Abstract
Colorectal cancer (CRC) is a malignant disease that is the second most common cancer worldwide. CRC arises from the complex interactions among a variety of genetic and environmental factors. To understand the mechanism of colon tumorigenesis, preclinical studies have developed various mouse models including carcinogen-induced and transgenic mice to recapitulate CRC in humans. Using these mouse models, scientific breakthroughs have been made on the understanding of the pathogenesis of this complex disease. Moreover, the availability of transgenic knock-in or knock-out mice further increases the potential of CRC mouse models. In this review, the overall features of carcinogen-induced (focusing on azoxymethane and azoxymethane/dextran sulfate sodium) and transgenic (focusing on ApcMin/+) mouse models, as well as their mechanisms to induce colon tumorigenesis, are explored. We also discuss limitations of these mouse models and their applications in the evaluation and study of drugs and treatment regimens against CRC. Through these mouse models, a better understanding of colon tumorigenesis can be achieved, thereby facilitating the discovery of novel therapeutic strategies against CRC.
1. Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related death worldwide [1]. CRC results from the progressive accumulation of genetic and epigenetic alterations that lead to the transformation of normal colon mucosa to adenocarcinoma. Approximate 90% of CRC cases are sporadic and occur in patients without genetic predisposition or family history of CRC [2], whereas around 2–5% of CRC cases are hereditary [3]. The two most common inherited CRC are hereditary nonpolyposis CRC and familial adenomatous polyposis (FAP). To date, the molecular mechanism in different stages of CRC development remains unclear. Thus, it is important to investigate the molecular initiation and progression of colon tumorigenesis using preclinical animal models.
The molecular mechanism of CRC progression has been identified since the establishment of the ApcMin/+ transgenic mouse model. Genetic mutation of adenomatous polyposis coli (APC) has been detected in 70% of patients with sporadic CRC [4], indicating a crucial role of APC mutation in colon tumorigenesis. Consistently, preclinical studies reported that Apc mutation can induce spontaneous formation of colon tumors in mice [5]. The ApcMin/+ transgenic mouse model is thus commonly used for research on colon tumorigenesis [6]. Meanwhile, deleting several other genes such as p53, Kirstein rat sarcoma viral oncogene homolog (KRAS), and phosphatase and tensin homolog (PTEN) have also been reported to contribute CRC development [7], although not all of these genetic alterations are similar to Apc mutation, which can induce spontaneous colon tumorigenesis. However, carcinogens are also widely applied to mice for studying CRC development. Azoxymethane (AOM) is the most used carcinogen to mimic the development of sporadic CRC [8,9]. Co-administration of AOM and dextran sulfate sodium (DSS) is also a common approach to induce colon tumorigenesis in mice, recapitulating the pathogenesis of colitis-associated CRC (CAC) [10].
The mechanism of CRC development is complex, since both genetic and environmental factors are involved [11]. Although in vitro cell cultures are efficient, they cannot accurately recapitulate the physiological conditions in human CRC. Hence, mouse models are necessary tools for preclinical studies of CRC. In this review, we summarize the overall features of the most commonly used carcinogen-induced (AOM and AOM/DSS) and transgenic (ApcMin/+) mouse models of CRC, with further elucidation on their mechanisms to induce colon tumorigenesis. We further evaluate limitations of these mouse models and how their applications in preclinical studies can facilitate clinical benefits.
2. Carcinogen-Induced Mouse Model of Colorectal Cancer
To date, a variety of carcinogen-induced CRC mouse models has been established: (1) AOM, methylazoxymethanol and 1,2-dimethylhydrazine (DMH); (2) heterocyclic amines including 2-amino-1-methyl-6-phenylimidazo and 2-amino-33-methylimidazo [4,5-f] quinoline; (3) aromatic amines including 3,2-dimethyl-4-aminobiphenyl; and (4) alkylating compounds including methylnitrosourea and N-methyl-N-nitro-N-nitrosoguanidine [6]. Among them, AOM is the most commonly used carcinogen to induce CRC in mice. Meanwhile, the combined application of AOM and colitogen DSS is also widely considered as another robust method to induce CRC in mice. In this section, the overall features of AOM and AOM/DSS mouse models as well as the mechanisms of how these carcinogens induce colon tumorigenesis are discussed.
2.1. AOM- or AOM/DSS-Induced Mouse Model of Colorectal Cancer
AOM is the metabolite of DMH. Compared to other carcinogens, AOM is more efficient in inducing colon carcinogenesis attributed by its high stability [12,13]. AOM specifically induces tumor formation in the colons of mice. Given by its high efficiency and reliability, AOM remains one of the most commonly used carcinogens to induce colon tumorigenesis in mice. To date, most studies provide a total of six AOM doses (10 mg/kg intraperitoneal injection per week), and mice can develop colon tumors about 24 weeks after receiving the last AOM injection [14] (Figure 1A).
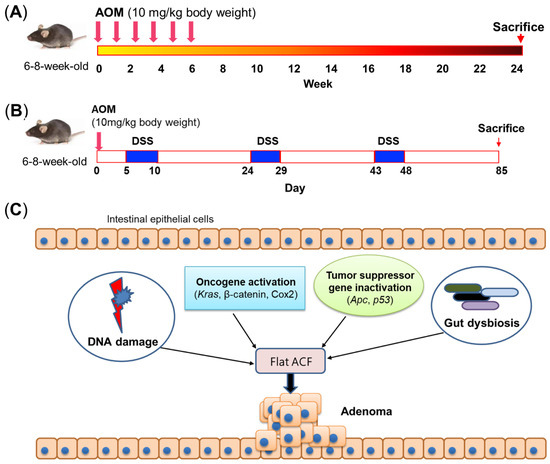
Figure 1.
Carcinogen-induced mouse models. (A) Schematic of commonly used method of AOM treatment. Mice are provided a total of 6 AOM doses (10 mg/kg intraperitoneal injection per week), and mice can develop colon adenomas about 24 weeks after the last AOM injection. (B) Schematic of commonly used method of AOM/DSS treatment. A single intraperitoneal injection of AOM (10 mg/kg body weight) is first provided, followed by three treatment cycles of 1–3% DSS in drinking water for 5 days and then regular water treatment for 14 days. Mice can develop colon tumors about 85 days after AOM injection. (C) Mechanism of AOM-induced colon tumorigenesis. AOM can induce DNA damage, activate oncogenes, and inactivate tumor suppressor genes to initiate colon tumorigenesis. In addition, the gut microbiota is also necessary for AOM-induced colon tumorigenesis. ACF, aberrant crypt foci; AOM, azoxymethane; Apc, adenomatous polyposis coli; COX2, cyclooxygenase 2; DSS, dextran sulfate sodium; Kras, Kirsten rat sarcoma viral oncogene homolog; p53, tumor protein p53.
