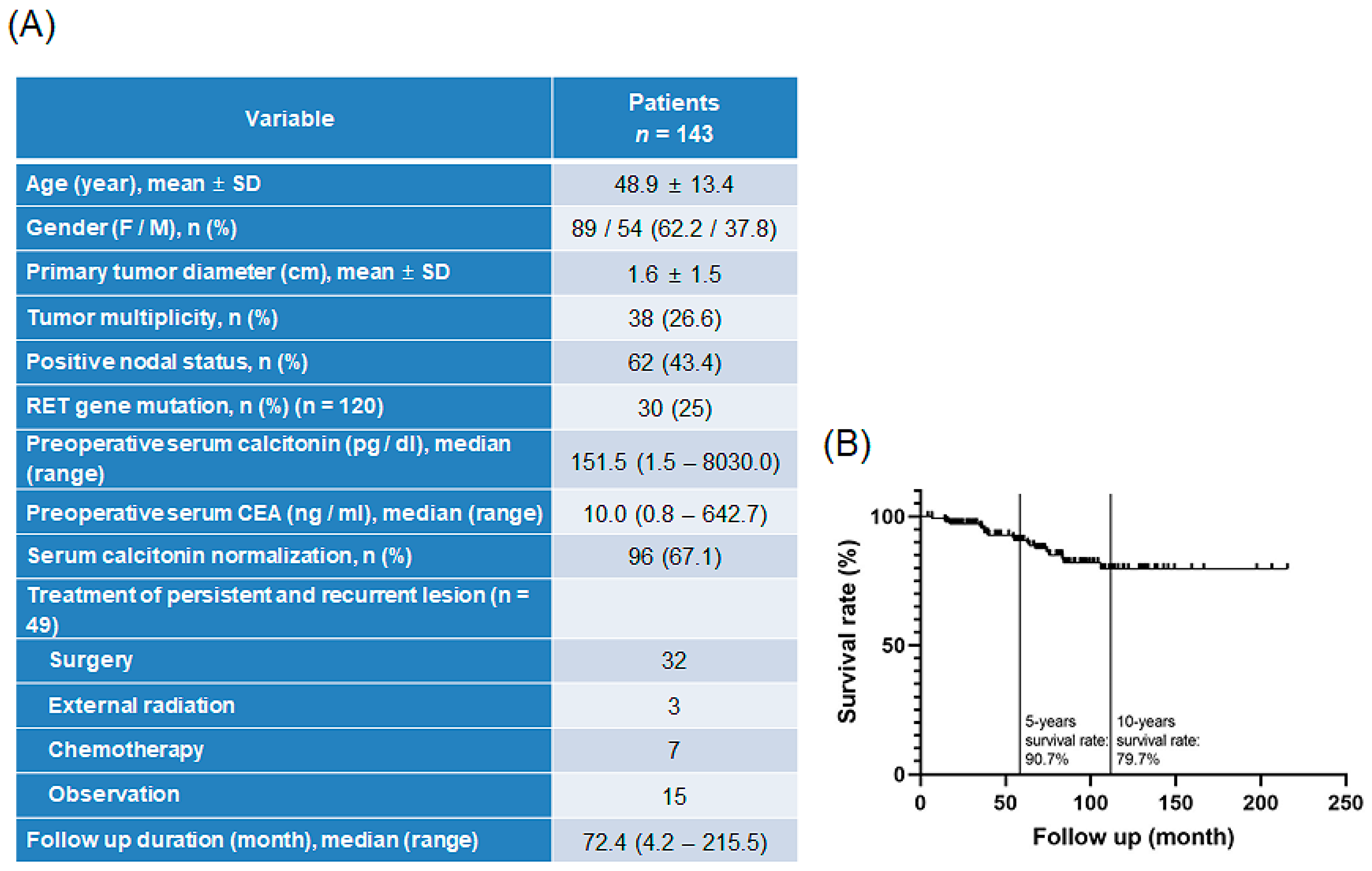
3.1. Patient Disease Characteristics
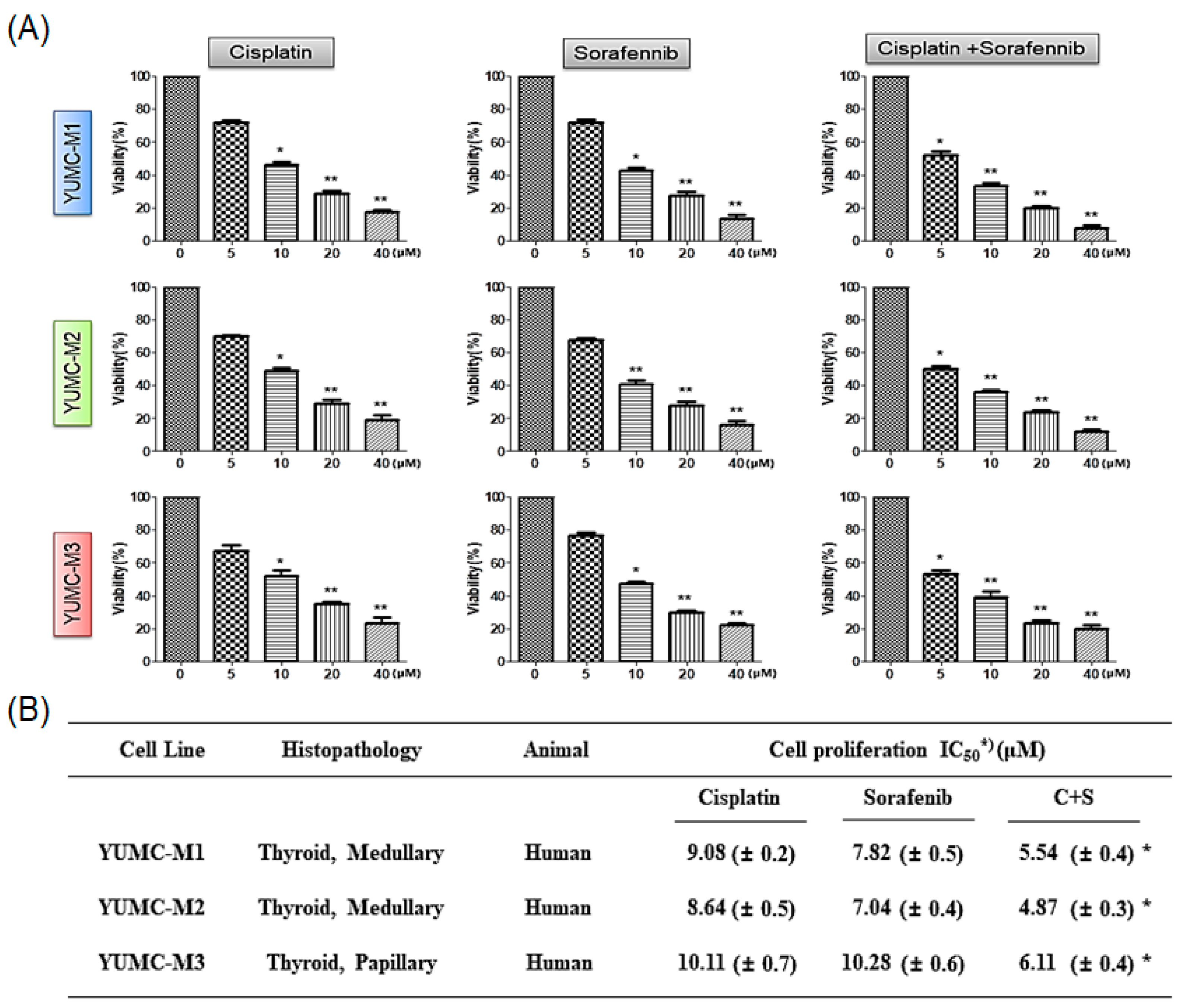
3.2. Features of Patient-Derived MTC Cell Lines
3.3. Combination of Cisplatin and Sorafenib Was More Efficacious Than Either Agent Alone
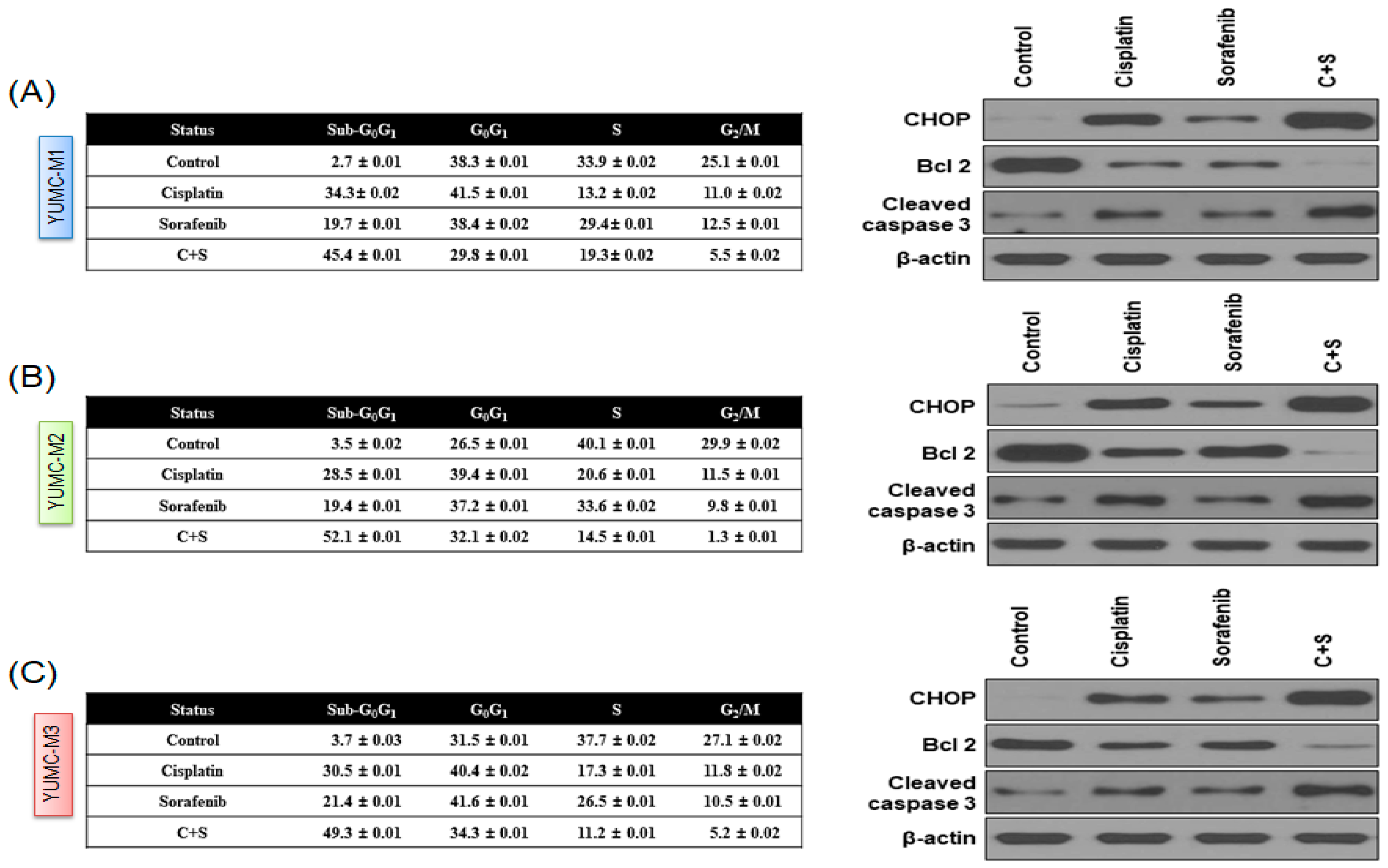
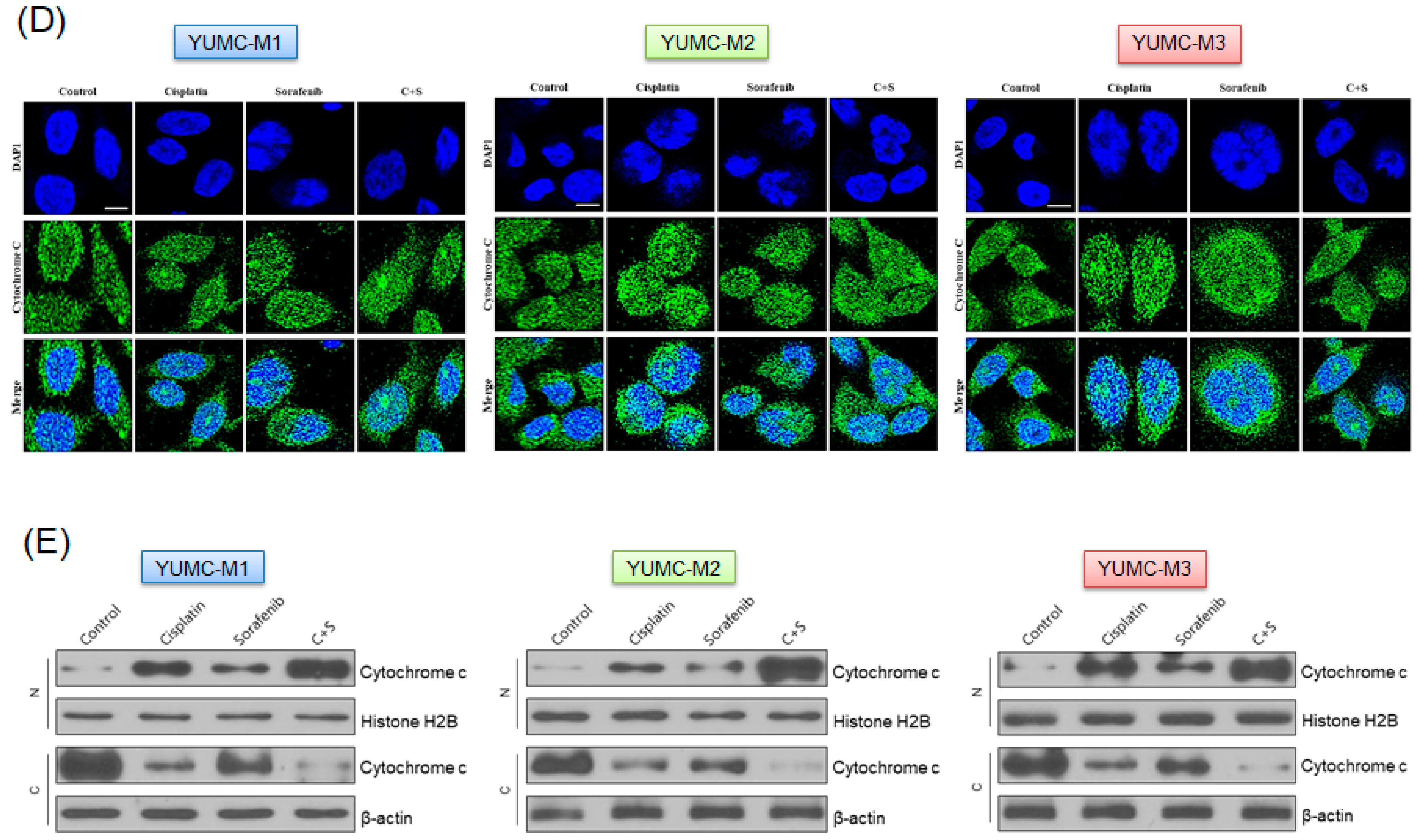
3.4. Cisplatin and Sorafenib Co-Treatment Increased Nuclear Translocation-Mediated Apoptosis in YUMC-M1, M2, and M3 Cells
3.5. Cisplatin and Sorafenib Co-Treatment Remarkably Restrained Tumor Growth in Mouse Xenograft Model with Patient-Derived MTC Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, H.J.; Shiah, H.S.; Chang, J.Y.; Su, W.C.; Chiang, N.J.; Chen, L.T. Phase i dose escalation study of sorafenib plus s-1 for advanced solid tumors. Sci. Rep. 2021, 11, 4834. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Lippard, S.J. Cisplatin: From DNA damage to cancer chemotherapy. Prog Nucleic Acid Res. Mol. Biol. 2001, 67, 93–130. [Google Scholar] [PubMed]
- Nikiforov, Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008, 21, S37–S43. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and treatment of patients with thyroid cancer. Am. Health Drug Benefits 2015, 8, 30–40. [Google Scholar]
- Fahiminiya, S.; de Kock, L.; Foulkes, W.D. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 2016, 375, 2306–2307. [Google Scholar]
- Owonikoko, T.K.; Chowdry, R.P.; Chen, Z.; Kim, S.; Saba, N.F.; Shin, D.M.; Khuri, F.R. Clinical efficacy of targeted biologic agents as second-line therapy of advanced thyroid cancer. Oncologist 2013, 18, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Viola, D.; Elisei, R. Management of medullary thyroid cancer. Endocrinol. Metab. Clin. 2019, 48, 285–301. