IgG N-glycosylation from Patients with Pemphigus Treated with Rituximab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. IgG Purification with ÄKTA-Start
2.3. N-glycome Analysis of IgG in Patients
2.4. Keratinocyte Dissociation Assay
2.5. Statistical Analysis
3. Results
3.1. Population of Patients
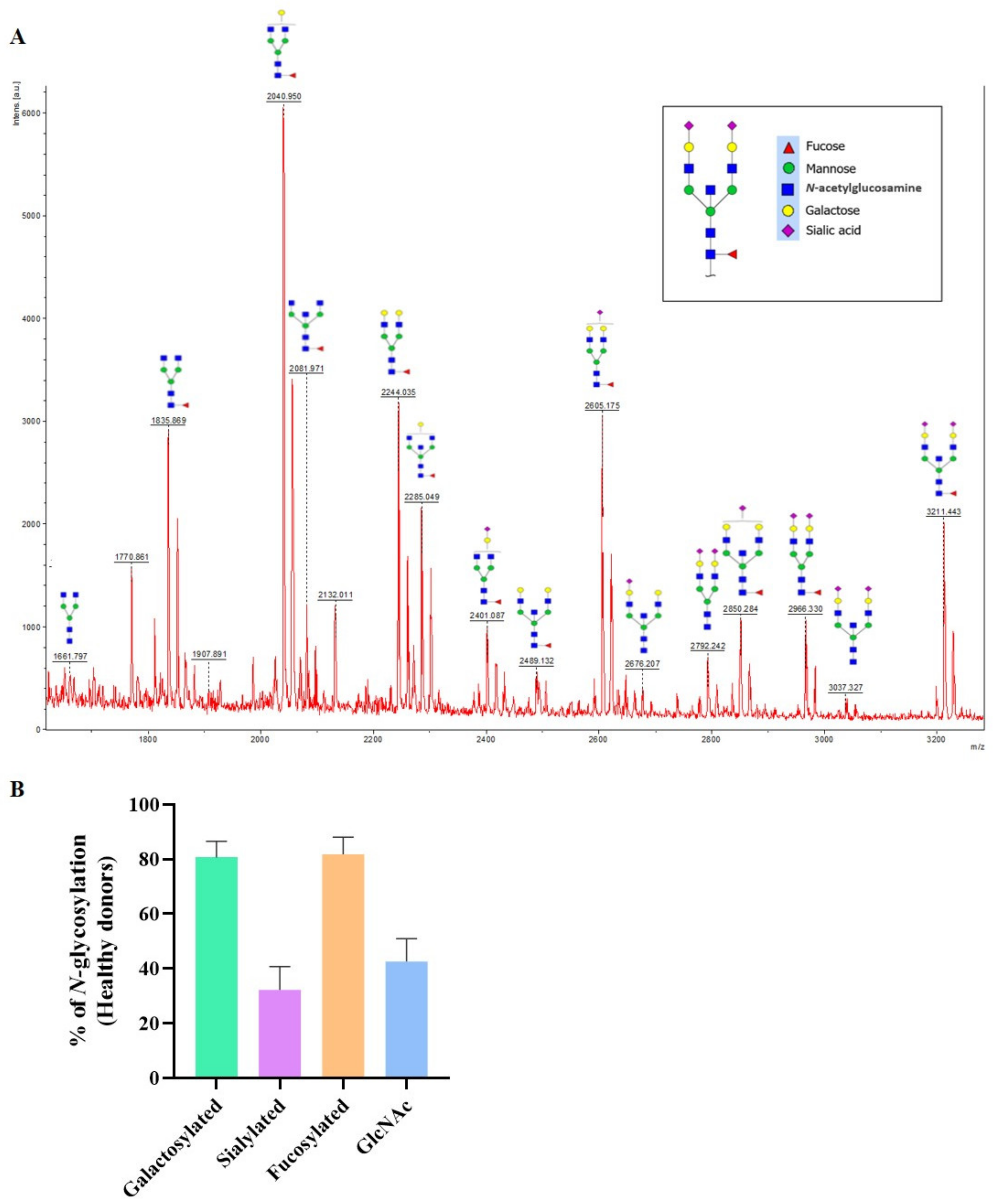
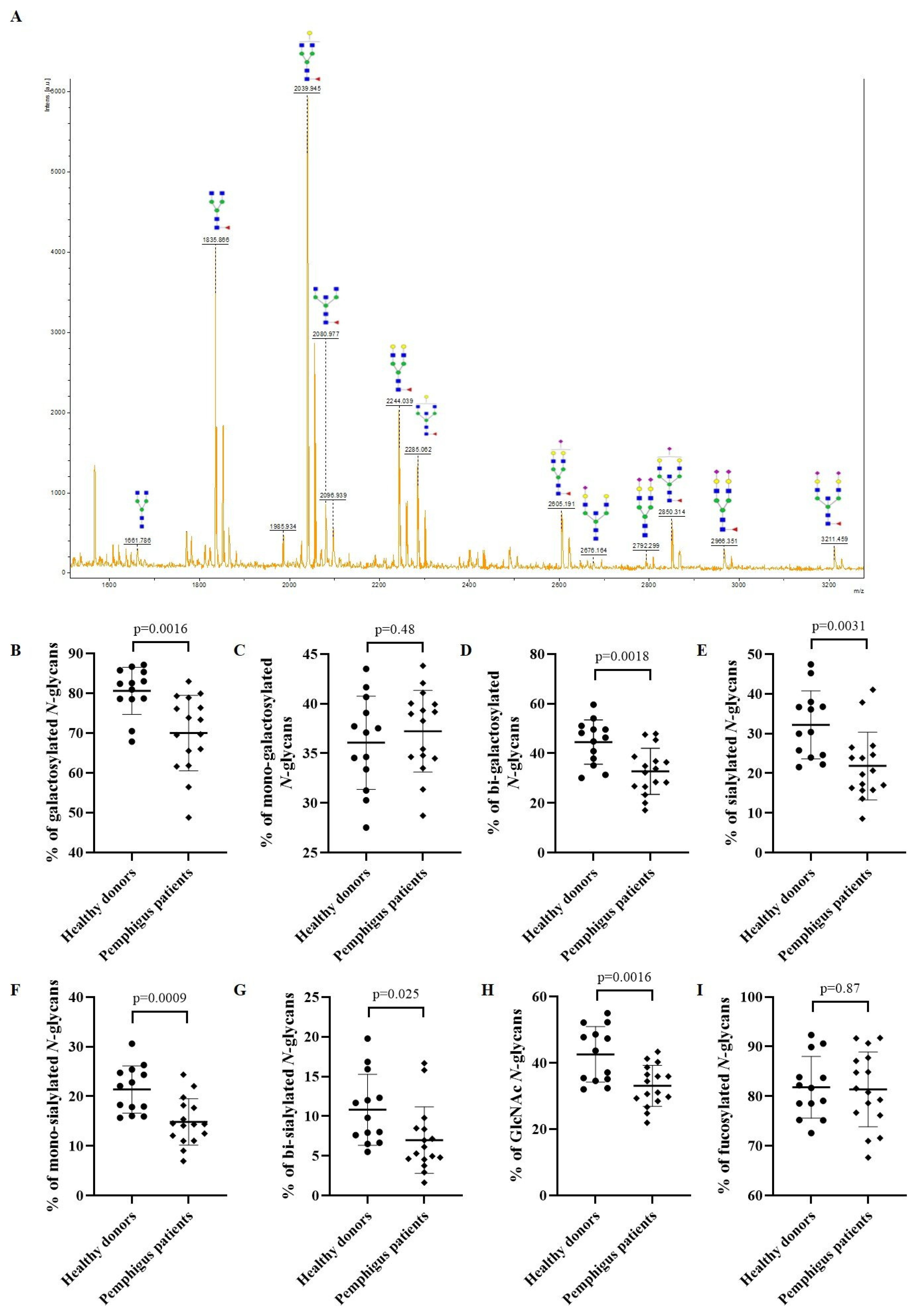
3.2. N-glycan Profile of Pemphigus Patients at Baseline Compared to Healthy Donors
3.3. Evolution of the IgG N-glycome of Pemphigus Patients under Treatment
3.3.1. Evolution of the IgG N-glycome under Treatment in the Whole Population of Patients
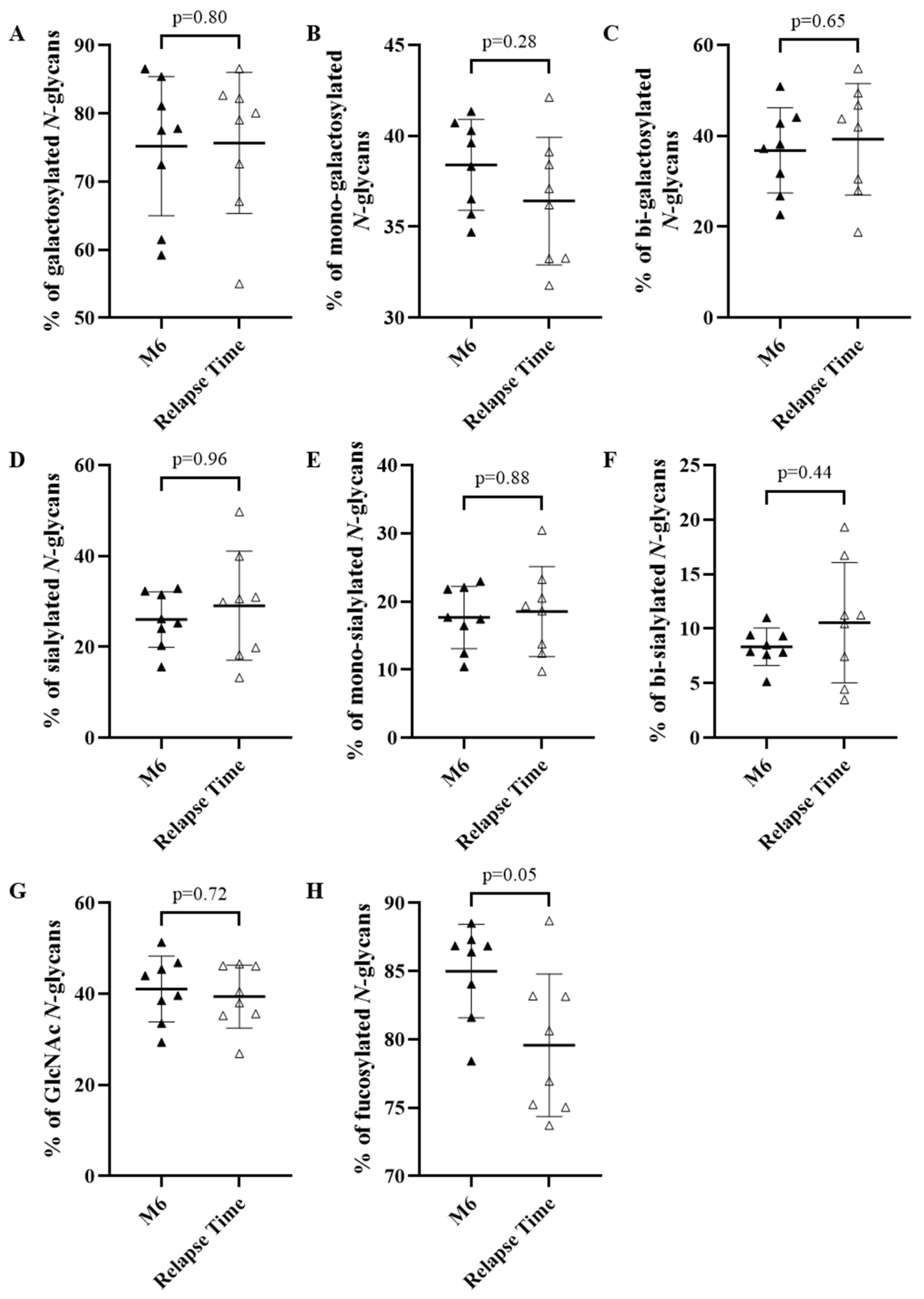
3.3.2. Evolution of the IgG N-glycome Depending on Response to Treatment
3.4. Pathogenic Activity of IgG of Relapsing and Non-Relapsing Pemphigus Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, X.; Aoki, V.; Mascaro, J.M.; Lopez-Swiderski, A.; Diaz, L.A.; Fairley, J.A. Mucosal and Mucocutaneous (Generalized) Pemphigus Vulgaris Show Distinct Autoantibody Profiles. J. Investig. Dermatol. 1997, 109, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet Lond. Engl. 2019, 394, 882–894. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Murrell, D.F. Pemphigus Vulgaris: An Evidence-Based Treatment Update. Drugs 2015, 75, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Maho-Vaillant, M.; Prost-Squarcioni, C.; Hebert, V.; Houivet, E.; Calbo, S.; Caillot, F.; Golinski, M.L.; Labeille, B.; Picard-Dahan, C.; et al. First-Line Rituximab Combined with Short-Term Prednisone versus Prednisone Alone for the Treatment of Pemphigus (Ritux 3): A Prospective, Multicentre, Parallel-Group, Open-Label Randomised Trial. Lancet Lond. Engl. 2017, 389, 2031–2040. [Google Scholar] [CrossRef]
- Mignard, C.; Maho-Vaillant, M.; Golinski, M.-L.; Balayé, P.; Prost-Squarcioni, C.; Houivet, E.; Calbo, S.B.; Labeille, B.; Picard-Dahan, C.; Konstantinou, M.P.; et al. Factors Associated With Short-Term Relapse in Patients With Pemphigus Who Receive Rituximab as First-Line Therapy: A Post Hoc Analysis of a Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 545–552. [Google Scholar] [CrossRef]