The relationship between inflammation and CRC has been well established since Crohn’s colitis and ulcerative colitis were recognized as being associated with increased risk of CRC [15]. In 2003, the AOM/DSS mouse model was the first reported to mimic CAC with 100% tumor incidence in the distal colon [10]. Currently, the most widely used approach is to intraperitoneally inject AOM (10 mg/kg body weight) once, followed by three treatment cycles of 1–3% DSS in drinking water for 5 days, and then regular water treatment for 14 days [16,17] (Figure 1B). Notably, the efficiency of tumor induction and tumor number depend on the dosage of AOM and/or concentration of DSS, which have greatly varied among CRC studies.
2.2. Sensitivity to Carcinogen and Tumor Characteristics in Different Mouse Strains
Mice with different genetic backgrounds have distinct sensitivity to AOM. Rosenberg et al. showed that the sensitivity to AOM is varied among different mouse strains [6]. A/J and SWR/J mice have great sensitivity to AOM with a high incidence of colon tumors. C57B/L6 and Balb/c mice have moderate sensitivity with relatively less incidence of colon tumors compared to A/J and SWR/J mice, whereas AOM administration in AKR/J and 129/SV mice cannot induce any formation of colon tumors [6]. The authors also evaluated features of AOM-induced tumors in different mouse strains. The tumor morphology was found to be similar among these mouse strains, while metastasis or invasion was not observed even in the mouse strain with high AOM sensitivity. AOM-induced tumors are exclusively located at the distal region of the mice colon. In A/J and SRJ/R mice, which are the strain with highest AOM sensitivity (develop ≥ 10 tumors per mouse within 8 weeks after AOM treatment), their colons are covered by multiple coalescing tumors with rectal bleeding, while C57BL/6 mice that have moderate AOM sensitivity can develop up to 5.5 tumors per mouse [6,18]. Moreover, histological features are also similar among mouse strains of which the intramucosal and expansile tumors are hypercellular with closely packed colon epithelial cells [19]. Tumor crypts are all composed of closely packed cells with indistinct cell borders, regardless of mouse strains. Infiltration of neoplastic cells into the muscular wall of the distal colon can also occur [19]. Contrastingly, although A/J and SWR/J mice have similar AOM sensitivity, histopathological progression varies among these two mouse strains. Tumors in SWR/J mice can develop features of carcinoma in situ with epithelial crypts and inter-glandular stroma 8 weeks after AOM treatment, while A/J mice have less tumor progression at 8 weeks of AOM treatment but with significantly larger tumor size [18]. No metastasis and invasion were observed even in the mouse strain with the highest AOM sensitivity [6], indicating that this model is suitable to study early-stage but not late-stage or metastatic CRC.
Similar to AOM, mice with different genetic backgrounds also have distinct sensitivity to AOM/DSS. A previous study treated four different mouse strains (BALB/c, C3H/HeN, C57BL/6N and DBA/2N) with AOM (10 mg/kg body weight) and 1% DSS [20]. The incidence of colon adenocarcinoma was reported to be 100% in BALB/c and 50% in C57BL/6N, but none was detected in the other two strains. Inflammation seems to be independent from the development of colon adenocarcinoma after AOM/DSS treatment, of which C3H/HeN has the most severe inflammation, followed by Balb/c and DBA/2N, whereas C57BL/6N has the least amount of inflammation, even though colon adenocarcinoma is present in this mouse strain. Moreover, the score of nitrotyrosine positivity was found to be Balb/c > C57BL/6N > C3H/HeN > DBA/2N. These results therefore indicate that the difference in sensitivity to AOM in distinct mouse strains can be attributed to responses toward nitrosative stress caused by DSS-induced inflammation [20].
In general, tumors developed in AOM/DSS-treated mice can recapitulate the main histopathological features of human CRC such as the predominant location of tumors in the middle and distal colon [10]. These tumors were histologically characterized as tubular adenoma or were well or moderately differentiated tubular adenocarcinoma [10]. AOM/DSS-treated mice show persistent colitis with diarrhea and colon bleeding, while there is no significant difference in colon length compared to untreated control mice [10]. In AOM/DSS-treated mice, colon tumors exhibit signs of colitis characterized by crypt architectural distortion and lamina propria inflammation, while a few spotted ulcers with regenerative changes can also occur on the colon mucosa [21]. Moreover, tumor invasion to submucosa, muscularis propria or serosa was observed in AOM/DSS-treated mice [10]. Importantly, these findings demonstrated that unlike AOM-induced tumors that are mostly adenoma, AOM/DSS treatment can induce formation of the full process of colon tumorigenesis, progressing from initial crypt proliferation to the final development of colon carcinoma.
2.3. Modeling Different Stages of Colon Tumorigenesis
Aberrant crypt foci (ACF) are putative preneoplastic lesions in the colon, and they have been observed in patients with CRC and in patients with FAP [22]. Previously, ACF were used as a short-term bioassay to evaluate the role of nutritional components at the early stage of colon tumorigenesis, even though ACF are not directly related to the early formation of tumors [23]. Two types of ACF have been characterized: classical elevated ACF whose small crypts are elevated from the surrounding epithelium and flat ACF without elevated structure. AOM can induce flat ACF with similar morphology and expressions of β-catenin and cyclin D1 as in CRC tumors, indicating a continuous progression from monocryptal dysplastic ACF to tumor formation, whereas these features were not observed in classical elevated ACF [23]. Moreover, flat ACF grows significantly faster than classical elevated ACF [23], further implying the close relationship between colon tumorigenesis and flat ACF instead of classical elevated ACF (Figure 1C). Given its association with the formation of flat ACF, AOM treatment is partially able to model the early stage of CRC development in mice.