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Randle, R.W.; Balentine, C.J.; Leverson, G.E.; Havlena, J.A.; Sippel, R.S.; Schneider, D.F.; Pitt, S.C. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2017, 161, 137–146. [Google Scholar] [CrossRef]
- Scollo, C.; Baudin, E.; Travagli, J.P.; Caillou, B.; Bellon, N.; Leboulleux, S.; Schlumberger, M. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2003, 88, 2070–2075. [Google Scholar] [CrossRef]
- Roman, S.; Lin, R.; Sosa, J.A. Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006, 107, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Call, J.A.; Caudill, J.S.; McIver, B.; Foote, R.L. A role for radiotherapy in the management of advanced medullary thyroid carcinoma: The mayo clinic experience. Rare Tumors 2013, 5, e37. [Google Scholar] [CrossRef]
- Vitale, G.; Dicitore, A.; Pepe, D.; Gentilini, D.; Grassi, E.S.; Borghi, M.O.; Gelmini, G.; Cantone, M.C.; Gaudenzi, G.; Misso, G.; et al. Synergistics activity of everolimus and 5-aza-2’-deoxycytidine in medullary thyroid carcinoma cell lines. Mol. Oncol. 2017, 11, 1007–1022. [Google Scholar] [CrossRef]
- Raoof, S.; Mulford, I.J.; Frisco-Cabanos, H.; Nangia, V.; Timonina, D.; Labrot, E.; Hafeez, N.; Bilton, S.J.; Drier, Y.; Ji, F.; et al. Targeting fgfr overcomes emt-mediated resistance in egfr mutant non-small cell lung cancer. Oncogene 2019, 38, 6399–6413. [Google Scholar] [CrossRef] [PubMed]
