The First Identification in Italy of SARS-CoV-2 Omicron BA.4 Harboring KSF141_del: A Genomic Comparison with Omicron Sub-Variants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Viral RNA Extraction
2.2. Library Preparation, Next-Generation Sequencing and Bioinformatics
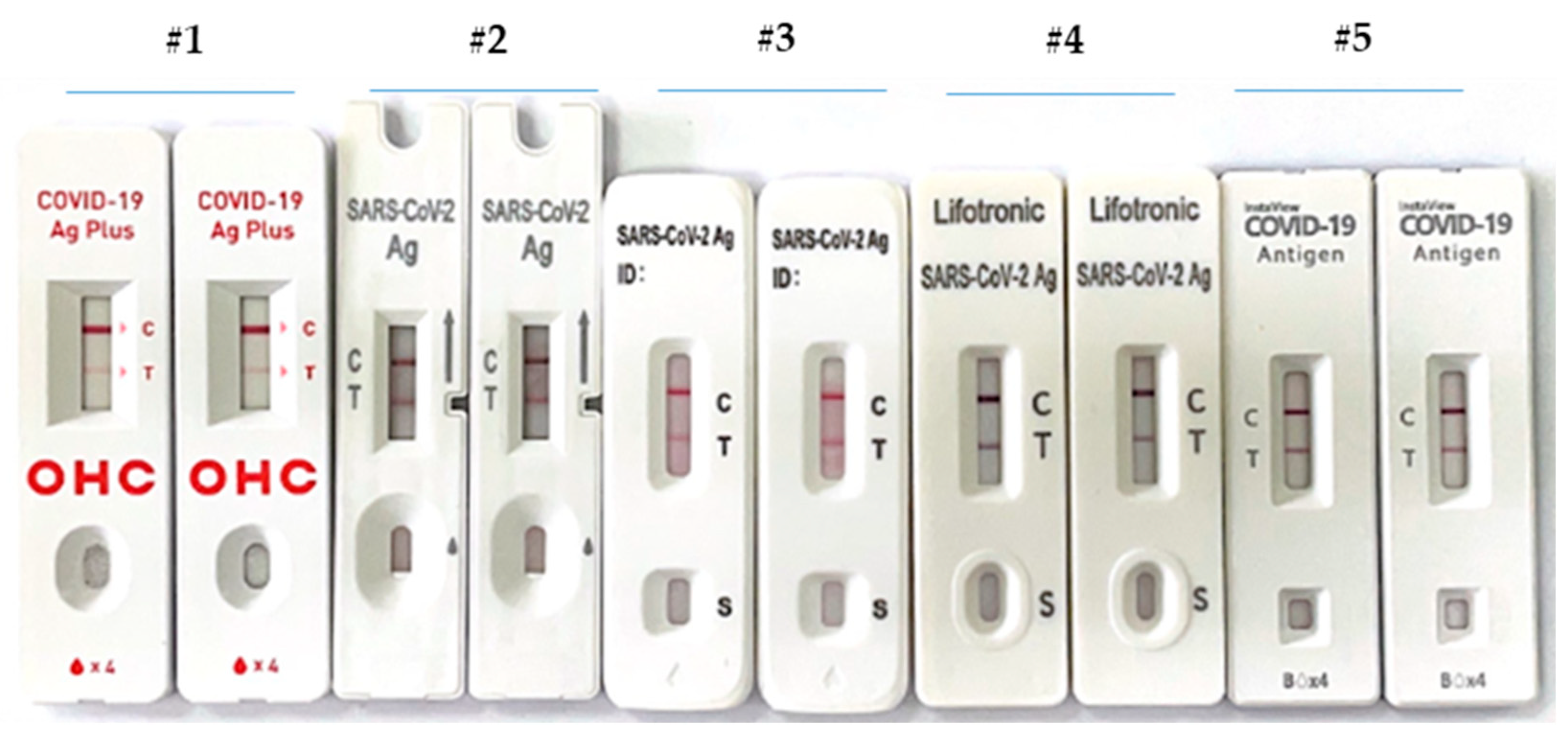
2.3. Rapid SARS-CoV-2 Antigen Detection Assays
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Toole, Á.; Scher, E.; Underwood, A.; Jackson, B.; Hill, V.; McCrone, J.T.; Colquhoun, R.; Ruis, C.; Abu-Dahab, K.; Taylor, B.; et al. Assignment of Epidemiological Lineages in an Emerging Pandemic Using the Pangolin Tool. Virus Evol. 2021, 7, veab064. [Google Scholar] [CrossRef] [PubMed]
- CDC. Science Brief: Omicron (B.1.1.529) Variant; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html (accessed on 2 December 2021).