- Passot, C.; Azzopardi, N.; Renault, S.; Baroukh, N.; Arnoult, C.; Ohresser, M.; Boisdron-Celle, M.; Gamelin, E.; Watier, H.; Paintaud, G.; et al. Influence of FCGRT Gene Polymorphisms on Pharmacokinetics of Therapeutic Antibodies. mAbs 2013, 5, 614–619. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Bas, M.; Terrier, A.; Jacque, E.; Dehenne, A.; Pochet-Béghin, V.; Beghin, C.; Dezetter, A.-S.; Dupont, G.; Engrand, A.; Beaufils, B.; et al. Fc Sialylation Prolongs Serum Half-Life of Therapeutic Antibodies. J. Immunol. 2019, 202, 1582–1594. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Alter, G. The Immunoregulatory Roles of Antibody Glycosylation. Trends Immunol. 2017, 38, 358–372. [Google Scholar] [CrossRef]
- van de Bovenkamp, F.S.; Hafkenscheid, L.; Rispens, T.; Rombouts, Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J. Immunol. 2016, 196, 1435–1441. [Google Scholar] [CrossRef]
- Cobb, B.A. The History of IgG Glycosylation and Where We Are Now. Glycobiology 2020, 30, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T. IgG Fc Glycosylation in Human Immunity. Curr. Top. Microbiol. Immunol. 2019, 423, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Dubé, R.; Rook, G.A.; Steele, J.; Brealey, R.; Dwek, R.; Rademacher, T.; Lennard-Jones, J. Agalactosyl IgG in Inflammatory Bowel Disease: Correlation with C-Reactive Protein. Gut 1990, 31, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Parekh, R.B.; Roitt, I.M.; Isenberg, D.A.; Dwek, R.A.; Ansell, B.M.; Rademacher, T.W. Galactosylation of IgG Associated Oligosaccharides: Reduction in Patients with Adult and Juvenile Onset Rheumatoid Arthritis and Relation to Disease Activity. Lancet Lond. Engl. 1988, 1, 966–969. [Google Scholar] [CrossRef]
