Conventional and Resin-Modified Glass Ionomer Cement Surface Characteristics after Acidic Challenges
Abstract
1. Introduction
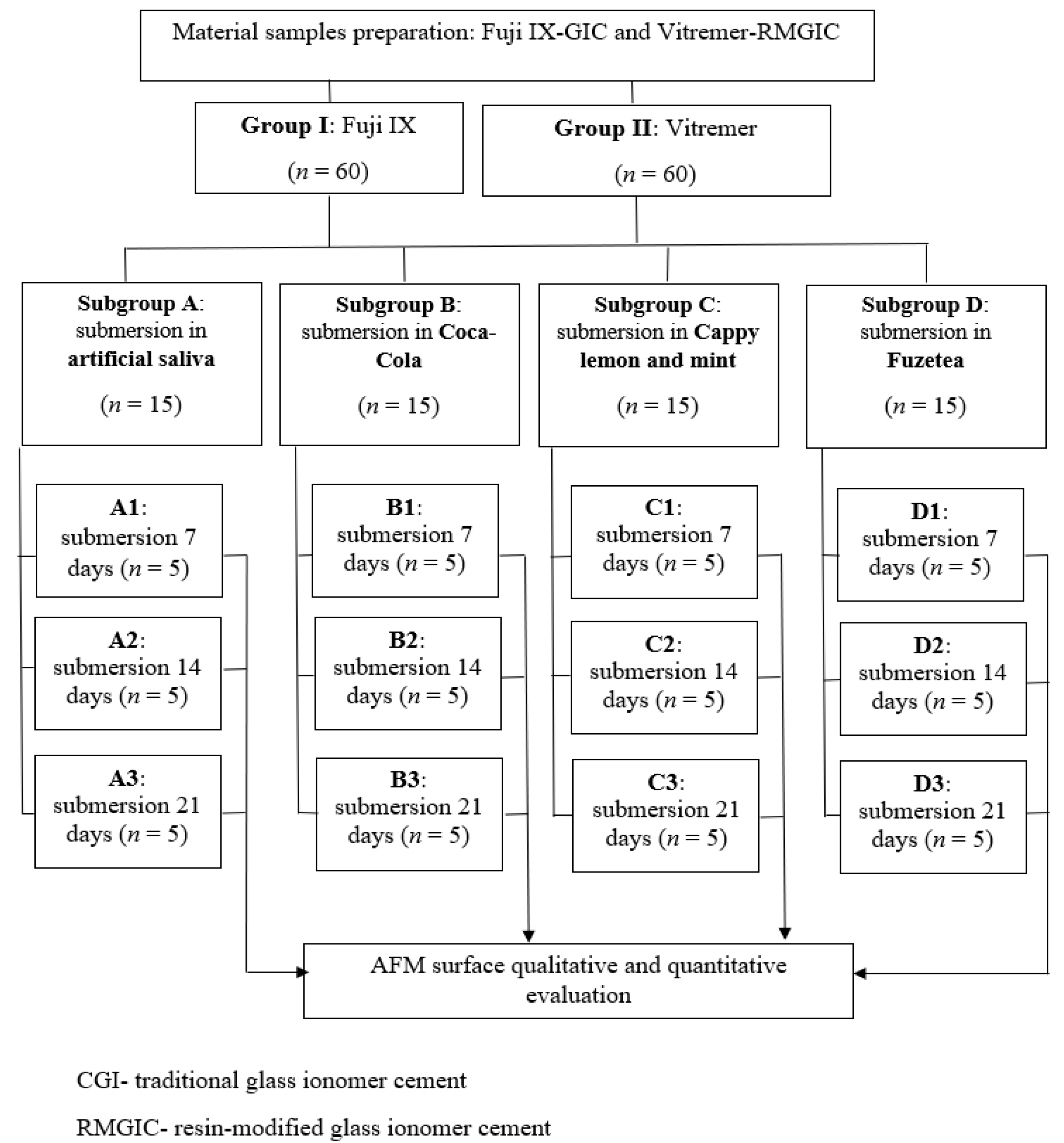
2. Materials and Methods
2.1. Material Specimen Preparation
2.2. Immersion Protocol in Artificial Saliva and Acidic Drinks
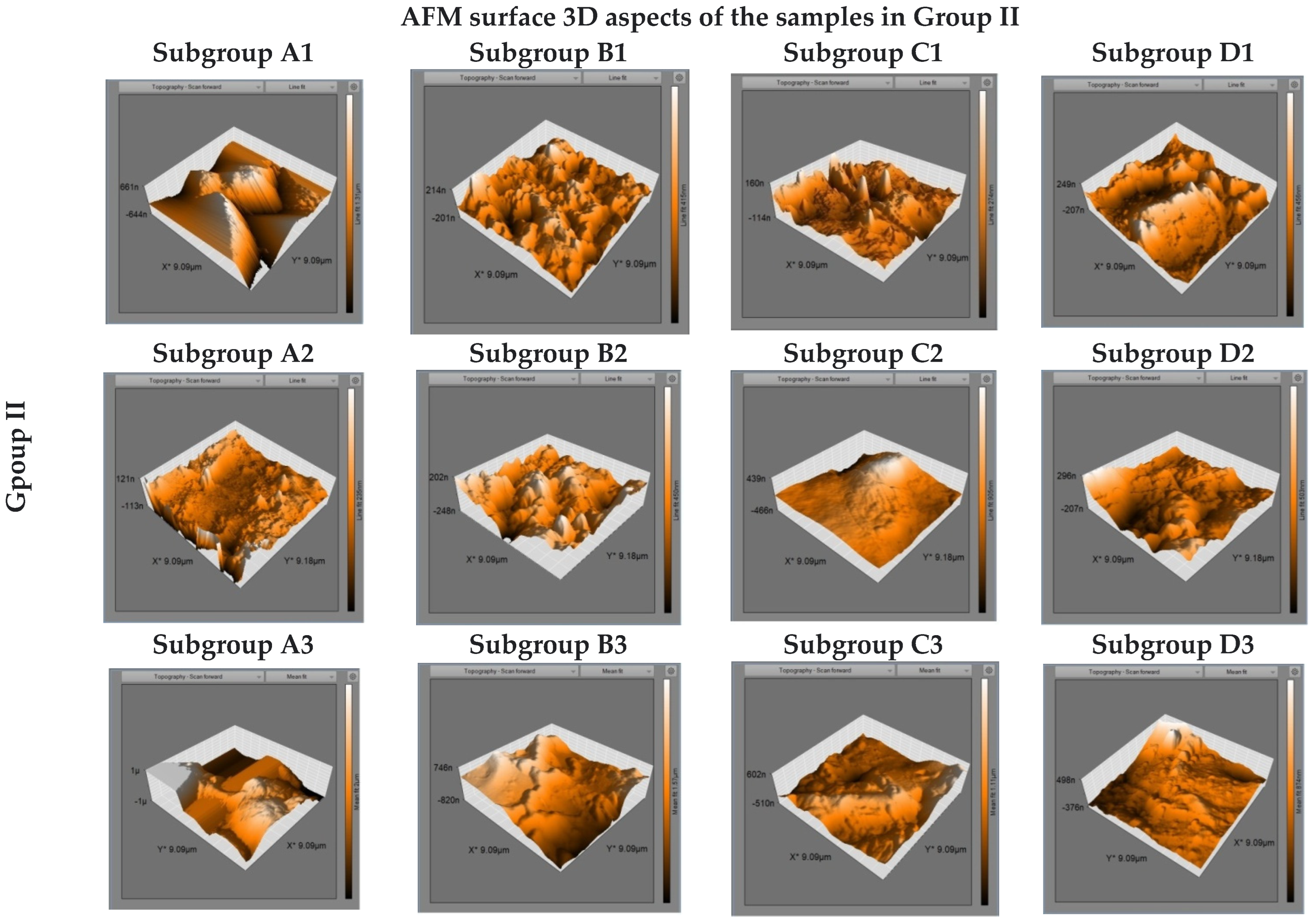
2.3. Surface Characteristics Evaluation
2.4. Statistical Analyses of the Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanzow, P.; Wegehaupt, F.J.; Attin, T.; Wiegand, A. Etiology and pathogenesis of dental erosion. Quintessence Int. 2016, 47, 275–278. [Google Scholar] [PubMed]
- Wongkhantee, S.; Patanapiradej, V.; Maneenut, C.; Tantbirojn, D. Effect of acidic food and drinks on surface hardness of enamel, dentine and tooth-coloured filling materials. J. Dent. 2006, 34, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W.; Millar, B.J.; Czarnecka, B.; Limanowska-Shaw, H. Storage of polyacid-modified resin composites (“compomers”) in lactic acid solution. Dent. Mater. 1999, 15, 413–416. [Google Scholar] [CrossRef]
- Bang, S.Y.; Kim, E.J.; Kim, H.J.; Nam, S.H.; Kim, Y.J. The effect of acidic drinks on the surface of tooth-colored restorative materials. J. Korean Acad. Pediatr. Dent. 2006, 33, 469–481. [Google Scholar]