As AOM alone can induce adenomas only, a novel transgenic mouse model with AOM treatment that can develop colon carcinoma to recapitulate sporadic CRC was recently established. CDX2P-Cre-Apc+/LoxP is first constructed, where the induction of CDX2P-Cre can help to heterozygously delete the Apc gene in mice colon epithelium. AOM (7.5 mg/kg body weight) is then injected into 10-week-old CDX2P-Cre-Apc+/LoxP mice, and these mice can develop 4–5 colon tumors at week 15 with no visible tumors in the small intestine [24]. Histopathological examination showed that colon tumors in these mice can range from well-differentiated adenomas to invasive adenocarcinomas and can exhibit many dominant characteristics of human colorectal adenocarcinomas such as increased nuclear-to-cytoplasmic ratio, heterochromatin and back-to-back gland formation, and tumor invasion into the muscularis mucosa. These observations therefore indicate that this newly established mouse model is capable of recapitulating human sporadic CRC.
2.4. Molecular Mechanisms of Carcinogen-Induced Colon Tumorigenesis
The activation of oncogenes and loss of tumor suppressor genes play important roles in human CRC development, as well as in carcinogen-induced tumor development. AOM-induced and AOM/DSS-induced colon tumorigenesis have similar mechanisms such as the activation of oncogenes (e.g., β-catenin) and inactivation of tumor suppressor genes (e.g., Apc and p53), probably due to the application of AOM in both models. Meanwhile, some reports showed the difference in molecular mechanisms between these two models, especially Kras and cyclooxygenase 2 (COX2). In general, each stage of carcinogen-induced colon tumorigenesis involves several functional pathways, for instance, Apc/β-catenin, Kras, c-Myc and global hypermethylation are activated in the early stage, while COX2 and iNOS are activated in the progression from adenoma to carcinoma [25] (Figure 2A).
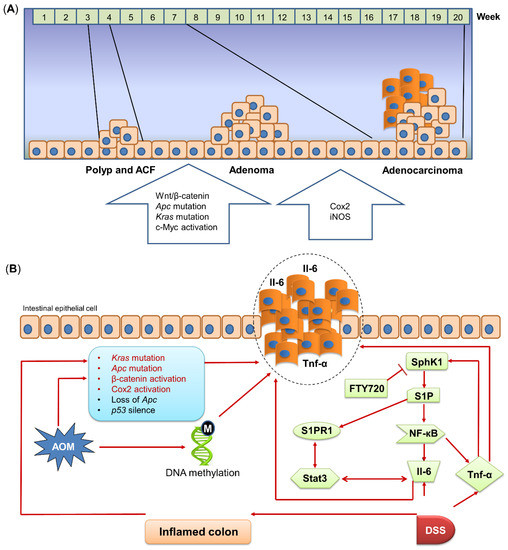
Figure 2.
Mechanism of AOM/DSS-induced mouse model. (A) Molecular alterations in different stages of AOM/DSS-induced colon tumorigenesis. AOM/DSS treatment induces the progression from polyps/ACFs and adenomas to adenocarcinomas. Different genes are correlated with each stage of AOM/DSS-induced tumorigenesis. (B) Mechanism of AOM/DSS-induced colon tumorigenesis. AOM induces DNA methylation and activates or mutates cancer-related pathways to initiate tumorigenesis. DSS treatment upregulates Il-6 and Tnf-α expressions in the colon. SphK1, which is induced by Tnf-α, drives the NF-кB/Stat3 pro-inflammatory pathway to induce inflammation and promote tumorigenesis. AOM, azoxymethane; Apc, adenomatous polyposis coli; COX2, cyclooxygenase 2; DSS, dextran sulfate sodium; Il-6, interleukin-6; iNOS, inducible nitric oxide synthase; Kras, Kirsten rat sarcoma viral oncogene homolog; NF-кB, nuclear factor kappa-light-chain-enhancer of activated B cells; p53, tumor protein p53; S1P, sphingosine-1-phosphate; S1PR1, sphingosine 1-phosphate receptor-1; SphK1, sphingosine kinase 1; Stat3, signal transducer and activator of transcription 3; Tnf-α, tumor necrosis factor-alpha; Wnt, wingless-related integration site.
KRAS is one of the most prominent proto-oncogenes in colon tumorigenesis belonging to the RAS family, and it controls various biological processes including survival, growth, proliferation, differentiation and apoptosis [26]. Activation of KRAS is associated with various oncogenic pathways including PI3K/AKT/mTOR signaling to promote proliferation and to suppress apoptosis of tumor cells [27]. In human CRC, 42.4% of patients involve KRAS mutation in codons 12 and 13 [28], while the frequency of Kras mutations in AOM-treated mise was reported to be 62% [29]. In contrast, the Kras mutation is not always present in the colon tumors of AOM/DSS-treated mice [30], consistent with the fact that Kras mutation less frequently occurs in colitis-associated colorectal tumors than sporadic tumors [31].
β-catenin, an intracellular signal transducer in the downstream of Wnt signaling, was found to be frequently activated in the colon of AOM-treated mice. β-catenin is involved in colon tumorigenesis under mutated or overexpressed status with a mutation in codons 33, 34, 37, 41, being G:C to A:T transitions in the tumors of AOM-treated mice [32]. β-catenin was also found to be activated in the colon of AOM/DSS-treated mice, while inhibition of β-catenin can protect the colon from AOM/DSS-induced tumorigenesis [25]. AOM can also cause β-catenin intracellular translocation from the cytoplasm to nucleus, leading to its activation [33]. These results collectively suggest that AOM and DSS induce hyperactivation of the oncogenic Wnt/β-catenin signaling, thereby accelerating colon tumorigenesis.
COX2 is crucial in regulating inflammatory response and lipid metabolism. It is well known that inflammation is a mediator of colon tumorigenesis [34], whereas altered lipid composition can disrupt intestinal epithelial barrier function [35]. Given its functional roles, COX2 can be associated with inflammation and lipid metabolism to contribute to colon tumorigenesis. It was reported that the COX2 mutation is undetected in the early stage of CRC, whereas in large colon adenocarcinoma, the incidence of COX2 mutation is around 80%, indicating that the COX2 mutation is pivotal in the late stage of CRC [36]. Ishikawa et al. found that COX2 is markedly increased in colon tumors, compared to the normal area of the colon in AOM/DSS-treated mice [37]. However, they also showed that COX2 is not required in CAC development, as the expression of COX2 is mainly located in tumor-infiltrating macrophages, fibroblasts, and endothelial cells instead of epithelial cells. Meanwhile, CAC can still be developed in COX2 knock-out mice after AOM/DSS treatment, thus demonstrating that COX2 mutation may not be essential for AOM/DSS-induced colon tumorigenesis. In comparison, although tumors in AOM-treated mice also have significantly overexpressed COX2, AOM treatment but without DSS administration fails to induce any colon tumors in COX2 knock-out mice [37]. These results indicate the distinct roles of COX2 in AOM/DSS-induced CAC and AOM-induced sporadic CRC, of which COX2 is only essential for AOM-induced colon tumorigenesis. Moreover, much more oncogenic pathways were reported to be activated after AOM or AOM/DSS administration. For example, AOM can enhance the activity of EGFR Tyr-k and proto-oncogene c-Myc to initiate colon carcinogenesis [9,38]. Other studies revealed that the Sphk1/S1P/S1PR1 axis plays a crucial role in CAC development, while using FTY720, a Sphk1 inhibitor, can significantly reduce colon tumorigenesis in AOM/DSS-treated mice [39,40,41] (Figure 2B).