- Espy, C.; Morelle, W.; Kavian, N.; Grange, P.; Goulvestre, C.; Viallon, V.; Chéreau, C.; Pagnoux, C.; Michalski, J.-C.; Guillevin, L.; et al. Sialylation Levels of Anti-Proteinase 3 Antibodies Are Associated with the Activity of Granulomatosis with Polyangiitis (Wegener’s). Arthritis Rheum. 2011, 63, 2105–2115. [Google Scholar] [CrossRef]
- Kemna, M.J.; Plomp, R.; van Paassen, P.; Koeleman, C.A.M.; Jansen, B.C.; Damoiseaux, J.G.M.C.; Cohen Tervaert, J.W.; Wuhrer, M. Galactosylation and Sialylation Levels of IgG Predict Relapse in Patients With PR3-ANCA Associated Vasculitis. eBioMedicine 2017, 17, 108–118. [Google Scholar] [CrossRef]
- Vučković, F.; Krištić, J.; Gudelj, I.; Teruel, M.; Keser, T.; Pezer, M.; Pučić-Baković, M.; Štambuk, J.; Trbojević-Akmačić, I.; Barrios, C.; et al. Association of Systemic Lupus Erythematosus with Decreased Immunosuppressive Potential of the IgG Glycome. Arthritis Rheumatol. 2015, 67, 2978–2989. [Google Scholar] [CrossRef]
- Albert, H.; Collin, M.; Dudziak, D.; Ravetch, J.V.; Nimmerjahn, F. In Vivo Enzymatic Modulation of IgG Glycosylation Inhibits Autoimmune Disease in an IgG Subclass-Dependent Manner. Proc. Natl. Acad. Sci. USA 2008, 105, 15005–15009. [Google Scholar] [CrossRef]
- Wada, R.; Matsui, M.; Kawasaki, N. Influence of N-Glycosylation on Effector Functions and Thermal Stability of Glycoengineered IgG1 Monoclonal Antibody with Homogeneous Glycoforms. mAbs 2019, 11, 350–372. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Schwab, I.; Biburger, M.; Krönke, G.; Schett, G.; Nimmerjahn, F. IVIg-Mediated Amelioration of ITP in Mice Is Dependent on Sialic Acid and SIGNR1. Eur. J. Immunol. 2012, 42, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Schwab, I.; Mihai, S.; Seeling, M.; Kasperkiewicz, M.; Ludwig, R.J.; Nimmerjahn, F. Broad Requirement for Terminal Sialic Acid Residues and FcγRIIB for the Preventive and Therapeutic Activity of Intravenous Immunoglobulins in Vivo. Eur. J. Immunol. 2014, 44, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Caillot, F.; Derambure, C.; Berkani, N.; Riou, G.; Maho-Vaillant, M.; Calbo, S.; Joly, P.; Musette, P. Long-Term Increase of Kcnn4 Potassium Channel Surface Expression on B Cells in Pemphigus Patients after Rituximab Treatment. J. Investig. Dermatol. 