Significant Decrease in Glycated Hemoglobin, 2h-Post-Load Glucose and High-Sensitivity C-Reactive Protein Levels in Patients with Abnormal Body Mass Index after Therapy with Manual Lymphatic Drainage
Abstract
:1. Introduction
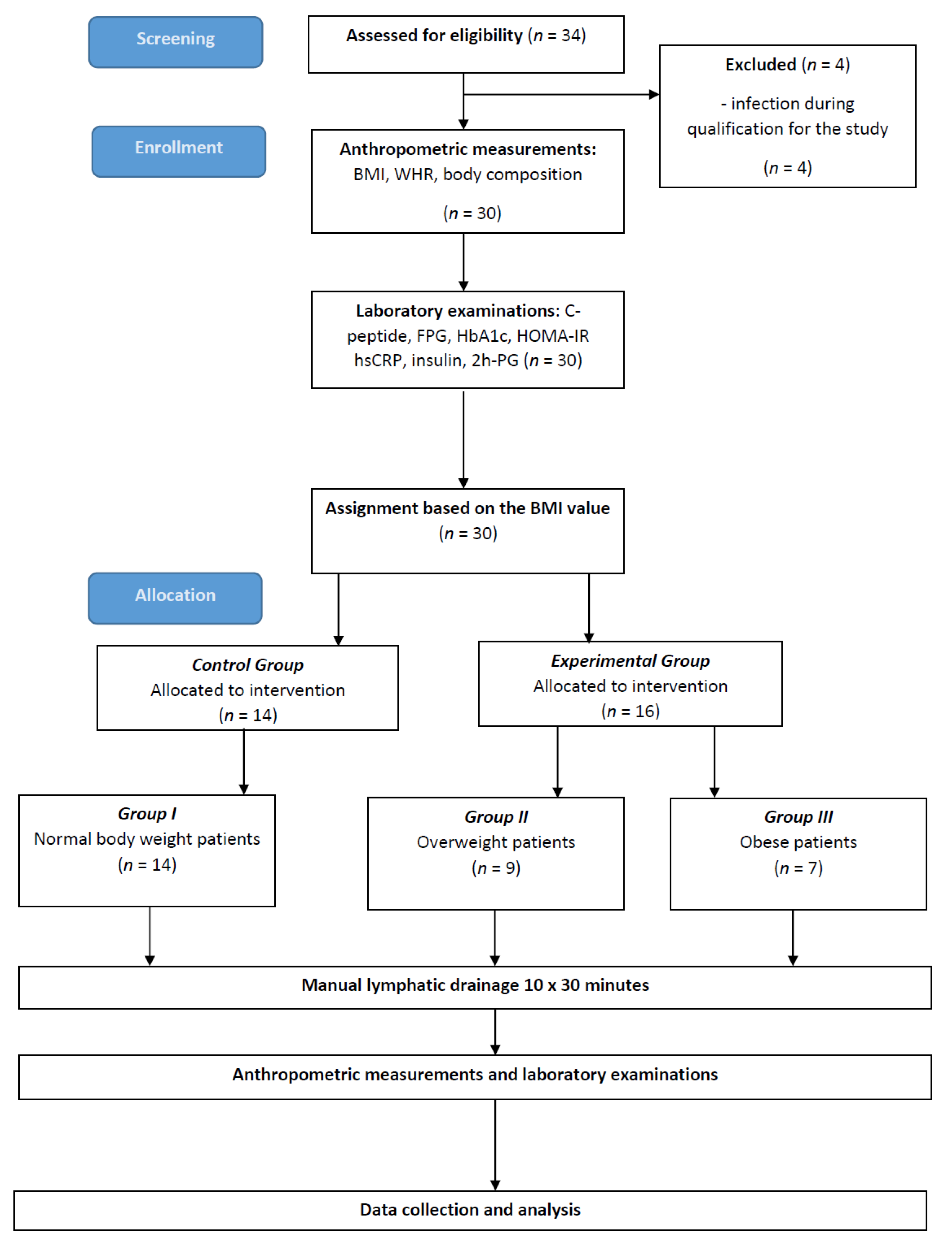
2. Materials and Methods
2.1. Data Collection
2.2. Anthropometric Measurements
2.3. Laboratory Examinations
2.4. Interventions
2.5. Statistical Methods
3. Results
3.1. Subjects’ Clinical Characteristics
3.2. The Correlations between the Level of C-peptide and BMI, WHR and the Level of Visceral Tissue in Patients with Normal Body Weight, Overweight and Obesity before and after MLD Therapy
3.3. The Correlations between the Level of HOMA-IR and BMI, WHR and the Level of Visceral Tissue in Patients with Normal Body Weight, Overweight and Obesity before and after MLD Therapy
3.4. The Correlations between the Level of hsCRP and BMI, WHR and the Level of Visceral Tissue in Patients with Normal Body Weight, Overweight and Obesity before and after MLD Therapy
3.5. The Characteristics of Patients with Normal Body Weight as a Control Group (Group I), Patients with Overweight (Group II) and Patients with Obesity (Group III)
3.6. Levels of Biochemical Parameters before (0′) and One Month (1′) after the Use of Manual Lymphatic Drainage Therapy in Patients with Normal Body Weight (Group I)
3.7. Levels of Biochemical Parameters before (0′) and One Month (1′) after the Use of Manual Lymphatic Drainage Therapy in Overweight Patients (Group II)
3.8. Levels of Biochemical Parameters in Subgroup III after the Use of Manual Lymphatic Drainage Therapy
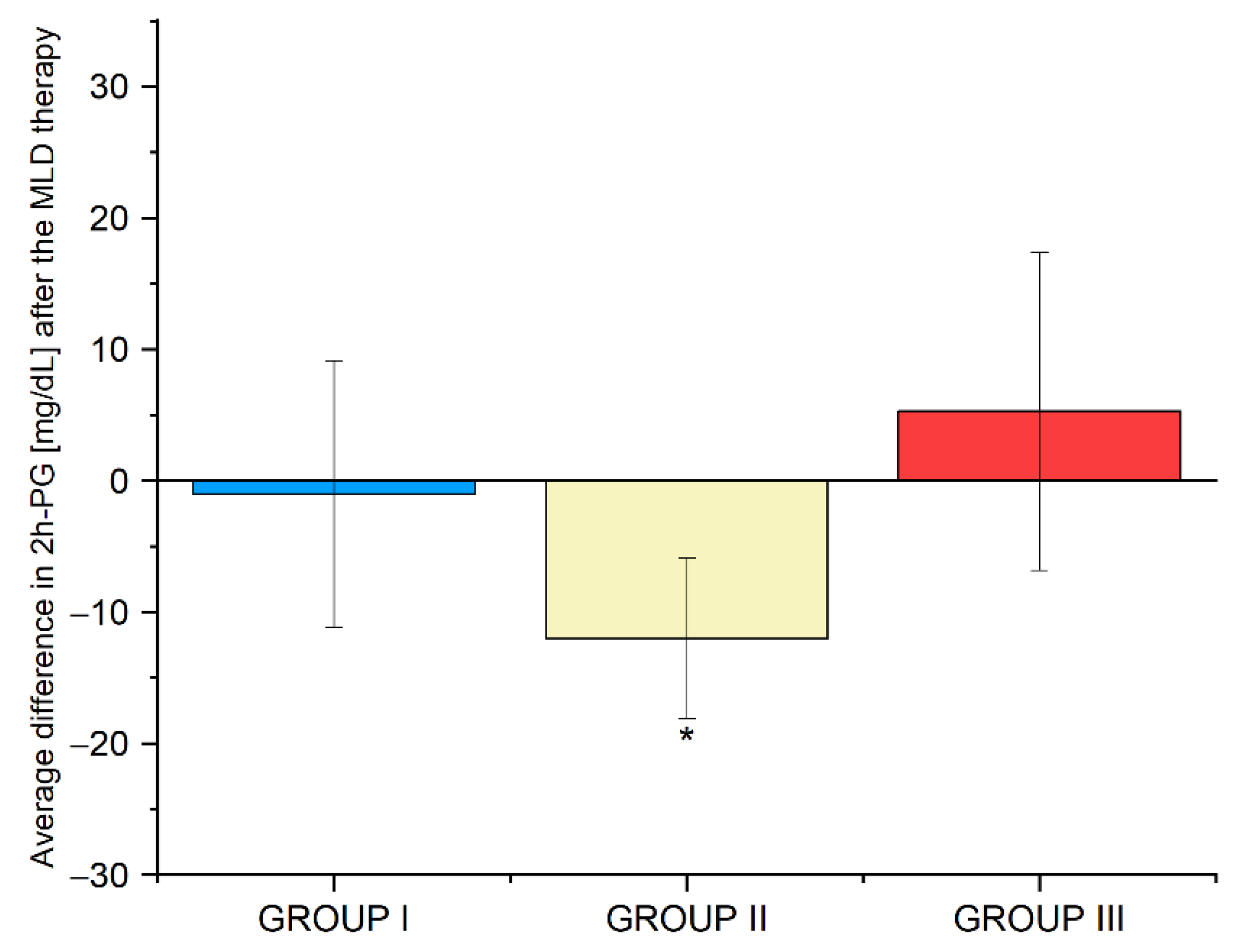
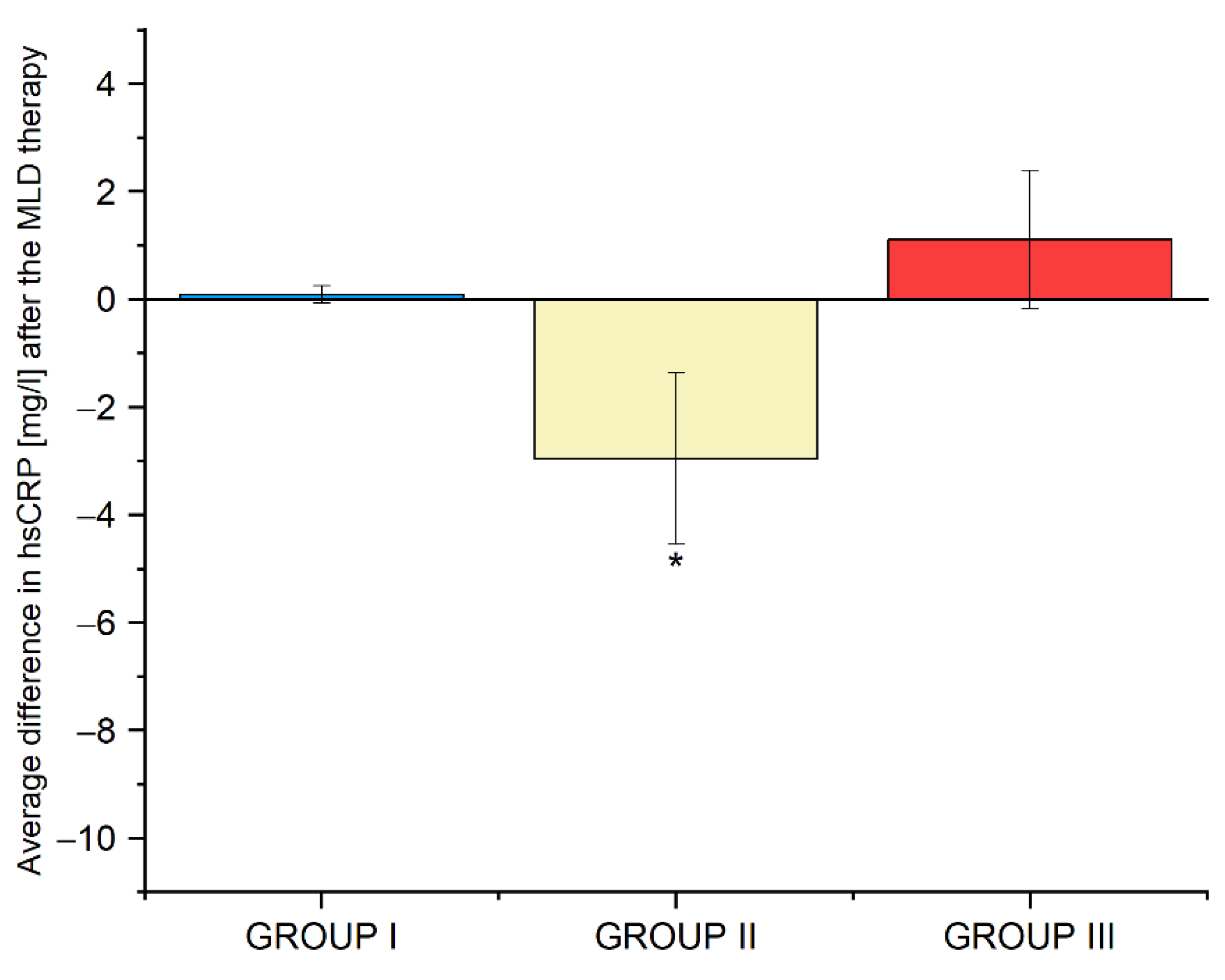
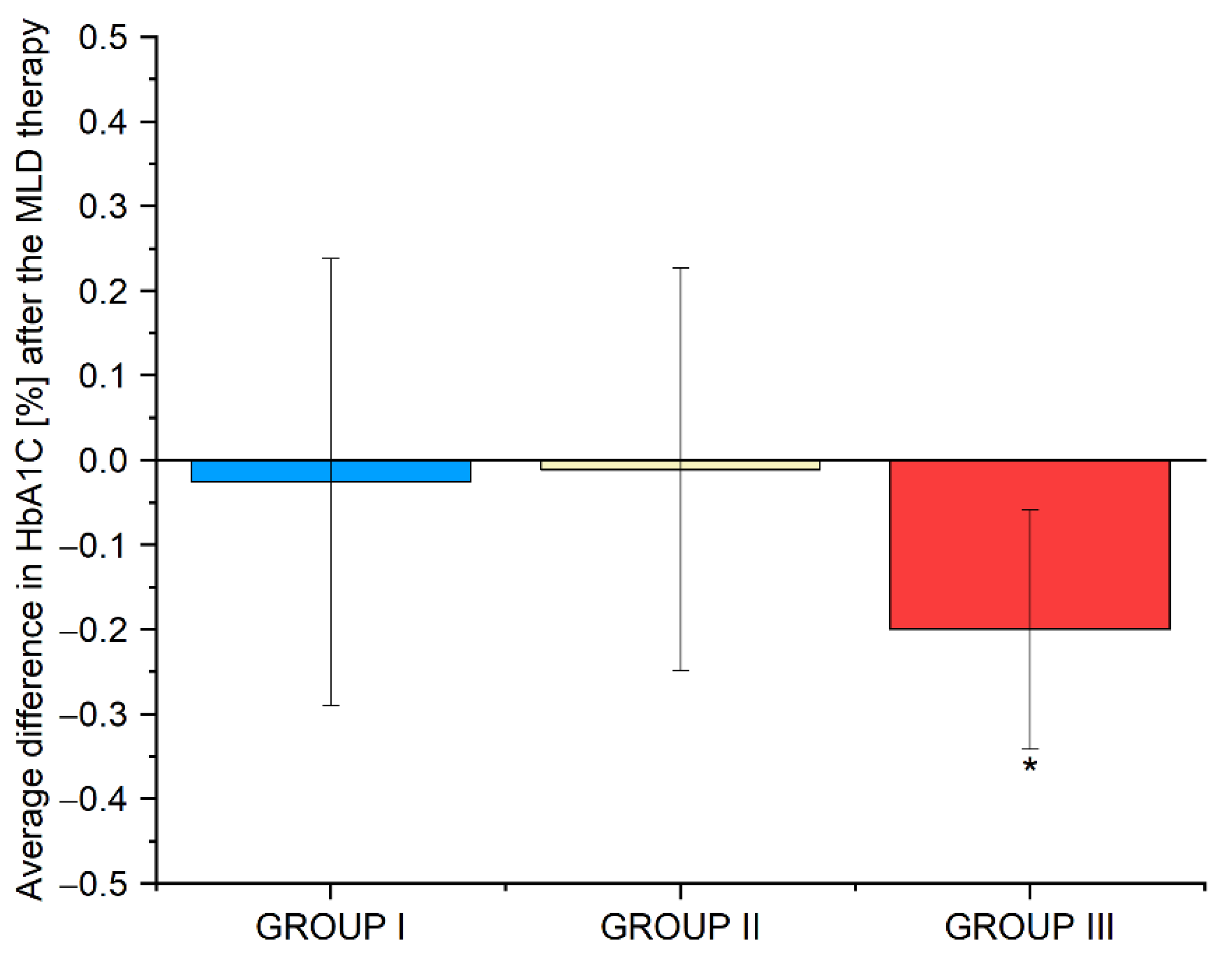
3.9. Average Differences in the Biochemical Parameters in Groups I, II and III before and after the Use of Manual Lymphatic Drainage Therapy
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| BMI | body mass index |
| CVD | cardiovascular disease |
| DECODE | Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe |
| DBP | diastolic blood pressure |
| eNOS | endothelial nitric oxide synthase |
| FPG | fasting plasma glucose |
| HbA1c | glycated hemoglobin |
| HOMA-IR | homeostatic model assessment–insulin resistance |
| hsCRP | high-sensitivity C-reactive protein |
| IGT | impaired glucose tolerance |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| IL-18 | interleukin-18 |
| MLD | manual lymphatic drainage |