- Pedrini, D.; Candido, M.S.M.; Rodrigues, A.L. Analysis of surface roughness of glass-ionomer cements and compomer. J. Oral Rehabil. 2003, 30, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Yadav, R.D.; Raisingan, D.; Jindal, D.; Mathur, R. A Comparative Analysis of Different Finishing and Polishing Devices on Nanofilled, Microfilled, and HybridComposite: A Scanning Electron Microscopy and Profilometric Study. Int. J. Clin. Pediatr. Dent. 2016, 9, 201–208. [Google Scholar] [CrossRef]
- Reis, A.F.; Giannini, M.; Lovadino, J.R.; dos Santos Dias, C.T. The effect of six polishing systems on the surface roughness of two packable resin-based composites. Am. J. Dent. 2002, 15, 193–197. [Google Scholar]
- Bajwa, N.K.; Pathak, A. Change in surface roughness of esthetic restorative materials after exposure to different immersion regimes in a cola drink. ISRN Dent. 2014, 2014, 353926. [Google Scholar] [CrossRef]
- Wilson, A.; Kent, B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef]
- Smith, D.C. DentalCements. Adv. Dent. Res. 1988, 2, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Silva, R.; Cabral, R.N.; Pascotto, R.C.; Borges, A.F.S.; Martins, C.C.; Navarro, M.F.L.; Sidhu, S.K.; Leal, S.C. Mechanical and optical properties of conventional restorative glass-ionomer cements—a systematic review. J. Appl. Oral Sci. 2019, 27, e2018357. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G.; Mulyani Zilm, P.S.; Gully, N.J. An in vitro investigation of marginal dentine caries abutting composite resin and glassionomer cement restorations. Aust. Dent. J. 2007, 52, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Tantbirojn, D.; Rusin, R.P.; Bui, H.T.; Mitra, S.B. Inhibition of dentin demineralization adjacent to a glass-ionomer/composite sandwich restoration. Quintessence Int. 2009, 40, 287–294. [Google Scholar] [PubMed]
- GC, Fuji IX: Technical Product Profile. Available online: https://www.gcamerica.com/products/operatory/GC_Fuji_IX_GP/FujiIX_GP_Brochure.pdf (accessed on 25 June 2022).
- 3M ESPE DentalProductsLaboratory, VitremerTri-Cure Glass IonomerSystem: TechnicalProductProfile, USA. 2012. Available online: https://multimedia.3m.com/mws/media/44495O/vitremertm-core-buildup-restorative.pdf (accessed on 25 June 2022).