2018, 138, 2666–2668. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.; Walet-Balieu, M.-L.; Schapman, D.; Golinski, M.-L.; Burel, C.; Barray, M.; Drouot, L.; Maho-Vaillant, M.; Hébert, V.; Boyer, O.; et al. Longitudinal Pathogenic Properties and N-Glycosylation Profile of Antibodies from Patients with Pemphigus after Corticosteroid Treatment. Biomedicines 2021, 9, 1411. [Google Scholar] [CrossRef]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lütteke, T.; O’Boyle, N.; Packer, N.H.; Stanley, P.; Toukach, P.; et al. Updates to the Symbol Nomenclature for Glycans Guidelines. Glycobiology 2019, 29, 620–624. [Google Scholar] [CrossRef]
- Ishii, K.; Harada, R.; Matsuo, I.; Shirakata, Y.; Hashimoto, K.; Amagai, M. In Vitro Keratinocyte Dissociation Assay for Evaluation of the Pathogenicity of Anti-Desmoglein 3 IgG Autoantibodies in Pemphigus Vulgaris. J. Investig. Dermatol. 2005, 124, 939–946. [Google Scholar] [CrossRef]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The Impact of Glycosylation on the Biological Function and Structure of Human Immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef]
- Wuhrer, M.; Stam, J.C.; van de Geijn, F.E.; Koeleman, C.A.M.; Verrips, C.T.; Dolhain, R.J.E.M.; Hokke, C.H.; Deelder, A.M. Glycosylation Profiling of Immunoglobulin G (IgG) Subclasses from Human Serum. Proteomics 2007, 7, 4070–4081. [Google Scholar] [CrossRef]
- Seeling, M.; Brückner, C.; Nimmerjahn, F. Differential Antibody Glycosylation in Autoimmunity: Sweet Biomarker or Modulator of Disease Activity? Nat. Rev. Rheumatol. 2017, 13, 621–630. [Google Scholar] [CrossRef]
- Biermann, M.H.C.; Griffante, G.; Podolska, M.J.; Boeltz, S.; Stürmer, J.; Muñoz, L.E.; Bilyy, R.; Herrmann, M. Sweet but Dangerous—The Role of Immunoglobulin G Glycosylation in Autoimmunity and Inflammation. Lupus 2016, 25, 934–942. [Google Scholar] [CrossRef]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G Glycosylation in Aging and Diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, Y.; Ewing, E.; van de Stadt, L.A.; Selman, M.H.J.; Trouw, L.A.; Deelder, A.M.; Huizinga, T.W.J.; Wuhrer, M.; van Schaardenburg, D.; Toes, R.E.M.; et al. Anti-Citrullinated Protein Antibodies Acquire a pro-Inflammatory Fc Glycosylation Phenotype Prior to the Onset of Rheumatoid Arthritis. Ann. Rheum. Dis. 2015, 74, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, C.; Grau, S.; Jäger, C.; Sondermann, P.; Brünker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique Carbohydrate-Carbohydrate Interactions Are Required for High Affinity Binding between FcgammaRIII and Antibodies Lacking Core Fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef]
- Scanlan, C.N.; Burton, D.R.; Dwek, R.A. Making Autoantibodies Safe. Proc. Natl. Acad. Sci. USA 2008, 105, 4081–4082. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of Fucose on Human IgG1 N-Linked Oligosaccharide Improves Binding to Human Fcgamma RIII and Antibody-Dependent Cellular Toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The Absence of Fucose but Not the Presence of Galactose or Bisecting N-Acetylglucosamine of Human IgG1 Complex-Type Oligosaccharides Shows the Critical Role of Enhancing Antibody-Dependent Cellular Cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar] [CrossRef]
- Gińdzieńska-Sieśkiewicz, E.; Radziejewska, I.; Domysławska, I.; Klimiuk, P.A.; Sulik, A.; Rojewska, J.; Gabryel-Porowska, H.; Sierakowski, S. Changes of Glycosylation of IgG in Rheumatoid Arthritis Patients Treated with Methotrexate. Adv. Med. Sci. 2016, 61, 193–197. [Google Scholar] [CrossRef]
- Maho-Vaillant, M.; Perals, C.; Golinski, M.-L.; Hébert, V.; Caillot, F.; Mignard, C.; Riou, G.; Petit, M.; Viguier, M.; Hertl, M.; et al. Rituximab and Corticosteroid Effect on Desmoglein-Specific B Cells and Desmoglein-Specific T Follicular Helper Cells in Pemphigus. J. Investig. Dermatol. 2021, 141, 2132–2140. [Google Scholar] [CrossRef]