| OGTT | oral glucose tolerance test |
| PDA | Polish Diabetes Association |
| SBP | systolic blood pressure |
| TNF-α | tumor necrosis factor-α |
| T1D | type 1 diabetes |
| T2DM | type 2 diabetes |
| VEGF | vascular endothelial growth factor |
| WHR | waist–hip ratio |
| 2h-PG | 2h-post-loadglucose |
References
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A Chronic Relapsing Progressive Disease Process. A Position Statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Stevens, G.A.; Singh, G.M.; Lu, Y.; Danaei, G.; Lin, J.K.; Finucane, M.M.; Bahalim, A.N.; McIntire, R.K.; Gutierrez, H.R.; Cowan, M.; et al. National, Regional, and Global Trends in Adult Overweight and Obesity Prevalences. Popul. Health Metr. 2012, 10, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.B.; Smith, M.S. Obesity Statistics. Prim. Care 2016, 43, 121–135. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories: A Systematic Review and Meta-Analysis. JAMA 2013, 309, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies from 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Akil, L.; Anwar Ahmad, H. Relationships between Obesity and Cardiovascular Diseases in Four Southern States and Colorado. J. Health Care Poor Underserved 2011, 22, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Adar, S.D.; D’Souza, J.; Mendelsohn-Victor, K.; Jacobs, D.R.; Cushman, M.; Sheppard, L.; Thorne, P.S.; Burke, G.L.; Daviglus, M.L.; Szpiro, A.A.; et al. Markers of Inflammation and Coagulation after Long-Term Exposure to Coarse Particulate Matter: A Cross-Sectional Analysis from the Multi-Ethnic Study of Atherosclerosis. Environ. Health Perspect. 2015, 123, 541–548. [Google Scholar] [CrossRef]
- Martins, M.V.; Ribeiro, A.Q.; Martinho, K.O.; Franco, F.S.; De Souza, J.D.; De Morais, K.B.D.; Leite, I.C.G.; Tinôco, A.L.A. Anthropometric Indicators of Obesity as Predictors of Cardiovascular Risk in the Elderly. Nutr. Hosp. 2015, 31, 2583–2589. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Ketema, E.B.; Kibret, K.T. Correlation of Fasting and Postprandial Plasma Glucose with HbA1c in Assessing Glycemic Control; Systematic Review and Meta-Analysis. Arch. Public Health 2015, 73, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Buring, J.E.; Shih, J.; Matias, M.; Hennekens, C.H. Prospective Study of C-Reactive Protein and the Risk of Future Cardiovascular Events among Apparently Healthy Women. Circulation 1998, 98, 731–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423. [Google Scholar] [CrossRef] [Green Version]