- Zivotic, M.; Tampe, B.; Muller, G.; Muller, C.; Lipkovski, A.; Xu, X.; Nyamsuren, G.; Zeisberg, M.; Markovic-Lipkovski, J. Modulation of ncam/fgfr1 signaling suppresses emt program in human proximal tubular epithelial cells. PLoS ONE 2018, 13, e0206786. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.S.; Akhand, S.S.; Wendt, M.K. Fgfr signaling maintains a drug persistent cell population following epithelial-mesenchymal transition. Oncotarget 2016, 7, 83424–83436. [Google Scholar] [CrossRef]
- Benekli, M.; Yalcin, S.; Ozkan, M.; Elkiran, E.T.; Sevinc, A.; Cabuk, D.; Coskun, H.S.; Oksuzoglu, B.; Bayar, B.; Akbulat, A.; et al. Efficacy of sorafenib in advanced differentiated and medullary thyroid cancer: Experience in a turkish population. OncoTargets Ther. 2015, 8, 1–5. [Google Scholar] [CrossRef][Green Version]
- Scherubl, H.; Raue, F.; Ziegler, R. Combination chemotherapy of advanced medullary and differentiated thyroid cancer. Phase ii study. J. Cancer Res. Clin. Oncol. 1990, 116, 21–23. [Google Scholar] [CrossRef]
- Yun, H.J.; Kim, M.; Kim, S.Y.; Fang, S.; Kim, Y.; Chang, H.S.; Chang, H.J.; Park, K.C. Effects of anti-cancer drug sensitivity-related genetic differences on therapeutic approaches in refractory papillary thyroid cancer. Int. J. Mol. Sci. 2022, 23, 699. [Google Scholar] [CrossRef]
- Kweon, S.S.; Shin, M.H.; Chung, I.J.; Kim, Y.J.; Choi, J.S. Thyroid cancer is the most common cancer in women, based on the data from population-based cancer registries, south korea. Jpn. J. Clin. Oncol. 2013, 43, 1039–1046. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Kim, J.; Gosnell, J.E.; Roman, S.A. Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol. 2020, 16, 17–29. [Google Scholar] [CrossRef]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on fundamental mechanisms of thyroid cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Wiltshire, J.J.; Drake, T.M.; Uttley, L.; Balasubramanian, S.P. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; De Vathaire, F.; Scollo, C.; Attard, M.; Giordano, C.; Arena, S.; Dardanoni, G.; Frasca, F.; Malandrino, P.; Vermiglio, F.; et al. Papillary thyroid cancer incidence in the volcanic area of sicily. J. Natl. Cancer Inst. 2009, 101, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Hazard, J.B. The c cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review. Am. J. Pathol. 1977, 88, 213–250. [Google Scholar]
- Song, H.; Lin, C.; Yao, E.; Zhang, K.; Li, X.; Wu, Q.; Chuang, P.T. Selective ablation of tumor suppressors in parafollicular c cells elicits medullary thyroid carcinoma. J. Biol. Chem. 2017, 292, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Onoda, N.; Ito, K.I.; Sugitani, I.; Takahashi, S.; Yamaguchi, I.; Kabu, K.; Tsukada, K. Sorafenib in japanese patients with locally advanced or metastatic medullary thyroid carcinoma and anaplastic thyroid carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 1142–1148. [Google Scholar] [CrossRef]
- Capdevila, J.; Trigo, J.M.; Aller, J.; Manzano, J.L.; Adrian, S.G.; Llopis, C.Z.; Reig, O.; Bohn, U.; Cajal, T.R.Y.; Duran-Poveda, M.; et al. Axitinib treatment in advanced rai-resistant differentiated thyroid cancer (dtc) and refractory medullary thyroid cancer (mtc). Eur. J. Endocrinol. 2017, 177, 309–317. [Google Scholar] [CrossRef]