- Schmidt, D.E.; de Haan, N.; Sonneveld, M.E.; Porcelijn, L.; van der Schoot, C.E.; de Haas, M.; Zwaginga, J.-J.; Wuhrer, M.; Vidarsson, G. IgG-Fc Glycosylation before and after Rituximab Treatment in Immune Thrombocytopenia. Sci. Rep. 2020, 10, 3051. [Google Scholar] [CrossRef]
- Van Beneden, K.; Coppieters, K.; Laroy, W.; De Keyser, F.; Hoffman, I.E.; Van den Bosch, F.; Vander Cruyssen, B.; Drennan, M.; Jacques, P.; Rottiers, P.; et al. Reversible Changes in Serum Immunoglobulin Galactosylation during the Immune Response and Treatment of Inflammatory Autoimmune Arthritis. Ann. Rheum. Dis. 2009, 68, 1360–1365. [Google Scholar] [CrossRef]
- Keser, T.; Vučković, F.; Barrios, C.; Zierer, J.; Wahl, A.; Akinkuolie, A.O.; Štambuk, J.; Nakić, N.; Pavić, T.; Periša, J.; et al. Effects of Statins on the Immunoglobulin G Glycome. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Xie, Y.; Li, Q.; Artegoitia, V.M.; Lebrilla, C.B.; Keim, N.L.; Adams, S.H.; Krishnan, S. Diet Affects Glycosylation of Serum Proteins in Women at Risk for Cardiometabolic Disease. Eur. J. Nutr. 2021, 60, 3727–3741. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Rahmöller, J.; Mertes, M.M.M.; Eiglmeier, S.; Lorenz, F.K.M.; Stoehr, A.D.; Braumann, D.; Lorenz, A.K.; Winkler, A.; Lilienthal, G.-M.; et al. Sialylated Autoantigen-Reactive IgG Antibodies Attenuate Disease Development in Autoimmune Mouse Models of Lupus Nephritis and Rheumatoid Arthritis. Front. Immunol. 2018, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Pagan, J.D.; Kitaoka, M.; Anthony, R.M. Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease. Cell 2018, 172, 564–577. [Google Scholar] [CrossRef]
- Pincetic, A.; Bournazos, S.; DiLillo, D.J.; Maamary, J.; Wang, T.T.; Dahan, R.; Fiebiger, B.-M.; Ravetch, J.V. Type I and Type II Fc Receptors Regulate Innate and Adaptive Immunity. Nat. Immunol. 2014, 15, 707–716. [Google Scholar] [CrossRef]
- Hébert, V.; Boulard, C.; Houivet, E.; Duvert Lehembre, S.; Borradori, L.; Della Torre, R.; Feliciani, C.; Fania, L.; Zambruno, G.; Camaioni, D.B.; et al. Large International Validation of ABSIS and PDAI Pemphigus Severity Scores. J. Investig. Dermatol. 2019, 139, 31–37. [Google Scholar] [CrossRef]