- Temelkova-Kurktschiev, T.S.; Koehler, C.; Henkel, E.; Leonhardt, W.; Fuecker, K.; Hanefeld, M. Postchallenge Plasma Glucose and Glycemic Spikes Are More Strongly Associated with Atherosclerosis than Fasting Glucose or HbA1c Level. Diabetes Care 2000, 23, 1830–1834. [Google Scholar] [CrossRef] [Green Version]
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose Tolerance and Cardiovascular Mortality: Comparison of Fasting and 2-Hour Diagnostic Criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- American Diabetes Association. Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S73–S84. [Google Scholar] [CrossRef]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; Huikuri, H.; et al. ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASDThe Task Force on Diabetes, Pre-Diabetes, and Cardiovascular Diseases of the European Society of Cardiology (ESC) and Developed in Collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar] [CrossRef] [Green Version]
- Khaw, K.T.; Wareham, N.; Luben, R.; Bingham, S.; Oakes, S.; Welch, A.; Day, N. Glycated Haemoglobin, Diabetes, and Mortality in Men in Norfolk Cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk). BMJ 2001, 322, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Wändell, P.E.; Ärnlöv, J.; Nixon Andreasson, A.; Andersson, K.; Törnkvist, L.; Carlsson, A.C. Effects of Tactile Massage on Metabolic Biomarkers in Patients with Type 2 Diabetes. Diabetes Metab. 2013, 39, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Bayat, D.; Mohammadbeigi, A.; Parham, M.; Mehrandasht, A.; Hashemi, M.; Mahlooji, K.; Asghari, M. The Effect of Massage on Diabetes and Its Complications: A Systematic Review. Crescent J. Med. Biol. Sci. 2019, 7, 22–28. [Google Scholar]
- Han, Y.; Lu, Z.; Chen, S.; Zhong, C.; Yan, M.; Wang, H.; Meng, M.; Liu, M. Abdominal Massage Alleviates Skeletal Muscle Insulin Resistance by Regulating the AMPK/SIRT1/PGC-1α Signaling Pathway. Cell Biochem. Biophys. 2021, 79, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Čížková, T.; Štěpán, M.; Daďová, K.; Ondrůjová, B.; Sontáková, L.; Krauzová, E.; Matouš, M.; Koc, M.; Gojda, J.; Kračmerová, J.; et al. Exercise Training Reduces Inflammation of Adipose Tissue in the Elderly: Cross-Sectional and Randomized Interventional Trial. J. Clin. Endocrinol. Metab. 2020, 105, 4510–4526. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.D.; Hespe, G.E.; Kataru, R.P.; García Nores, G.D.; Savetsky, I.L.; Torrisi, J.S.; Gardenier, J.C.; Dannenberg, A.J.; Mehrara, B.J. Obesity-induced Lymphatic Dysfunction Is Reversible with Weight Loss. J. Physiol. 2016, 594, 7073. [Google Scholar] [CrossRef] [PubMed]