- Brett, C.M.A.; Trandafir, F. Thecorrosion of dental amalgam in artificial salivas:an electrochemical impedance study. J. Electroanal. Chem. 2004, 572, 347–354. [Google Scholar] [CrossRef]
- Özenen, D.O.; Sungurtekin Ekci, E.; Özenen, G.; Karatas, M.O. Effect of Common Daily Acidic Beverages on the Surface Roughness. Glass Phys. Chem. 2019, 45, 496–502. [Google Scholar] [CrossRef]
- Miličević, A.; Goršeta, K.; van Duinen, R.N.; Glavina, D. SurfaceRoughness of Glass Ionomer Cements afterApplication of Different PolishingTechniques. ActaStomatol. Croat. 2018, 52, 314–321. [Google Scholar] [CrossRef]
- Tărăboanță, I.; Stoleriu, S.; Nica, I.; Georgescu, A.; Gamen, A.C.; Maftei, G.A.; Andrian, S. Roughness variation of a nanohybrid composite resin submitted to acid andabrasive challenges. IJMD 2020, 24, 182–187. [Google Scholar]
- Qutub, O. In Vitro Comparison of Sports Drinks’ Effect on the Hardness of Different Temporary Restorative Materials. Int. J. Pharm. Res. Allied Sci. 2018, 7, 7–12. [Google Scholar]
- Abdelmegid, F.; Salama, F.S.; Al-Jameel, M.M.; Al-Rasheed, T.T.; El-Sharawy, M.A. Effects of fruit drinks on surface roughness of two esthetic restorative materials. Stomatologija. 2019, 21, 47–52. [Google Scholar]
- de Luca Cunha, C.M.B.; Wambier, L.M.; Dias, G.F.; Reis, A.; Alves, F.B.T.; Rodrigues Chibinski, A.C.; Stadler Wambier, D. In Vitro Evaluation of the Impact of Erosive/Abrasive Challenge in Glass Ionomer Cements. Biomed. J. Sci. Technol. Res. 2017, 1, 1263–1266. [Google Scholar]
- Poggio, C.; Viola, M.; Mirando, M.; Chiesa, M.; Beltrami, R.; Colombo, M. Microhardness of different esthetic restorative materials: Evaluation and comparison after exposure to acidic drink. Dent. Res. J. 2018, 15, 166–172. [Google Scholar] [CrossRef]
- Perera, D.; Yu, S.C.; Zeng, H.; Meyers, I.A.; Walsh, L.J. Acid Resistance of Glass Ionomer Cement RestorativeMaterials. Bioengineering 2020, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Tărăboanță, I.; Stoleriu, S.; Gurlui, S.; Nica, I.; Tărăboanță-Gamen, A.C.; Iovan, A.; Andrian, S. The Influence of Abrasiveand Acidic Aggressions on the Surface Condition of Flowable Composite Resin. Materials 2022, 15, 1000. [Google Scholar] [CrossRef] [PubMed]
- Čulina, M.Z.; Rajić, V.B.; Šalinović, I.; Klarić, E.; Marković, L.; Ivanišević, A. Influence of pH Cycling on ErosiveWearand Color Stability of High-Viscosity Glass Ionomer Cements. Materials 2022, 15, 923. [Google Scholar] [CrossRef]
- Aliping-McKenzie, M.; Linden, R.W.A.; Nicholson, J.W. The physical properties of conventional and resin-modified glass-ionomer dental cements stored in saliva, proprietary acidic beverages, saline and water. Biomaterials 2003, 24, 4063–4069. [Google Scholar] [CrossRef]
- Almuhaiza, M. Glass-Ionomer Cements in Restorative Dentristry: A Critical Appraisal. J. Contemp. Dent. Pract. 2016, 17, 331–336. [Google Scholar] [CrossRef]
- Balan, A.; Savin, C.; Sandu, A.; Stoleriu, S. Study Regarding the Behaviour of Glass-ionomer Cements in Different Acidic Solutions. Mat. Plast. 2016, 53, 100–103. [Google Scholar]
- Aliping-McKenzie, M.; Linden, R.W.; Nicholson, J.W. Theeffect of Coca-Cola and fruit juices on the surface hardness of glass-ionomersand ‘compomers’. J. Oral. Rehabil. 2004, 31, 1046–1052. [Google Scholar] [CrossRef]
- Al-Taee, L.; Deb, S.; Banerjee, A. An in vitro assessment of the physical properties of manually-mixed and encapsulated glass-ionomer cements. BDJ Open 2020, 6, 12. [Google Scholar] [CrossRef]