- Coloma, M.J.; Trinh, R.K.; Martinez, A.R.; Morrison, S.L. Position Effects of Variable Region Carbohydrate on the Affinity and in Vivo Behavior of an Anti-(1-->6) Dextran Antibody. J. Immunol. 1999, 162, 2162–2170. [Google Scholar]
- Knezević, A.; Polasek, O.; Gornik, O.; Rudan, I.; Campbell, H.; Hayward, C.; Wright, A.; Kolcic, I.; O’Donoghue, N.; Bones, J.; et al. Variability, Heritability and Environmental Determinants of Human Plasma N-Glycome. J. Proteome Res. 2009, 8, 694–701. [Google Scholar] [CrossRef]
- Hennig, R.; Cajic, S.; Borowiak, M.; Hoffmann, M.; Kottler, R.; Reichl, U.; Rapp, E. Towards Personalized Diagnostics via Longitudinal Study of the Human Plasma N-Glycome. Biochim. Biophys. Acta 2016, 1860, 1728–1738. [Google Scholar] [CrossRef]
- Shikata, K.; Yasuda, T.; Takeuchi, F.; Konishi, T.; Nakata, M.; Mizuochi, T. Structural Changes in the Oligosaccharide Moiety of Human IgG with Aging. Glycoconj. J. 1998, 15, 683–689. [Google Scholar] [CrossRef]
- Parekh, R.; Roitt, I.; Isenberg, D.; Dwek, R.; Rademacher, T. Age-Related Galactosylation of the N-Linked Oligosaccharides of Human Serum IgG. J. Exp. Med. 1988, 167, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Krištić, J.; Vučković, F.; Menni, C.; Klarić, L.; Keser, T.; Beceheli, I.; Pučić-Baković, M.; Novokmet, M.; Mangino, M.; Thaqi, K.; et al. Glycans Are a Novel Biomarker of Chronological and Biological Ages. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, V.; Desmyter, L.; Liu, X.-E.; Cardelli, M.; Franceschi, C.; Federico, A.; Libert, C.; Laroy, W.; Dewaele, S.; Contreras, R.; et al. N-Glycomic Changes in Serum Proteins during Human Aging. Rejuvenation Res. 2007, 10, 521–531. [Google Scholar] [CrossRef] [PubMed]


| Relapse (n = 16) | |||
|---|---|---|---|
| Characteristic | Yes | No | p-Value |
| Number | 8 | 8 | NA |
| Age, mean (SEM) | 47.0 (4.7) | 54.8 (5.8) | 0.32 |
| Gender, n (%) | |||