- Varaliová, Z.; Vlasák, R.; Čížková, T.; Gojda, J.; Potočková, J.; Šiklová, M.; Krauzová, E.; Štěpán, M.; Bülow, J.; Štich, V.; et al. Lymphatic Drainage Affects Lipolytic Activity of Femoral Adipose Tissue in Women. Int. J. Obes. 2020, 44, 1974–1978. [Google Scholar] [CrossRef]
- Arngrim, N.; Simonsen, L.; Holst, J.J.; Bülow, J. Reduced Adipose Tissue Lymphatic Drainage of Macromolecules in Obese Subjects: A Possible Link between Obesity and Local Tissue Inflammation? Int. J. Obes. 2013, 37, 748–750. [Google Scholar] [CrossRef] [Green Version]
- Antoniak, K.; Zorena, K.; Hansdorfer-Korzon, R.; Wojtowicz, D.; Kozíński, M. Favourable Changes in C-Peptide, C-Reactive Protein and Lipid Profile, and Improved Quality of Life in Patients with Abnormal Body Mass Index after the Use of Manual Lymphatic Drainage: A Case Series with Three-Month Follow-Up. Medicina 2022, 58, 273. [Google Scholar] [CrossRef]
- Antoniak, K.; Hansdorfer-Korzon, R.; Mrugacz, M.; Zorena, K. Adipose Tissue and Biological Factors. Possible Link between Lymphatic System Dysfunction and Obesity. Metabolites 2021, 11, 617. [Google Scholar] [CrossRef]
- de Godoy, J.M.P.; Santana, K.R.; Godoy, M.d.F.G. Lymphoscintigraphic Evaluation of Manual Lymphatic Therapy: The Godoy & Godoy Technique. Phlebology 2015, 30, 39–44. [Google Scholar] [CrossRef]
- Kurz, W.; Kurz, R.; Litmanovitch, Y.I.; Romanoff, H.; Pfeifer, Y.; Sulman, F.G. Effect of Manual Lymphdrainage Massage on Blood Components and Urinary Neurohormones in Chronic Lymphedema. Angiology 1981, 32, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Chen, Q.; Peng, K.; Deng, L.; He, L.; Hou, Y.; Zhang, Y.; Guo, J.; Mei, Z.; Li, L. Manual Lymphatic Drainage for Lymphedema in Patients after Breast Cancer Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 2020, 99, e23192. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.C.; Maus, E.A.; Rasmussen, J.C.; Marshall, M.V.; Adams, K.E.; Fife, C.E.; Smith, L.A.; Chan, W.; Sevick-Muraca, E.M. Assessment of Lymphatic Contractile Function after Manual Lymphatic Drainage Using Near-Infrared Fluorescence Imaging. Arch. Phys. Med. Rehabil. 