In addition to oncogenes, AOM also affects tumor suppressor genes to promote colon tumorigenesis. In 80–90% of CRC patients, the initial step of tumorigenesis is the loss of tumor suppressor gene APC [9], while in mice, AOM induces Apc mutation, including downregulating its expression and shortening the length of Apc [42,43]. The loss of Apc was also observed in the colon tumor of mice given AOM/DSS. Kishimoto et al. demonstrated that inhibition of COX2 can increase the expression of Apc in the colon of rats treated with AOM/DSS, indicating an inverse correlation between Apc and inflammation [44]. These results suggest that the loss of Apc is associated with inflammation, a risk factor of colon tumorigenesis. The direct interplay between Apc and COX2 in AOM/DSS-induced CAC development remains unclear.
Another important tumor suppressor gene affected by AOM is p53, which has increased sensitivity to AOM under null status [45]. However, unlike Apc, the expression of p53 is increased after AOM treatment but with reduced activity [46]. Mechanistically, AOM-induced COX2 upregulation can interact with p53 to inhibit p53-dependent transcription [47]. Another study also revealed that COX2 products can cause p53 accumulation in the cytosol to inhibit p53-dependent apoptosis [48]. These results indicate that the suppressed activity of p53 is associated with COX2 upregulation induced by AOM (Figure 1C). Similarly, p53 was also found to be elevated in AOM/DSS-treated mice [49]. Since DSS alone cannot upregulate p53 [17], the increased expression of p53 may result from AOM treatment. Altogether, carcinogen treatment can induce molecular alterations at the genetic level, contributing to colon tumorigenesis and CAC development.
2.5. Gut Microbiota Is Pivotal for Carcinogen-Induced Colon Tumorigenesis
Recent studies showed that the gut microbiota play a pivotal role in AOM-induced CRC mouse models. Colon tumor formation in AOM-treated germ-free IL10−/− mice is greatly abrogated compared to conventional AOM-treated IL10−/− mice due to the impaired inflammatory response [50]. Our previous study also demonstrated that antibiotics administration markedly reduces AOM-induced colon tumor number in mice fed with a high-fat diet [51]. It is well known that inflammation is important for colon tumorigenesis [15]. Bacteria invasion into epithelial cells is essential for inflammation to occur, since antibiotics administration can inactivate ATF6 and STAT3 signaling due to the lack of bacteria invasion [52]. Furthermore, the microbial community within a colorectal neoplasia can be heterogenous, and such heterogeneity is associated with colon tumorigenesis [53]. Moreover, single-cell sequencing revealed the distinct signature of immune cells in different regions of colon tumors [54], which may be associated with the heterogeneous intratumoral microbiota. These findings indicate that bacteria-mediated inflammation is a key step in the initiation of AOM-induced colon tumorigenesis (Figure 1C).
3. Transgenic Mouse Model of Colorectal Cancer
In addition to the carcinogen-induced CRC mouse model, transgenic mice are also widely used to study colon tumorigenesis. In particular, as APC mutation occurs in over 90% of CRC patients, different transgenic mice have been established with Apc mutation being the centrality of these models. In this section, we explore the overall features of the Apc mutant mouse model as well as other transgenic mice for the study of spontaneous colon tumorigenesis.
3.1. Apc Mutant Mouse Model of Colorectal Cancer
As Apc mutation is considered to be the initial step in FAP progression, many mouse models have been developed to mimic FAP by carrying Apc mutation (Figure 3A). In 1990, the first Apc mutant mouse model was generated by mutagen N-ethyl-N-nitrosourea, leading to the mutant, multiple intestinal neoplasia (Min), which carries a truncation mutation at codon 850 [9,55]. Heterozygotic ApcMin/+ C57BL/6 mice can develop over 100 adenomas in the small intestine with a few adenomas developed in the colon [56]. ApcMin/+ mice have many genetic and phenotypic similarities to patients with FAP, with 90% identical in orthology. However, ApcMin/+ mice mostly develop intestinal adenomas in contrast to patients with FAP, which predominantly develop colon lesions. A histological study showed that colon tumors generated from the Apc mutation are benign adenomas [57]; hence, this model is suitable for studying the early stage of CRC instead of the late stage (Figure 3A). Using gene knock-out approaches, several Apc mutations in other loci have been generated. Mice carrying ApcΔ716, ApcΔ14 or Apc1638N, containing truncating mutations at codon 716, 14 or 1638, respectively, were shown to have varied tumor numbers, indicating the difference in sensitivity among different mutation loci of the Apc gene [9,38]. Apc1638N seems to be insensitive to Apc mutation, as mice carrying Apc1638N only develop a few tumors in the small intestine and no tumors in the colon, while tumor numbers in the small intestine and colon of mice carrying ApcΔ716 and ApcΔ14 are significantly higher. Nevertheless, although more mouse models targeting the Apc gene have been developed, ApcMin/+ remains the most commonly used transgenic mouse model of CRC [38].
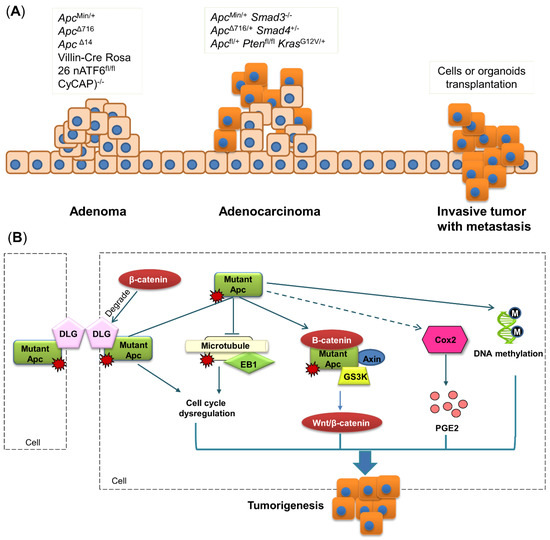
Figure 3.