| Male | 3 (37.5) | 1 (12.5) | 0.57 |
| Female | 5 (62.5) | 7 (87.5) | |
| Type of pemphigus, n (%) | |||
| Vulgaris | 6 (75) | 8 (100) | 0.47 |
| Foliaceus | 2 (25) | 0 (0) | |
| PDAI score, mean (SEM) | 54.2 (14.0) | 37.4 (29.3) | 0.35 |
| B cell frequency, mean (SEM), % | |||
| Baseline | 19.5 (4.5) | 9.1 (0.5) | 0.10 |
| Month 6 | 0.9 (0.5) | 0.1 (0.0) | 0.20 |
| Month 12 | NA | 4.5 (1.5) | NA |
| Anti-DSG1 auto-Ab level, mean (SEM), IU/mL | |||
| Baseline | 568.6 (298.0) | 176.0 (70.1) | 0.22 |
| Month 6 | 9.0 (6.1) | 1.0 (0.0) | 0.21 |
| Month 12 | NA | 1.0 (0.0) | NA |
| Anti-DSG3 auto-Ab level, mean (SEM), IU/mL | |||
| Baseline | 1615.3 (559.6) | 815.0 (181.2) | 0.19 |
| Month 6 | 247.3 (180.4) | 6.6 (1.8) | 0.20 |
| Month 12 | NA | 6.6 (1.9) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Font, G.; Walet-Balieu, M.-L.; Petit, M.; Burel, C.; Maho-Vaillant, M.; Hébert, V.; Chan, P.; Fréret, M.; Boyer, O.; Joly, P.; et al. IgG N-glycosylation from Patients with Pemphigus Treated with Rituximab. Biomedicines 2022, 10, 1774. https://doi.org/10.3390/biomedicines10081774
Font G, Walet-Balieu M-L, Petit M, Burel C, Maho-Vaillant M, Hébert V, Chan P, Fréret M, Boyer O, Joly P, et al. IgG N-glycosylation from Patients with Pemphigus Treated with Rituximab. Biomedicines. 2022; 10(8):1774. https://doi.org/10.3390/biomedicines10081774
Chicago/Turabian StyleFont, Guillaume, Marie-Laure Walet-Balieu, Marie Petit, Carole Burel, Maud Maho-Vaillant, Vivien Hébert, Philippe Chan, Manuel Fréret, Olivier Boyer, Pascal Joly, and et al. 2022. "IgG N-glycosylation from Patients with Pemphigus Treated with Rituximab" Biomedicines 10, no. 8: 1774. https://doi.org/10.3390/biomedicines10081774
APA StyleFont, G., Walet-Balieu, M.-L., Petit, M., Burel, C., Maho-Vaillant, M., Hébert, V., Chan, P., Fréret, M., Boyer, O., Joly, P., Calbo, S., Bardor, M., & Golinski, M.-L. (2022). IgG N-glycosylation from Patients with Pemphigus Treated with Rituximab. Biomedicines, 10(8), 1774. https://doi.org/10.3390/biomedicines10081774