2011, 92, 756–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgrado, J.P.; Vandermeeren, L.; Vankerckhove, S.; Valsamis, J.B.; Malloizel-Delaunay, J.; Moraine, J.J.; Liebens, F. Near-Infrared Fluorescence Lymphatic Imaging to Reconsider Occlusion Pressure of Superficial Lymphatic Collectors in Upper Extremities of Healthy Volunteers. Lymphat. Res. Biol. 2016, 14, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R. Low-Frequency Vibrotherapy Considerably Improves the Effectiveness of Manual Lymphatic Drainage (MLD) in Patients with Lipedema: A Two-Armed, Randomized, Controlled Pragmatic Trial. Physiother. Theory Pract. 2020, 36, 63–70. [Google Scholar] [CrossRef]
- Thompson, B.; Gaitatzis, K.; Janse de Jonge, X.; Blackwell, R.; Koelmeyer, L.A. Manual Lymphatic Drainage Treatment for Lymphedema: A Systematic Review of the Literature. J. Cancer Surviv. 2021, 15, 244–258. [Google Scholar] [CrossRef]
- Palazzin, E.P.; Kikuchi, N.H.; Schittler, R.F.; Ruaro, J.A.; Fréz, A.R.; Marquetti, M.d.G.K. Effect of Lymphatic Drainage on Blood and Urinary Glucose in Patients with Type 1 Diabetes Mellitus: Preliminary Study [Abstract in English]. Sci. Med. 2012, 22, 97–101. [Google Scholar]
- de Sire, A.; Inzitari, M.T.; Moggio, L.; Pinto, M.; de Sire, G.; Supervia, M.; Petraroli, A.; Rubino, M.; Carbotti, D.; Succurro, E.; et al. Effects of Intermittent Pneumatic Compression on Lower Limb Lymphedema in Patients with Type 2 Diabetes Mellitus: A Pilot Randomized Controlled Trial. Medicina 2021, 57, 1018. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. 2021 Guidelines on the Management of Patients with Diabetes. A Position of Diabetes Poland. Clin. Diabetol. 2021, 10, 1–113. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Foldi, M.; Strossenreuther, R.H.K. Foundations of Manual Lymph Drainage; Elsevier Mosby: Maryland Heights, MI, USA, 2005; ISBN 9780323070416. [Google Scholar]
- Yosten, G.L.C.; Maric-Bilkan, C.; Luppi, P.; Wahren, J. Physiological Effects and Therapeutic Potential of Proinsulin C-Peptide. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E955–E968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, E.; Watt, M.J.; Nowell, C.J.; Quach, T.; Simpson, J.S.; De Melo Ferreira, V.; Agarwal, S.; Chu, H.; Srivastava, A.; Anderson, D.; et al. Mesenteric Lymphatic Dysfunction Promotes Insulin Resistance and Represents a Potential Treatment Target in Obesity. Nat. Metab. 2021, 3, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Ernaga Lorea, A.; Hernández Morhain, M.C.; Ollero García-Agulló, M.D.; Martínez de Esteban, J.P.; Iriarte Beroiz, A.; Gállego Culleré, J. Prognostic Value of Blood Glucose in Emergency Room and Glycosylated Hemoglobin in Patients Who Have Suffered an Acute Cerebro-Vascular Event. Med. Clin. 2017, 149, 17–23. [Google Scholar] [CrossRef]