Transgenic mouse models. (A) Schematic of gene mutation in each stage of colon tumorigenesis. Single gene mutation mostly induces the development of adenomas, while multiple gene mutations can promote the formation of adenocarcinomas. Metastasis is rarely observed in transgenic mouse models, and its appearance in mice requires transplantation of cancer cells or organoids. (B) Apc mutation results in the activation of a destruction complex consisting of β-catenin, Apc, Axin and GS3K. The EB1-microtubule complex and DLG are destructed by mutant Apc, causing cell-cycle dysregulation. Apc mutation also promotes DNA methylation and COX2 activation. All these alterations induced by Apc mutation can lead to colon tumorigenesis. Apc, adeno A recent study matous polyposis coli; COX2, cyclooxygenase 2; CyCAP, cyclophilin C-associated protein; DLG, discs-large; EB1, end-binding protein 1; GSK3, glycogen synthase kinase 3; Kras, Kirsten rat sarcoma viral oncogene homolog; nATF6, activating transcription factor 6; PGE2, prostaglandin E2; PTEN, phosphatase and tensin homolog; Wnt, wingless-related integration site.
Gene functional studies have identified numerous genes that impact CRC progression. The application of Cre-Loxp makes it possible to delete or overexpress a gene in a specific organ. By combining Cre-Loxp gene modification with ApcMin/+ mice, various mouse models with multiple genetic mutations can be established. LGR5, which is a G-protein coupled receptor binding R-spondin to enhance WNT signaling, was shown to be a marker of intestinal stem cells (ISCs) [58]. Using Cre-Loxp to delete Apc in mice Lgr5+ ISCs leads to rapid formation of intestinal adenomas, strongly indicating that Lgr5+ ISCs are the cells of origin for intestinal cancer. Moreover, ApcMin/+ and ApcΔ716 only induce mostly the formation of adenomas, but not invasive tumors, whereas mice carrying combined ApcMin/+ with Smad3−/− as well as ApcΔ716/+ with Smad4+/− can develop colon adenocarcinoma [59,60]. Another study explored the impact of p53 on colon tumor invasion by utilizing Cre-Loxp to generate Apcfl/+p53fl/+ and Apcfl/+p53R172H/+ mice, which show 25% and 100% stroma invasion, respectively [61]. These results therefore indicate the tumor-suppressive role of p53 as well as the diverse impacts of p53 with different mutant locus. For oncogene Kras in which its mutation occurs in early CRC, mice with both mutant KrasG12V/+ and Apc develop invasive intestinal carcinoma, while mice with mutant KrasG12V/+ alone need a much longer duration (>500 days) to develop carcinoma [62]. Collectively, these findings potentiate the importance of the ApcMin/+ mouse model in the study of colon tumorigenesis by demonstrating the initiative role Apc mutation in CRC development.
Recent studies show that the gut microbiota play pivotal roles in CRC initiation and progression. The enrichment of enterotoxigenic Bacteroides fragilis and colibactin-expressing Escherichia coli was observed in patients with FAP, which were also reported in ApcMin/+ mice [63,64], further indicating that the Apc mutant mouse model can accurately recapitulate human FAP. Meanwhile, ApcMin/+ mice fail to develop colon tumors under germ-free condition due to their abolished inflammatory response [65]. However, when these mice are transferred to a specific pathogen-free environment, colon tumors can be reintroduced. Hence, these findings indicate that tumor formation in ApcMin/+ mice needs the presence of an intact gut microbiota.
3.2. Molecular Mechanism of Apc Mutation-Induced Colon Tumorigenesis
Tumors in ApcMin+ mice are mainly adenomas that usually have either polypoid or crateriform appearance with compression or adjacent tissues. The mechanism of how Apc mutation induces the formation of colon adenoma has been explored for a long time. In general, the Apc gene has four binding sites: β-catenin binding site, end-binding protein 1 (EB1) binding site, microtubule binding site, and discs-large (DLG) binding site [66] (Figure 3B).
β-catenin is involved in the destruction complex together with APC, Axin and GS3K, and this complex is important for WNT/β-catenin signaling [9]. Normally, the destruction complex is inactive with β-catenin maintained at a low level, whereas alteration of this complex can lead to tumorigenesis [67]. Accumulated studies have revealed that β-catenin is activated with knock-out or mutant Apc [68], driving the increased level of β-catenin and promoting colon tumorigenesis (Figure 3B).
A recent study showed that EB1 expression is increased in human tumor samples, whereas APC expression in the same sample is reduced [69]. This inverse relationship between Apc and EB1 is also present in murine models, of which a previous study reported that EB1 expression is two-fold higher in rats containing the germline Apc mutation, compared to wild-type controls [70]. Mechanically, EB1 was identified to be associated with Apc carboxyl terminus [69]. The Apc-EB1 complex can connect microtubule spindles with kinetochores to regulate microtubule stability [69]. Given its involvement in cell cycle regulation, EB1 is considered to play a crucial role in colon tumorigenesis (Figure 3B).
DLG is located at cell–cell contacts where it acts as a scaffold to interact with other proteins [71]. DLG has been identified as a tumor suppressor essential for regulating cell polarity and proliferation [72]. DLG can form a complex with Apc, and this complex plays a crucial role in suppressing cell proliferation by blocking cell cycle progression from the G0/G1 to S phase [71], whereas these anti-tumorigenic activities can become weaker if Apc is mutated or truncated [71]. The ability of DLG to disturb the transformation of cell growth is also hampered by β-catenin. Subbaiah et al. found that overexpressed β-catenin drives tumorigenesis by enhancing DLG degradation, while ablated β-catenin can raise the stability of DLG [72]. These results collectively indicate that the altered activity of DLG induced by Apc mutation can contribute to colon tumorigenesis (Figure 3B).