- Bala, O.; Arisu, H.D.; Yikilgan, I.; Arslan, S.; Gullu, A. Evaluation of surface roughness and hardness of different glassionomer cements. Eur. J. Dent. 2012, 6, 79–86. [Google Scholar] [PubMed]
- Guedes, A.P.A.; Oliveira-Reis, B.; Catelan, A.; Suzuki, T.Y.U.; Briso, A.L.F.; Dos Santos, P.H. Mechanical and surface properties analysis of restorative materials submitted to erosive challenges in situ. Eur. J. Dent. 2018, 12, 559–565. [Google Scholar] [CrossRef] [PubMed][Green Version]
- WanBakar, W.; McIntyre, J. Susceptibility of selected tooth-coloured dental materials to damage by common erosive acids. Aust. Dent. J. 2008, 53, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zimehl, R.; Hannig, M. Non metallic restorative materials based on glass ionomer cements—Recent trends and developments. Colloids Surf. A Physicochem. Eng. Asp. 2000, 163, 55–62. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Gabric, D.; Guclu, Z.A.; Miletic, I.; Coleman, N.J. Assessment of the Impact of the Addition of Nanoparticles on the Properties of Glass–Ionomer Cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef]
- Beltrame, A.; Noschang, R.; Lacerda, D.; Souza, L.C.; Almeida, I.C.S. Are grape juices more erosive than orange juices? Eur. Arch. Paediatr. Dent. 2017, 18, 263–270. [Google Scholar] [CrossRef]
- Effendi, M.C.; Nugraeni, Y.; Hartami, E.; Ummah, A.N. Changes in the Surface Roughness of Glass Ionomer Cement and Zirconomer after Immersion in Carbonated Beverages. J. Dent. Ind. 2020, 27, 85–90. [Google Scholar]
- Dissenha, R.M.S.; Lara, J.S.; Shitsuka, C.; Raggio, D.P.; Bonecker, M.; Corre, F.N.P.; Imparto, J.C.P.; Correa, M.S.N.P. Assessment of Glass Ionomer Cements (GIC) Restorations after Acidic Erosive Challenges: An in vitro Study. Braz. Res. Ped. Dent. and Int. Clin. 2016, 16, 323–330. [Google Scholar]
- Enam, F.; Mursalat, M.; Guha, U.; Aich, N.; Anik, M.I.; Nisha, N.S.; Esha, A.A.; Khan, M.S. Dental erosion potential of beverages and bottled drinking water in Bangladesh. Int. J. Food Prop. 2017, 20, 2499–2510. [Google Scholar] [CrossRef]
- Andrian, S.; Munteanu, B.; Tărăboanţă, I.; Negraia, D.; Nica, P.E.; Stoleriu, S.; Nica, I. Surface roughness after finishing and polishing of a restorative nanocomposite material. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017; pp. 101–104. [Google Scholar]
- Hamouda, I.; Ibrahim, D.; Alwakeel, E. Influence of Sport Beverages on the Properties of Dental Restorative Glass Ionomers. Int. J. Dent. Oral Health. 2016, 2, 1–8. [Google Scholar]
- Hemalatha; Nagar, P. A Compartive Evaluation of the Effect of Sports and Fruit Drinks on the Surface Roughness of Nanofilled Composite and Light Cure GIC—An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2018, 11, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja Upadhya, P.; Kishore, G. Glass ionomer cement: The different generations. Trends Biomater. Artif. Organs. 2005, 18, 158–165. [Google Scholar]
- Maganur, P.; Satish, V.; Prabhakar, A.R.; Namineni, S. Effect of soft drinks and fresh fruit juice on surface roughness of commonly used restorative materials. Int. J. Clin. Pediatr. Dent. 2015, 8, 1–5. [Google Scholar]
- Zaki, D.Y.I.; Hamzawy, E.M.A.; Halim, S.A.E.; Amer, M.A. Effect of simulated gastric juice on surface characteristics of direct esthetic restorations. Aust. J. Basic Appl. Sci. 2012, 6, 686–694. [Google Scholar]