- Mishra, N.; Saxena, S.; Shukla, R.K.; Singh, V.; Meyer, C.H.; Kruzliak, P.; Khanna, V.K. Association of Serum N(ε)-Carboxy Methyl Lysine with Severity of Diabetic Retinopathy. J. Diabetes Complicat. 2016, 30, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, A.; Kiejna, A.; Cichoń, E.; Jodko-Modlińska, A.; Obrębski, M.; Kokoszka, A. Relations of Well-Being, Coping Styles, Perception of Self-Influence on the Diabetes Course and Sociodemographic Characteristics with HbA1c and BMI Among People with Advanced Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 407–418. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New Insights into Oxidative Stress and Inflammation during Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Fonseca, F.A.H.; de Oliveira Izar, M.C. High-Sensitivity C-Reactive Protein and Cardiovascular Disease Across Countries and Ethnicities. Clinics 2016, 71, 235–242. [Google Scholar] [CrossRef]
- Rehal, S.; Kataru, R.P.; Hespe, G.E.; Baik, J.E.; Park, H.J.; Ly, C.; Shin, J.Y.; Mehrara, B.J. Regulation of Lymphatic Function and Injury by Nitrosative Stress in Obese Mice. Mol. Metab. 2020, 42, 101081. [Google Scholar] [CrossRef] [PubMed]
- Thangaswamy, S.; Bridenbaugh, E.A.; Gashev, A.A. Evidence of Increased Oxidative Stress in Aged Mesenteric Lymphatic Vessels. Lymphat. Res. Biol. 2012, 10, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, S.S.; Yeo, W.; Lee, Y.M.; Mo, K.F.; Tse, K.Y.; Tse, S.M.; Ho, F.P.; Kwan, W.H. Predictors of Lymphedema in Patients with Breast Cancer Undergoing Axillary Lymph Node Dissection in Hong Kong. Nurs. Res. 2008, 57, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.M.; Yeun, Y.R.; Kim, H.Y.; Kim, S.J. Effects of Manual Lymph Drainage for Abdomen on the Brain Activity of Subjects with Psychological Stress. J. Phys. Ther. Sci. 2017, 29, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The Role of the Autonomic Nervous System in the Pathophysiology of Obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef] [Green Version]