Besides genetic modification, recent studies also reported that ApcMin/+ mice exhibit extensive aberrant DNA methylation in intestinal adenoma, which is a hallmark of CRC [73]. Homozygous mutation of Apc can lead to decreased DNA methylation at the promoters of genes implicated in intestinal cell fate specification, such as hoxd13a and pitx2, thereby contributing to CRC development [74]. The Apc mutation also affects the DNA demethylase system in mice, including cytidine deaminases Aid and Apobec2a, thymine glycosylase Mbd4, and DNA repair protein Gadd45α, whereas all these epigenetic alterations were observed in human adenoma with germline Apc mutation [75]. Meanwhile, the Apc mutation can alter the gut microbiota, and furthermore, retinoic acid is metabolite derived from gut bacteria [76,77]. Hence, it is possible that mutant Apc alters the gut microbiota and their metabolites to modulate DNA methylation. Taken together, these results indicate that Apc loss can induce DNA methylation to facilitate the initiation of CRC development (Figure 3B).
3.3. Cre-Loxp-Based Mouse Models of Colorectal Cancer
Since the establishment of Cre-Loxp technology in the 1990s, deleting any genes in any tissue of interest has become plausible, of which Cre can mediate the recombination of Loxp sites. To date, tamoxifen-inducible Cre-ERT2 is the most commonly used approach to generate conditional knock-out or knock-in mice. Generally, mice carrying tissue-specific inducible Cre-ERT2 is inter-crossed with mice carrying the target gene, which is flanked by Loxp recombination sites (Figure 4). The Cre enzyme is then activated by tamoxifen administration, resulting in conditional addition of the target gene in specific tissue [78]. This technology has been frequently applied to mouse models as aforementioned. Mice carrying the knock-out or knock-in gene of interest can be treated with AOM or AOM/DSS or can be inbred with ApcMin/+ mice to determine the effect of a specific gene in CRC development and therapeutics.
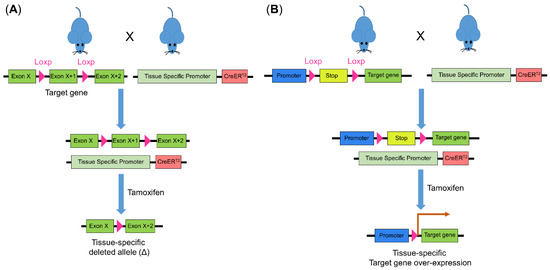
Figure 4.
Cre-Loxp recombination system for genetic modulation in mice. The introduction of Loxp to flank the target gene creates a conditional knock-out prerequisite. Mice with the introduced Loxp is inter-crossed with mice carrying Cre whose promoter is tissue specific. Using tamoxifen, Cre is activated and (A) leads to target gene deletion or (B) deletion of the stop motif and subsequent overexpression of the target gene. Cre, Cre recombinase.
ATF6 is a basic leucine zipper transcription factor belonging to the CREB and ATF family of transcription factors [79]. Upon endoplasmic reticulum stress, ATF6 induces the transcription of genes encoding chaperones and enzymes that facilitate protein folding and maturation. Previously, ATF6 activation was reported in CRC patients [80], but its exact role in CRC development is still unclear. To address this question, a Villin-Cre Rosa 26 nATF6fl/fl mouse model was established with the overexpression of activated ATF6 specifically in intestinal epithelial cells [52]. These mice spontaneously develop adenomas in the large intestine within 12 weeks. Similar to AOM-treated and ApcMin/+ mice, tumor formation in this transgenic mouse model also needs the presence of an intact gut microbiota [52]. In addition, CyCAP is a widely expressed secreted glycoprotein that modulates host response to bacterial endotoxins [81]. A previous study identified that CyCAP is a murine orthologue of the TAA90K/Mac-2-binding protein, which can suppress endotoxin signaling in the colon mucosa of mice [82]. In contrast, CyCAP−/− mice spontaneously develop colon mucosal hyperplasia within 8 weeks, indicating that this transgenic mouse model is suitable to study the early stage of colon tumorigenesis.
4. Colorectal Cancer Metastasis Mouse Models
Metastasis is a main cause of death for patients with CRC; hence, utilizing mouse models to recapitulate the clinical characteristics is crucial for studying the underlying mechanism and for developing effective treatment against metastasis. Although substantial CRC mouse models have been established, models that can develop features of metastasis remain rare. Here, several mouse models of metastasis are discussed.
4.1. Cell and Organoid Xenotransplantation
The xenograft model refers to the injection of cancer cell lines into mice. Xenograft transplantation readily induces tumor invasion and metastasis, which depends on the site of inoculation. Subcutaneous injection of cancer cells is commonly used in xenograft models due to its convenience and high success rate to induce tumor formation, yet it fails to produce metastasis, while orthotopic injection of CRC cells into specific organs such as caecum, tail vein, and spleen can lead to metastasis in liver, lung, and bones [83]. In general, to evaluate metastasis, human cells with ectopic expression of an oncogene are injected to nude mice or mice with severe combined immunodeficiency (SCID). For example, a previous study injected the CRC cell line HCT116 with ectopic RNF6 expression into nude mice and reported a 100% incidence rate of lung metastasis in these xenograft mice [84], indicating that RNF6 can promote the invasion and migration of CRC cells. Notably, a major problem of xenograft models is the incomplete tumor microenvironment, of which the tumor barrier such as the basement membrane is lacking in xenograft mice. Moreover, as nude and SCID mice are immunocompromised, the xenograft model with the injection of cancer cell lines cannot accurately recapitulate metastasis in human patients.
To overcome the problems of xenograft mice with cell transplantation, another patient-derived orthotopic xenograft (PDOX) model has been recently established [85]. Tumor cells are first extracted from patients undergoing resection for colorectal adenocarcinoma and are tagged by luciferase. The collected luciferase-tagged tumor cells are then inoculated with stroma cells to physiologically mimic the tumor microenvironment, followed by being orthotopically injected into the rectum of male NOD/SCID mice. As tumor cells are tagged by luciferase, metastasis can be monitored by an imaging machine. Upon sacrifice, tumors were detected in 96.9% of PDOX mice. Histological examination demonstrated a similarity of architecture between xenografts and the originated tumors in patients, together with the occurrence (53.1%) of metastases in the liver and lung. Importantly, the mortality of PDOX mice was reported to be 0%, indicating that PDOX is a safe and efficient mouse model to recapitulate CRC metastasis.