- Badra, V.V.; Faraoni, J.J.; Ramos, R.P.; Palma-Dibb, R.G. Influence of different beverages on the microhardness and surface roughness of resin composites. Oper. Dent. 2005, 30, 213–219. [Google Scholar]
- Sari, P.; Herda, E.; Damiyanti, M. Variables affecting conventional glass ionomer cement surface roughness after exposure to Coca-Cola®. J. Phys. Conf. Ser. 2018, 1073, 032029. [Google Scholar] [CrossRef]
- Khurram, M.; Zafar, K.J.; Qaisar, A.; Atiq, T.; Khan, S.A. Restorative dental materials; a comparative evaluation of surface microhardness of three restorative materials when exposed to acidic beverages. Profess. Med. J. 2018, 25, 140–149. [Google Scholar]


| Material | Manufacturer | Type | Batch/Shade | Composition |
|---|---|---|---|---|
| Fuji IX GP | GC Corporation, Tokyo, Japan | Traditionl Glass ionomer Cement | 1810021/A3 | Powder: Fluro alumino silicate glass, Polyacrylic acid powder. |
| Liquid: Polyacrylic acid | ||||
| Polybasic carboxylic acid | ||||
| Vitremer | 3M ESPE, St. Paul, MN, USA | Resin-Modified Glass ionomer Cement | N999128/A3 | Powder: fluoroaminosilicate glass, potassium persulfate, ascorbic acid. |
| Liquid: aqueous solution of a polycarboxylic acid modified with pendant methacrylate groups, water, HEMA, photoinitiators. |
| Chemical Compound | Weight (on 1 L) |
|---|---|
| KCl | 1.5 g |
| NaHCO3 | 1.5 g |
| NaH2PO4 | 0.5 g |
| KSCN | 0.5 g |
| Lactic acid | 0.7 g |
| Acidic Beverages | Composition |
|---|---|
| Coca-Cola Green lemon Zero sugar (S.C. Coca-Cola HBC România S.R.L., Voluntari,. Ilfov) | Water, Carbon dioxide, Lemon juice from concentrate (0.5%), Phosphoric acid, Sodium citrate, Sugar, Caffeine, Aspartame, Sodium cyclamate, Acesulfame, Sulfate ammoniacal additives (E150d) |
| Lemonade by Cappy lemon and mint (S.C. Coca-Cola HBC România S.R.L., Voluntari, Ilfov) | Water, Lemon juice from concentrate (11%), Sugar, Sodium citrate/E331, Lemon and mint natural aromas, Ascorbic acid, Caroten |
| Fuzetea Green tea with Green lemon and mint (S.C. Coca-Cola HBC România S.R.L., Voluntari, Ilfov) | Water, Sugar, Fructose, Citric acid, Green Tea Extract (0.1%), Lemon juice from concentrate (0.1%), Mint extract (0.01%), Sodium citrate, Ascorbic acid |
| Subgroups | Mean ± Std. Deviation | |
|---|---|---|
| Group I | Group II | |
| A1 | 0.34140 ± 0.118423 | 0.02147 ± 0.012867 |
| B1 | 0.49267 ± 0.037023 | 0.04507 ± 0.011919 |
| C1 | 0.38233 ± 0.061864 | 0.03100 ± 0.007416 |
| D1 | 0.40840 ± 0.011975 | 0.05653 ± 0.006424 |
| A2 | 0. 47440 ± 0.018995 | 0.02047 ± 0.008879 |
| B2 | 0.59200 ± 0.047485 | 0.05480 ± 0.018876 |
| C2 | 0.49700 ± 0.056729 | 0.02887 ± 0.011090 |
| D2 | 0.47413 ± 0.019291 | 0.05440 ± 0.005680 |
| A3 | 0.42533 ± 0.098306 | 0.02193 ± 0.009169 |
| B3 | 0.48313 ± 0.022077 | 0.03500 ± 0.007010 |
| C3 | 0.47440 ± 0.018995 | 0.02707 ± 0.011622 |
| D3 | 0.39513 ± 0.028553 | 0.03007 ± 0.007667 |
| Group I—07 Days | Group I—14 Days | Group I—21 Days | Group II—7 Days | Group II—14 Days | Group II—21 Days | ||
|---|---|---|---|---|---|---|---|
| N | 60 | 60 | 60 | 60 | 60 | 60 | |
| Normal Parameters ab | Mean | 0.42270 | 0.50938 | 0.02852 | 0.03852 | 0.03963 | 0.02852 |
| Std. Deviation | 0.093089 | 0.062241 | 0.010023 | 0.016650 | 0.019413 | 0.010023 | |
| Most Extreme Differences | Absolute | 0.170 | 0.250 | 0.077 | 0.074 | 0.085 | 0.077 |