- Kheiroddin, P.; Rasihashemi, S.Z.; Estiar, M.A.; Mahmudian, B.; Halimi, M.; Mousavi, F.; Nemati, M.; Sakhinia, E. Ret gene analysis in patients with medullary thyroid carcinoma. Clin. Lab. 2016, 62, 871–876. [Google Scholar] [CrossRef]
- Oczko-Wojciechowska, M.; Czarniecka, A.; Gawlik, T.; Jarzab, B.; Krajewska, J. Current status of the prognostic molecular markers in medullary thyroid carcinoma. Endocr. Connect. 2020, 9, R251–R263. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Santoro, M.; Schlumberger, M. The importance of the ret gene in thyroid cancer and therapeutic implications. Nat. Rev. Endocrinol. 2021, 17, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Castellone, M.D.; Melillo, R.M. Ret-mediated modulation of tumor microenvironment and immune response in multiple endocrine neoplasia type 2 (men2). Endocr.-Relat. Cancer 2018, 25, T105–T119. [Google Scholar] [CrossRef]
- Nozhat, Z.; Hedayati, M. Medullary thyroid carcinoma: A review on ethical considerations in treatment of children. J. Pediatric Endocrinol. Metab. JPEM 2016, 29, 633–639. [Google Scholar] [CrossRef]
- Zedenius, J. Is somatic ret mutation a prognostic factor for sporadic medullary thyroid carcinoma? Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 432–433. [Google Scholar] [CrossRef]
- Paepegaey, A.C.; Cochand-Priollet, B.; Louiset, E.; Sarfati, P.O.; Alifano, M.; Burnichon, N.; Bienvenu-Perrard, M.; Lahlou, N.; Bricaire, L.; Groussin, L. Long-term control of hypercortisolism by vandetanib in a case of medullary thyroid carcinoma with a somatic ret mutation. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.; Frilling, A. 27-bp deletion in the ret proto-oncogene as a somatic mutation associated with medullary thyroid carcinoma. J. Mol. Med. 1998, 76, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.S.; Evans, D.B.; Fareau, G.G.; Carroll, T.; Yen, T.W. Effect of prophylactic central compartment neck dissection on serum thyroglobulin and recommendations for adjuvant radioactive iodine in patients with differentiated thyroid cancer. Ann. Surg. Oncol. 2012, 19, 4217–4222. [Google Scholar] [CrossRef]
- Priya, S.R.; Dravid, C.S.; Digumarti, R.; Dandekar, M. Targeted therapy for medullary thyroid cancer: A review. Front. Oncol. 2017, 7, 238. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Hu, M.I.; Jimenez, C. Medullary thyroid cancer in the era of tyrosine kinase inhibitors: To treat or not to treat—And with which drug—Those are the questions. J. Clin. Endocrinol. Metab. 2014, 99, 4390–4396. [Google Scholar] [CrossRef]
- Schneider, T.C.; Abdulrahman, R.M.; Corssmit, E.P.; Morreau, H.; Smit, J.W.; Kapiteijn, E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: Final results of a phase ii trial. Eur. J. Endocrinol. 2012, 167, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Waguespack, S.G.; Bronstein, Y.; Williams, M.D.; Feng, L.; Hernandez, M.; Lopez, A.; Sherman, S.I.; Busaidy, N.L. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: The M. D. Anderson experience. J. Clin. Endocrinol. Metab. 2010, 95, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Wan, J.; Morse, P.T.; Zurek, M.P.; Turner, A.A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell Biol. 2020, 121, 105704. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Soares, P.; Lima, J.; Preto, A.; Castro, P.; Vinagre, J.; Celestino, R.; Couto, J.P.; Prazeres, H.; Eloy, C.; Maximo, V.; et al. Genetic alterations in poorly differentiated and undifferentiated thyroid carcinomas. Curr. Genom. 2011, 12, 609–617. [Google Scholar] [CrossRef]