Currently, the PDOX mouse model has been widely applied in the evaluation of cancer drug efficiency. For example, using PDOX mice, the combination of temozolomide, pazopanib and FOLFOX (oxaliplatin, leucovorin and 5-fluorouracil) was reported to be an effective treatment for CRC through inhibiting lymphangiogenesis [86]. However, the long establishment period of the PDOX model has been an obstacle for its utilization. PDOX mice also lack stromal compounds, including fibroblasts and blood vessels, resulting in the absence of interaction between tumor stroma and the tumor microenvironment [38]. To address these issues, a modified model was recently developed by first co-transplanting patient-derived organoids with endothelial colony-forming cells (ECFC) into NRGA-immunodeficient mice (PDOXwE), which was then sub-cultured in Balb/c mice [87]. PDOXwE mice have fast tumor growth, meanwhile maintaining the primary characteristics of patient-derived organoids. The formation of new blood vessels was also observed in PDOXwE mice. Mechanistically, ECFCs are circulating endothelial progenitor cells contributing to neovascularization in several pathophysiological conditions [88]. These findings thus indicate that the PDOXwE model can recapitulate the tumor microenvironment more accurately than common PDOX mouse models. Additionally, similar sensitivity to chemotherapy drugs between PDOXwE and PDO mice was also observed [87], implying the potential of these mouse models as important tools for the development of individualized therapy. Nevertheless, it is noteworthy to highlight that although genotypic characteristics of the primary tumor in patients are highly preserved in PDOX mice, changes in copy number can still occur after xenotransplantation [38]. Hence, before the popularization of using a PDOX mouse model for drug screening, further investigations are necessary to confirm the consistency between the PDOX result and clinical patient response.
In general, the above models greatly facilitate the investigation of genes that contribute to CRC development and metastasis as well as the selection of most appropriate therapeutic options. However, several limitations still remain to be solved. For instance, the gut microbiota plays a pivotal role in CRC progression, and accumulated evidence has reported the importance of the microbiota in cancer metastasis [89]. Although orthotopic injection of organoids into mice has provided new approach for the study of metastasis, it is still different from the actual metastasis that occurs in human. Extensive studies are required to advance organoid cultures to include more components of the tumor microenvironment, such as immune cells, stroma cells, and intratumoral microbes, thereby establishing organoid models that can more accurately recapitulate the metastatic conditions in human.
4.2. Transgenic Mouse Model of Metastasis
In addition to transplantation mouse models, metastasis can also be induced in transgenic mice, although the latency is long with low penetrance. These transgenic mouse models are mainly characterized by hyperplastic lesions and serrated histology of intestinal epithelium [55]. It was reported that heterozygous deletion of BrafLSL–V637E/+ leads to full progression from serrated hyperplasia, adenoma, and finally to metastatic carcinoma [90]. However, the latency of this transgenic model is long, and the metastasis rate is only 20%. Metastasis was also observed in the Tp53R172H/+ mouse model, yet again, these transgenic mice exhibit long latency with a low metastasis rate [55,90], whereas another study developed a mouse model with KrasG12V/+ and Ptenfl/fl deletion, and these mice have a higher metastasis rate (41%) compared to other transgenic mouse models; however, the latency is still long [91]. Altogether, unless major breakthroughs are achieved, transgenic mouse models remain a minor alternative of xenotransplantation in the study of metastasis.
5. Conclusions and Future Perspectives
Mouse models have been used as robust tools in the study of CRC for over 40 years. For example, the AOM-induced CRC mouse model can recapitulate sporadic CRC with many similar features as observed in CRC patients. However, most of the current CRC mouse models can only induce the formation of adenomas but not adenocarcinomas in the distal colon. Given that invasive adenocarcinoma is the main cause of death in CRC patients, it is urgent to develop and popularize mouse models that can develop adenocarcinoma for preclinical studies. Fortunately, advances in Cre-Loxp technology has provided many new opportunities. The CDX2P-Cre-Apc+/LoxP mouse model with AOM treatment was recently established, which can develop colon adenocarcinomas with similar histopathological characteristics as in human sporadic CRC. However, a major downside of this mouse model is the deletion of the Apc gene in colon epithelial cells; thus, the development of sporadic CRC cannot be achieved. The next goal of researchers should focus on developing mouse models that can recapitulate sporadic human CRC, thereby allowing more accurate evaluation of drug treatment.
Apc mutation has been identified in the initial step of colon tumorigenesis, and many CRC mouse models have been established with the Apc mutation being the centrality of these models. To date, a variety of transgenic mouse models that target different loci of Apc have been developed. However, tumors developed in these models are mostly located in the small intestine instead of the colon. As we all know, tumors in CRC patients are actually located in the colon. and tumorigenesis in human small intestines is rare. Hence, it is important to develop transgenic mice with specific colon tumorigenesis. This would allow for more precise recapitulation of colon tumorigenesis in humans, further facilitating the impact evaluation of drugs and other treatments in CRC patients.
One of the major goals of mouse models is to recapitulate the pathology of human diseases in order to produce accurate models for testing the efficacy and safety of treatments. In the late stage of human CRC, metastasis frequently occurs, leading to high mortality and recurrence. It is therefore urgent to understand the mechanism of metastasis, thereby developing treatments against metastasis for clinical benefits. Currently, the most commonly used mouse model of metastasis is established by transplantation or orthotopic injection of CRC cells or organoids into nude or SCID mice. Although metastases are developed in these models, they cannot accurately represent the real metastasis that occurs in humans due to the lack of multiple components such as immune cells of the tumor microenvironment. Further studies are necessary to establish mouse models that can accurately recapitulate human metastasis, thus facilitating accurate evaluation of drug treatment.
In summary, with these advanced mouse models, improved mechanistic understanding of colon tumorigenesis can be achieved, thereby providing valuable preclinical findings that can be potentially translated into clinical benefits for CRC patients. Nevertheless, the current mouse models still need to be improved to more precisely mimic human CRC.