- Kaptchuk, T.J.; Kelley, J.M.; Conboy, L.A.; Davis, R.B.; Kerr, C.E.; Jacobson, E.E.; Kirsch, I.; Schyner, R.N.; Bong, H.N.; Nguyen, L.T.; et al. Components of Placebo Effect: Randomised Controlled Trial in Patients with Irritable Bowel Syndrome. BMJ 2008, 336, 999–1003. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Subjects |
|---|---|
| Age [years] | 40 ± 12 |
| SBP [mmHg] | 120 ± 5 |
| DBP [mmHg] | 88 ± 8 |
| BMI [kg/m2] | 27 ± 5 |
| WHR | 0.92 ± 0.16 |
| Visceral adipose tissue [LVL] | 7 ± 4 |
| FPG [mg/dL] | 91 ± 15 |
| 2h-PG [mg/dL] | 93 ± 20 |
| Insulin [uIU/mL] | 10 ± 8.0 |
| C-peptide [ng/mL] | 2.3 ± 1.9 |
| HbA1c [%] | 5.3 ± 0.4 |
| hsCRP [mg/L] | 2.7 ± 5 |
| HOMA-IR | 2.6 ± 2.1 |
| Parameter | I Normal Body Weight (n = 14) | II Overweight (n = 9) | III Obesity (n = 7) |
|---|---|---|---|
| Age [years] | 37 ± 13 | 40 ± 11 | 46 ± 10 |
| SBP [mmHg] | 120 ± 4 | 125 ± 3 | 139 ± 3 |
| DBP [mmHg] | 74 ± 6 | 81 ± 7 | 83 ± 6 |
| Body mass index [BMI] [kg/m2] | 22.0 ± 2.0 | 28.0 ± 2.0 | 34.0 ± 2.0 |
| Waist–hip ratio [WHR] | 0.78 ± 0.075 (normal) | 1.0 ± 0.0 (abdominal obesity) | 1.2 ± 0.1 (abdominal obesity) |
| Visceral adipose tissue [LVL] | 4 ± 2 | 8 ± 4 | 12 ± 4 |
| Patients with Normal Body Weight (Group I; n = 14) | |||
|---|---|---|---|
| Parameter | 0′ (Before MLD Therapy) | 1′ (One Month after MLD Therapy) | p-Value * |
| Age [years] | 37 ± 13 | 37 ± 13 | ns |
| BMI [kg/m2] | 22 ± 2 | 22 ± 2 | ns |
| Waist–hip ratio [WHR] | 0.78 ± 0.075 (normal) | 0.78 ± 0.075 (normal) | ns |
| Visceral adipose tissue [LVL] | 4 ± 2 | 4 ± 2 | ns |
| FPG [mg/dL] | 85 ± 8 | 85 ± 10 | 0.960 |
| 2h-PG [ mg/dL] | 77 ± 16 | 76 ± 15 | 0.864 |
| Insulin [uIU/mL] | 8.3 ± 7.8 | 4.9 ± 1.7 | 0.122 |
| C-peptide [ng/mL] | 1.9 ±1.8 | 1.3 ± 0.3 | 0.220 |
| HbA1C [%] | 5.2 ± 0.4 | 5.1 ± 0.4 | 0.613 |
| hsCRP [mg/L] | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.562 |
| HOMA-IR | 2 ± 2 | 1.1 ± 0.4 | 0.112 |
| Patients with Overweight (Group II) | |||
|---|---|---|---|
| Parameter | 0′ (Before MLD Therapy) | 1′ (One Month after MLD Therapy) | p-Value * |
| Age [years] | 40 ± 11 | 40 ± 11 | ns |
| Body mass index BMI [kg/ m2] | 28.0 ± 2.0 | 28.0 ± 2.0 | ns |
| Waist–hip ratio [WHR] | 1.0 ± 0.0 (abdominal obesity) | 1.0 ± 0.0 (abdominal obesity) | ns |
| Visceral adipose tissue [LVL] | 8 ± 4 | 8 ± 4 | ns |
| FPG [mg/dL] | 97 ± 9 | 100 ± 10 | 0.661 |
| 2h-PG [mg/dL] | 112 ± 12 | 100 ± 11 | 0.050 * |
| Insulin [uIU/mL] | 12.0 ± 10.9 | 10 ± 4.7 | 0.544 |
| C-peptide [ng/mL] | 3.0 ± 2.6 | 2.0 ± 0.7 | 0.390 |