- Viola, D.; Valerio, L.; Molinaro, E.; Agate, L.; Bottici, V.; Biagini, A.; Lorusso, L.; Cappagli, V.; Pieruzzi, L.; Giani, C.; et al. Treatment of advanced thyroid cancer with targeted therapies: Ten years of experience. Endocr.-Relat. Cancer 2016, 23, R185–R205. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-J.; Lim, J.-H.; Kim, S.-Y.; Kim, S.-M.; Park, K.-C. Discovery of Pharmaceutical Composition for Prevention and Treatment in Patient-Derived Metastatic Medullary Thyroid Carcinoma Model. Biomedicines 2022, 10, 1901. https://doi.org/10.3390/biomedicines10081901
Yun H-J, Lim J-H, Kim S-Y, Kim S-M, Park K-C. Discovery of Pharmaceutical Composition for Prevention and Treatment in Patient-Derived Metastatic Medullary Thyroid Carcinoma Model. Biomedicines. 2022; 10(8):1901. https://doi.org/10.3390/biomedicines10081901
Chicago/Turabian StyleYun, Hyeok-Jun, Jin-Hong Lim, Sang-Yong Kim, Seok-Mo Kim, and Ki-Cheong Park. 2022. "Discovery of Pharmaceutical Composition for Prevention and Treatment in Patient-Derived Metastatic Medullary Thyroid Carcinoma Model" Biomedicines 10, no. 8: 1901. https://doi.org/10.3390/biomedicines10081901
APA StyleYun, H.-J., Lim, J.-H., Kim, S.-Y., Kim, S.-M., & Park, K.-C. (2022). Discovery of Pharmaceutical Composition for Prevention and Treatment in Patient-Derived Metastatic Medullary Thyroid Carcinoma Model. Biomedicines, 10(8), 1901. https://doi.org/10.3390/biomedicines10081901