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 17 January 2022).
- CDC. Science Brief: Omicron (B.1.1.529) Variant. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html (accessed on 4 December 2021).
- Enhancing Readiness for Omicron (B.1.1.529): Technical Brief and Priority Actions for Member States. Available online: https://www.who.int/docs/default-source/coronaviruse/20211217-global-technical-brief-and-priority-action-on-omicron_latest-2.pdf?sfvrsn=bdd8297c_12 (accessed on 11 January 2022).
- Majumdar, S.; Sarkar, R. Mutational and phylogenetic analyses of the two lineages of the Omicron variant. J. Med. Virol. 2021, 94, 1777–1779. [Google Scholar] [CrossRef] [PubMed]
- Desingu, P.A.; Nagarajan, K.; Dhama, K. Emergence of omicron third lineage ba. 3 and its importance. J. Med. Virol. 2022, 94, 1808–1810. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Chan, J.F.W.; Hu, B.; Chai, Y.; Yuen, T.T.T.; Yin, F.; Huang, X.; Yoon, C.; Hu, J.C.; Liu, H.; et al. Attenuated replication and pathogenicity of SARS-CoV-2 B. 1.1. 529 Omicron. Nature 2022, 603, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Han, W.; Li, J.; Xu, S.; Wang, Y.; Li, Z.; Wang, Y.; Zhang, C.; Huang, Z.; Cong, Y. Molecular basis of SARS-Cov-2 omicron variant receptor engagement and antibody evasion and neutralization. bioRxiv 2022. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer bnt162b2 neutralization. Nature 2021, 602, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped sars-cov-2 variant omicron. Emerg. Microbes Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the omicron variant of Sars-Cov-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The omicron variant is highly resistant against anti-body-mediated neutralization–implications for control of the COVID-19 pandemic. Cell 2021, 185, 447–456. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV-2. Nat. Commun. 2020, 12, 2144. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiong, J.; Sun, Z.; Hu, J.; Thilakavathy, K.; Chen, M.; Zhao, Q.; Feng, Y.; Jiang, Q.; Xiong, C. Omicron: A chimera of two early SARS-CoV-2 lineages. Signal Transduct. Target. Ther. 2022, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, M.; Minchella, P.; Tallerico, R.; Talotta, I.; Peronace, C.; Gallelli, L.; Di Mizio, G.; Cione, E. Comparison of Allplex™ 2019-nCoV and TaqPath™ COVID-19 Assays. Reports 2022, 5, 14. [Google Scholar] [CrossRef]
- Korf, I. Gene finding in novel genomes. BMC Bioinform. 2004, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Paden, C.R.; Tao, Y.; Queen, K.; Zhang, J.; Li, Y.; Uehara, A.; Tong, S. Rapid, Sensitive, Full-Genome Sequencing of Severe Acute Respiratory Syndrome Coronavirus. Emerg. Infect. Dis. 2020, 26, 2401–2405. [Google Scholar] [CrossRef]
- Rambaut, A.; Holmes, E.C.; O’Toole, Á.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.J.; Mayer, C.; Poch, O.; Thompson, J.D. Characterization of accessory genes in coronavirus genomes. Virol. J. 2020, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, W.; Narayanan, K.; Huang, C.; Lokugamage, K.; Ikegami, T.; Ito, N.; Kubo, H.; Makino, S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 12885–12890. [Google Scholar] [CrossRef] [Green Version]