| HbA1c [%] | 5.3 ± 0.3 | 5.3 ± 0.3 | 0.931 |
| hsCRP [mg/L] | 5.2 ± 8.4 | 2.3 ± 1.7 | 0.041 * |
| HOMA-IR | 3.1 ± 2.5 | 2.5 ± 1.1 | 0.493 |
| Patients with Obesity (Group III) | |||
|---|---|---|---|
| Parameter | 0′ (before MLD Therapy) | 1′ (One Month after MLD Therapy) | p-Value * |
| Age [years] | 46 ± 10 | 46 ±10 | ns |
| Body mass index (BMI) [kg/ m2] | 34.0 ± 2.0 | 34.0 ± 2.0 | ns |
| Waist–hip ratio [WHR] | 1.2 ± 0.1 (abdominal obesity) | 1.2 ± 0.1 (abdominal obesity) | ns |
| Visceral adipose tissue [LVL] | 12 ± 4 | 12 ± 4 | ns |
| FPG [mg/dL] | 97 ± 26 | 94 ± 18 | 0 |
| 2h-PG [mg/dL] | 99 ± 14 | 106 ± 18 | 0.433 |
| Insulin [uIU/mL] | 11.0 ± 3.8 | 11.5 ± 4.3 | 0.852 |
| C-peptide [ng/mL] | 2.3 ± 0.8 | 2.3 ± 1.0 | 0.950 |
| HbA1c [%] | 5.4 ± 0.6 | 5.2 ± 0.6 | 0.013* |
| hsCRP [mg/L] | 2.5 ± 2.9 | 4.0 ± 4.0 | 0.480 |
| HOMA-IR | 3.5 ± 2.0 | 3.2 ± 1.1 | 0.581 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniak, K.; Zorena, K.; Jaskulak, M.; Hansdorfer-Korzon, R.; Mrugacz, M.; Koziński, M. Significant Decrease in Glycated Hemoglobin, 2h-Post-Load Glucose and High-Sensitivity C-Reactive Protein Levels in Patients with Abnormal Body Mass Index after Therapy with Manual Lymphatic Drainage. Biomedicines 2022, 10, 1730. https://doi.org/10.3390/biomedicines10071730
Antoniak K, Zorena K, Jaskulak M, Hansdorfer-Korzon R, Mrugacz M, Koziński M. Significant Decrease in Glycated Hemoglobin, 2h-Post-Load Glucose and High-Sensitivity C-Reactive Protein Levels in Patients with Abnormal Body Mass Index after Therapy with Manual Lymphatic Drainage. Biomedicines. 2022; 10(7):1730. https://doi.org/10.3390/biomedicines10071730
Chicago/Turabian StyleAntoniak, Klaudia, Katarzyna Zorena, Marta Jaskulak, Rita Hansdorfer-Korzon, Małgorzata Mrugacz, and Marek Koziński. 2022. "Significant Decrease in Glycated Hemoglobin, 2h-Post-Load Glucose and High-Sensitivity C-Reactive Protein Levels in Patients with Abnormal Body Mass Index after Therapy with Manual Lymphatic Drainage" Biomedicines 10, no. 7: 1730. https://doi.org/10.3390/biomedicines10071730
APA StyleAntoniak, K., Zorena, K., Jaskulak, M., Hansdorfer-Korzon, R., Mrugacz, M., & Koziński, M. (2022). Significant Decrease in Glycated Hemoglobin, 2h-Post-Load Glucose and High-Sensitivity C-Reactive Protein Levels in Patients with Abnormal Body Mass Index after Therapy with Manual Lymphatic Drainage. Biomedicines, 10(7), 1730. https://doi.org/10.3390/biomedicines10071730