| Positive | 0.106 | 0.250 | 0.077 | 0.062 | 0.085 | 0.077 | |
| Negative | –0.170 | –0.099 | –0.058 | –0.074 | –0.071 | –0.058 | |
| Kolmogorov-Smirnov Z | 1.314 | 1.933 | 0.600 | 0.576 | 0.657 | 0.600 | |
| Asymp. Sig, (2-tailed) | 0.063 | 0.001 | 0.864 | 0.895 | 0.781 | 0.864 | |
| Paired Diffrences | t | df | Sig(2-Tailed) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Diffrences | |||||
| Lower | Upper | |||||||
| Pair 1 IA1–IIA1 | 0.319933 | 0.116402 | 0.030055 | 0.255472 | 0.384395 | 10.645 | 14 | 0 |
| Pair 2 IB1–IIB1 | 0.4476 | 0.040771 | 0.010527 | 0.425022 | 0.470178 | 42.519 | 14 | 0 |
| Pair 3 IC1–IIC1 | 0.351333 | 0.062256 | 0.016074 | 0.316857 | 0.38581 | 21.857 | 14 | 0 |
| Pair 4 ID1–IID1 | 0.351867 | 0.014667 | 0.003787 | 0.343744 | 0.359989 | 92.914 | 14 | 0 |
| Pair 5 IA2–IIA2 | 0.453933 | 0.023998 | 0.006196 | 0.440643 | 0.467223 | 73.258 | 14 | 0 |
| Pair 6 IB2–IIB2 | 0.5372 | 0.059514 | 0.015366 | 0.504242 | 0.570158 | 34.959 | 14 | 0 |
| Pair 7 IC2–IIC2 | 0.468133 | 0.054797 | 0.014148 | 0.437788 | 0.498479 | 33.087 | 14 | 0 |
| Pair 8 ID2–IID2 | 0.419733 | 0.020232 | 0.005224 | 0.408529 | 0.430938 | 80.347 | 14 | 0 |
| Pair 9 IA3–IIA3 | 0.4034 | 0.093493 | 0.02414 | 0.351625 | 0.455175 | 16.711 | 14 | 0 |
| Pair 10 IB3–IIB3 | 0.448133 | 0.023213 | 0.005994 | 0.435278 | 0.460988 | 74.769 | 14 | 0 |
| Pair 11 IC3–IIC3 | 0.447333 | 0.02562 | 0.006615 | 0.433145 | 0.461521 | 67.624 | 14 | 0 |
| Pair 12 ID3–IID3 | 0.365067 | 0.029651 | 0.007656 | 0.348646 | 0.381487 | 47.684 | 14 | 0 |
| Subgroups | Groups | Subgroups | Groups | Subgroups | Groups | |||
|---|---|---|---|---|---|---|---|---|
| I | II | I | II | I | II | |||
| A1-B1 | 0.000 * | 0.000 * | A2–B2 | 0.000 * | 0.000 * | A3–B3 | 0.001 * | 0.001 * |
| A1-C1 | 0.386 | 0.056 | A2–C2 | 0.403 | 0.242 | A3–C3 | 0.413 | 0.413 |
| A1-D1 | 0.000 * | 0.000 * | A2–D2 | 1.000 | 0.000 * | A3–D3 | 0.077 | 0.077 |
| B1-C1 | 0.000 * | 0.002 * | B2–C2 | 0.000 * | 0.000 | B3–C3 | 0.088 | 0.088 |
| B1-D1 | 0.891 | 0.000 * | B2–D2 | 0.000 * | 1.000 | B3–D3 | 0.448 | 0.448 |
| C1-D1 | 0.004 * | 0.003 * | C2–D2 | 0.393 | 0.000 * | C3–D3 | 0.800 | 0.800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, I.; Stoleriu, S.; Iovan, A.; Tărăboanță, I.; Pancu, G.; Tofan, N.; Brânzan, R.; Andrian, S. Conventional and Resin-Modified Glass Ionomer Cement Surface Characteristics after Acidic Challenges. Biomedicines 2022, 10, 1755. https://doi.org/10.3390/biomedicines10071755
Nica I, Stoleriu S, Iovan A, Tărăboanță I, Pancu G, Tofan N, Brânzan R, Andrian S. Conventional and Resin-Modified Glass Ionomer Cement Surface Characteristics after Acidic Challenges. Biomedicines. 2022; 10(7):1755. https://doi.org/10.3390/biomedicines10071755
Chicago/Turabian StyleNica, Irina, Simona Stoleriu, Alexandru Iovan, Ionuț Tărăboanță, Galina Pancu, Nicoleta Tofan, Răzvan Brânzan, and Sorin Andrian. 2022. "Conventional and Resin-Modified Glass Ionomer Cement Surface Characteristics after Acidic Challenges" Biomedicines 10, no. 7: 1755. https://doi.org/10.3390/biomedicines10071755
APA StyleNica, I., Stoleriu, S., Iovan, A., Tărăboanță, I., Pancu, G., Tofan, N., Brânzan, R., & Andrian, S. (2022). Conventional and Resin-Modified Glass Ionomer Cement Surface Characteristics after Acidic Challenges. Biomedicines, 10(7), 1755. https://doi.org/10.3390/biomedicines10071755