- Clark, L.K.; Green, T.J.; Petit, C.M. Structure of Nonstructural Protein 1 from SARS-CoV-2. J. Virol. 2021, 95, e02019-20. [Google Scholar] [CrossRef] [PubMed]
- Wathelet, M.G.; Orr, M.; Frieman, M.B.; Baric, R.S. Severe Acute Respiratory Syndrome Coronavirus Evades Antiviral Signaling: Role of nsp1 and Rational Design of an Attenuated Strain. J. Virol. 2007, 81, 11620–11633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Y.-Q.; Mo, Q.; Wang, J.; Deng, F.; Wang, H.; Ning, Y.-J. SARS-CoV-2 nsp1: Bioinformatics, Potential Structural and Functional Features, and Implications for Drug/Vaccine Designs. Front. Microbiol. 2020, 11, 587317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Balaji, S.; Lomakin, I.B.; Xiong, Y. Coronavirus Nsp1: Immune Response Suppression and Protein Expression Inhibition. Front. Microbiol. 2021, 12, 752214. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Moller, R. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e1039. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Zarate, S.; Eberl, S.; Gladue, D.P.; Novella, I.; Borca, M.V. Positive Selection of ORF1ab, ORF3a, and ORF8 Genes Drives the Early Evolutionary Trends of SARS-CoV-2 during the 2020 COVID-19 Pandemic. Front. Microbiol. 2020, 11, 550674. [Google Scholar] [CrossRef]
- Ghasemi, S.; Harmooshi, N.N.; Rahim, F. Diagnostic utility of antigen detection rapid diagnostic tests for COVID-19: A systematic review and meta-analysis. Diagn. Pathol. 2022, 17, 36. [Google Scholar] [CrossRef]
- Peronace, C.; Tallerico, R.; Colosimo, M.; De Fazio, M.; Pasceri, F.; Talotta, I.; Panduri, G.; Pintomalli, L.; Oteri, R.; Calantoni, V.; et al. BA.1 Omicron Variant of SARS-CoV-2: First Case Reported in Calabria Region, Italy. COVID 2022, 2, 16. [Google Scholar] [CrossRef]
- Bekliz, M.; Adea, K.; Essaidi-Laziosi, M.; Sacks, J.A.; Escadafal, C.; Kaiser, L.; Eckerle, I. SARS-CoV-2 antigen-detecting rapid tests for the delta variant. Lancet Microbe 2021, 3, e90. [Google Scholar] [CrossRef]
- Peronace, C.; Tallerico, R.; Colosimo, M.; Sacco, V.; Talarico, R.; De Fazio, M.; Pasceri, F.; Talotta, I.; Panduri, G.; Kim, J.-H.; et al. Validation of GeneFinder COVID-19 Ag Plus Rapid Test and Its Potential Utility to Slowing Infection Waves: A Single-Center Laboratory Evaluation Study. Diagnostics 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.; Claas, E.C.; Breijer, S.; Vaessen, N. Comparison of the GeneFinderTM COVID-19 Plus RealAmp Kit on the sample-to-result Platform ELITe InGenius to the national reference method: An added value of N gene target detection? J. Clin. Virol. 2020, 132, 104632. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). What You Need to Know about the New Omicron COVID-19 Variant. Available online: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2021/12/what-you-needto-know-about-the-new-omicron-covid-19-variant (accessed on 9 December 2021).
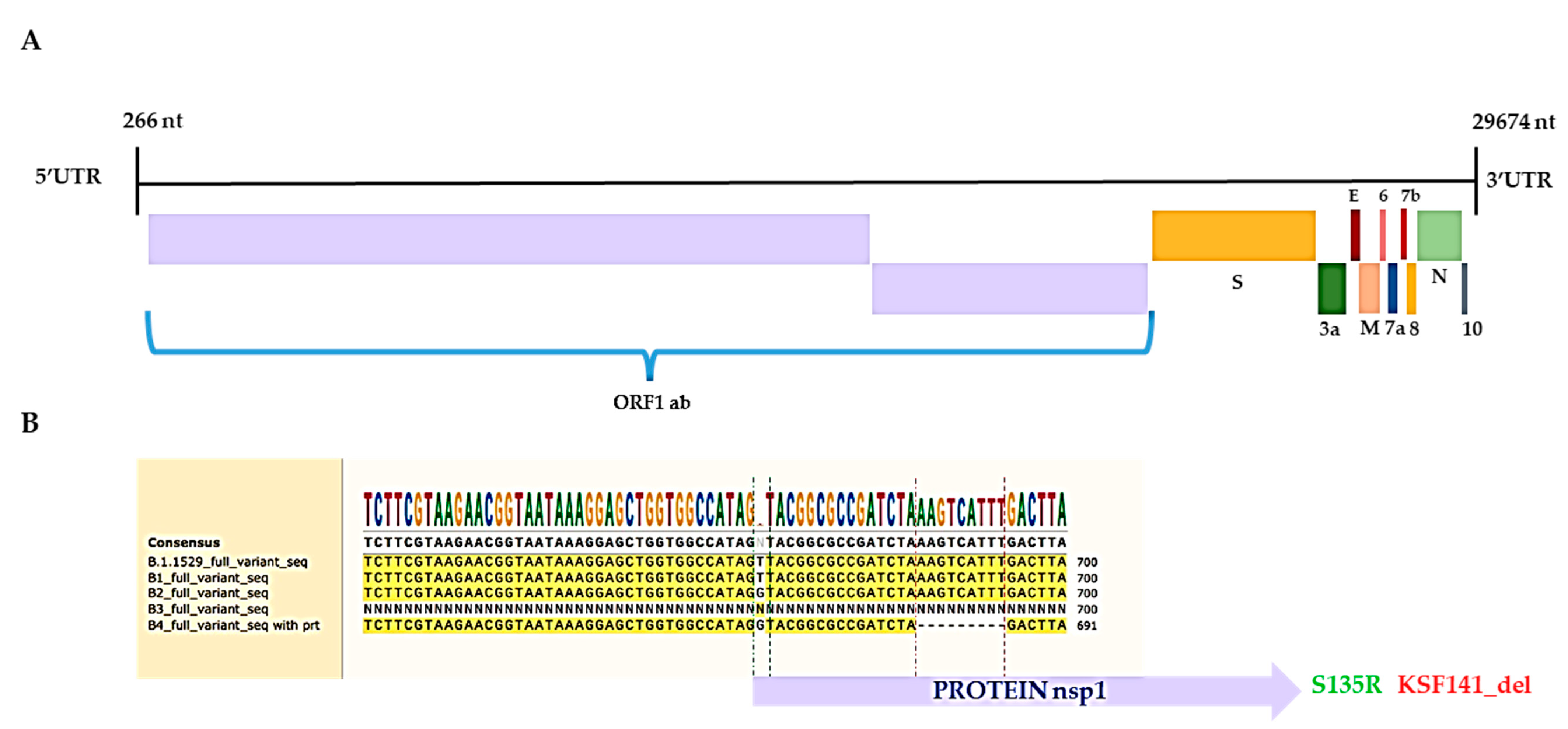

| Protein Mutations | ||||||||
|---|---|---|---|---|---|---|---|---|
| ORF1ab | S | ORF3a | E | M | ORF6 | ORF7b | N | |
| BA.1.1.529 | A555; K38R; F106; SL1265I_del; T492I; P132H; LSGF105F_del; I189V; V57; P323L; N600; | A67V; IHV68I_del; T95I; GVYY142D_del; NL211I_del; D215EPED; R346K; S371L; S373P; S375F; K417N; N440K; G446S; F456; T547K; D614G; H655Y; N679K; P681H; N764K; N856K; Q954H; N969K; D1146 | T64 | D3G; Q19E; A63T; | L18 | P13L; GERS30G_del; R203K; G204R; | ||
| BA.1 | E563D; K38R; F106; SL1265I_del; T492I; P132H; LSGF105F_del; I189V; V57; P323L; N600 | A67V; IHV68I_del; T95I; GVYY142D_del; NL211I_del; D215EPED; S371L; S373P; S375F; K417N; N440K; G446S; T547 | T64 | T9I | D3G; Q19E; A63T | R20 | L18 | P13L; GERS30G_del; R203K; G204R |
| BA.2 | S135R; T24I; F106; G489S; A534; A1526V; L264F; V290; T327I; T492I; D48; R131; P132H; SGF106_del; F251L; R252T; Y253S; I65; S11; P323L; L758; I258; R392C; I42V; T112I; E145 | T19I; LPPA24S_del; G142D; V213G; D405N; R408S; K417N; N440K; S477N; T478K; E484A; Q493R; Q498R; N501Y; Y505H; T547K; D614G; H655Y; N679K; P681H; N764K; D796Y; Q954H; N969K; D1146 | T64; T223I | T9I | Q19E; A63T; F112 | R20; D61L | L18 | P13L; GERS30G_del; R203K; G204R |
| BA.3 | R27C; K38R; F106; L264F; V290; T327I; R131; P132H; SGF106_del; P323L; L758; R392C; I42V; E145 | T19I; LPPA24S_del; G142D; S371F; S373P; S375F; T376A; N440K; F456; D614G; H655Y; N679K; P681H; D796Y; Q954H | T64 | T9I | F112 | D61L | L18 | P13L |
| BA.4 | S135R; KSF141_del; T24I; F106; G489S; A534; L264F; V290; T327I; L417; T492I; D48; R131; P132H; SGF106_del; E23; I65; S11; P323L; L758; R392C; I42V; T112I; E145 | T19I; LPPA24S_del; IHV68I_del; G142D; V213G; S371F; S373P; S375F; T376A; D405N; R408S; K417N; N440K; L452R; T478K; E484A; F486V; Q498R; N501Y; Y505H; D614G; S640F; H655Y; N679K; P681H; N764K; D796Y; Q954H; N969K; D1146 | T64; T223I | T9I | Q19E; A63T; F112 | R20; D61L | L11F; L18 | P13L; GERS30G_del; P151S; R203K; G204R; S413R; S416L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peronace, C.; Tallerico, R.; Colosimo, M.; Fazio, M.D.; Pasceri, F.; Talotta, I.; Panduri, G.; Pintomalli, L.; Oteri, R.; Calantoni, V.; et al. The First Identification in Italy of SARS-CoV-2 Omicron BA.4 Harboring KSF141_del: A Genomic Comparison with Omicron Sub-Variants. Biomedicines 2022, 10, 1839. https://doi.org/10.3390/biomedicines10081839
Peronace C, Tallerico R, Colosimo M, Fazio MD, Pasceri F, Talotta I, Panduri G, Pintomalli L, Oteri R, Calantoni V, et al. The First Identification in Italy of SARS-CoV-2 Omicron BA.4 Harboring KSF141_del: A Genomic Comparison with Omicron Sub-Variants. Biomedicines. 2022; 10(8):1839. https://doi.org/10.3390/biomedicines10081839
Chicago/Turabian StylePeronace, Cinzia, Rossana Tallerico, Manuela Colosimo, Marco De Fazio, Federica Pasceri, Ilenia Talotta, Giuseppina Panduri, Letizia Pintomalli, Rosaria Oteri, Valeria Calantoni, and et al. 2022. "The First Identification in Italy of SARS-CoV-2 Omicron BA.4 Harboring KSF141_del: A Genomic Comparison with Omicron Sub-Variants" Biomedicines 10, no. 8: 1839. https://doi.org/10.3390/biomedicines10081839
APA StylePeronace, C., Tallerico, R., Colosimo, M., Fazio, M. D., Pasceri, F., Talotta, I., Panduri, G., Pintomalli, L., Oteri, R., Calantoni, V., Fiorillo, M. T., Caroleo, M. C., Curcio, R., Dolce, V., Cione, E., & Minchella, P. (2022). The First Identification in Italy of SARS-CoV-2 Omicron BA.4 Harboring KSF141_del: A Genomic Comparison with Omicron Sub-Variants. Biomedicines, 10(8), 1839. https://doi.org/10.3390/biomedicines10081839