Author Contributions
C.L. collected the data and drafted the manuscript; H.C.-H.L. and X.Z. discussed the content and revised the manuscript; J.Y. supervised and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by RGC Research Impact Fund Hong Kong (R4632-21F), RGC-CRF Hong Kong (C4039-19GF and C7065-18GF), RGC-GRF Hong Kong (14110819, 14111621), and Vice-Chancellor’s Discretionary Fund CUHK. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, I.H.; Kim, J.S.; Kim, S.W.; Kim, J.G.; Oh, S.T.; Kang, W.K.; Lee, M.A. Different clinical characteristics in sporadic young-age onset colorectal cancer. Medicine 2016, 95, e4840. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M. Genetic testing for high-risk colon cancer patients. Gastroenterology 2003, 124, 1574–1594. [Google Scholar] [CrossRef]
- Aoki, K.; Taketo, M.M. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J. Cell Sci. 2007, 120, 3327–3335. [Google Scholar] [CrossRef]
- Rosenberg, D.W.; Giardina, C.; Tanaka, T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 2009, 30, 183–196. [Google Scholar] [CrossRef]
- Normanno, N.; Tejpar, S.; Morgillo, F.; De Luca, A.; Van Cutsem, E.; Ciardiello, F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat. Rev. Clin. Oncol. 2009, 6, 519–527. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.F. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol. Ther. 2009, 8, 1313–1317. [Google Scholar] [CrossRef]
- Stastna, M.; Janeckova, L.; Hrckulak, D.; Kriz, V.; Korinek, V. Human Colorectal Cancer from the Perspective of Mouse Models. Genes 2019, 10, 788. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef]
- Diergaarde, B.; Braam, H.; Vasen, H.F.; Nagengast, F.M.; van Muijen, G.N.; Kok, F.J.; Kampman, E. Environmental factors and colorectal tumor risk in individuals with hereditary nonpolyposis colorectal cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2007, 5, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, A.; Shank, R.C.; Delker, D.A.; Povey, A.; Cooper, D.P.; Rosenberg, D.W. Initial levels of azoxymethane-induced DNA methyl adducts are not predictive of tumor susceptibility in inbred mice. Toxicol. Appl. Pharmacol. 1998, 150, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Guda, K.; Cui, H.; Garg, S.; Dong, M.; Nambiar, P.R.; Achenie, L.E.; Rosenberg, D.W. Multistage gene expression profiling in a differentially susceptible mouse colon cancer model. Cancer Lett. 2003, 191, 17–25. [Google Scholar] [CrossRef]
- Davies, G.R.; Rampton, D.S. Eicosanoids: Role in gastrointestinal inflammation and cancer. Eur. J. Gastroenterol. Hepatol. 1997, 9, 1033–1044. [Google Scholar] [CrossRef]
- Wilson, J.E.; Petrucelli, A.S.; Chen, L.; Koblansky, A.A.; Truax, A.D.; Oyama, Y.; Rogers, A.B.; Brickey, W.J.; Wang, Y.; Schneider, M.; et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat. Med. 2015, 21, 906–913. [Google Scholar] [CrossRef]
- Gupta, J.; del Barco Barrantes, I.; Igea, A.; Sakellariou, S.; Pateras, I.S.; Gorgoulis, V.G.; Nebreda, A.R. Dual function of p38alpha MAPK in colon cancer: Suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 2014, 25, 484–500. [Google Scholar] [CrossRef]
- Bissahoyo, A.; Pearsall, R.S.; Hanlon, K.; Amann, V.; Hicks, D.; Godfrey, V.L.; Threadgill, D.W. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice: Effects of dose, route, and diet. Toxicol. Sci. 2005, 88, 340–345. [Google Scholar] [CrossRef]
- Nambiar, P.R.; Girnun, G.; Lillo, N.A.; Guda, K.; Whiteley, H.E.; Rosenberg, D.W. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int. J. Oncol. 2003, 22, 145–150. [Google Scholar] [CrossRef]
- Suzuki, R.; Kohno, H.; Sugie, S.; Nakagama, H.; Tanaka, T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis 2006, 27, 162–169. [Google Scholar] [CrossRef]
- Snider, A.J.; Bialkowska, A.B.; Ghaleb, A.M.; Yang, V.W.; Obeid, L.M.; Hannun, Y.A. Murine Model for Colitis-Associated Cancer of the Colon. Methods Mol. Biol. 2016, 1438, 245–254. [Google Scholar] [PubMed]
- Wargovich, M.J.; Brown, V.R.; Morris, J. Aberrant crypt foci: The case for inclusion as a biomarker for colon cancer. Cancers 2010, 2, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, J.E.; Loberg, E.M.; Olstorn, H.B.; Knutsen, H.; Steffensen, I.L.; Alexander, J. Flat dysplastic aberrant crypt foci are related to tumorigenesis in the colon of azoxymethane-treated rat. Cancer Res. 2005, 65, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Souris, J.S.; Zhang, H.J.; Dougherty, U.; Chen, N.T.; Waller, J.V.; Lo, L.W.; Hart, J.; Chen, C.T.; Bissonnette, M. A novel mouse model of sporadic colon cancer induced by combination of conditional Apc genes and chemical carcinogen in the absence of Cre recombinase. Carcinogenesis 2019, 40, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Garrel, S.; Gerber, M.; Maitra, R.; Goel, S. Therapeutic Targets of KRAS in Colorectal Cancer. Cancers 2021, 13, 6233. [Google Scholar] [CrossRef]
- Vaughn, C.P.; Zobell, S.D.; Furtado, L.V.; Baker, C.L.; Samowitz, W.S. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer 2011, 50, 307–312. [Google Scholar] [CrossRef]
- Erdman, S.H.; Wu, H.D.; Hixson, L.J.; Ahnen, D.J.; Gerner, E.W. Assessment of mutations in Ki-ras and p53 in colon cancers from azoxymethane- and dimethylhydrazine-treated rats. Mol. Carcinog. 1997, 19, 137–144. [Google Scholar] [CrossRef]
- Pan, Q.; Lou, X.; Zhang, J.; Zhu, Y.; Li, F.; Shan, Q.; Chen, X.; Xie, Y.; Su, S.; Wei, H.; et al. Genomic variants in mouse model induced by azoxymethane and dextran sodium sulfate improperly mimic human colorectal cancer. Sci. Rep. 2017, 7, 25. [Google Scholar] [CrossRef]
- Saraggi, D.; Fassan, M.; Mescoli, C.; Scarpa, M.; Valeri, N.; Michielan, A.; D’Inca, R.; Rugge, M. The molecular landscape of colitis-associated carcinogenesis. Dig. Liver Dis. 2017, 49, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, H.; Watanabe, M.; Ushijima, T.; Toyota, M.; Imai, K.; Weisburger, J.H.; Sugimura, T.; Nagao, M. Specific 5′-GGGA-3′-->5′-GGA-3′ mutation of the Apc gene in rat colon tumors induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Proc. Natl. Acad. Sci. USA 1995, 92, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Guda, K.; Upender, M.B.; Belinsky, G.; Flynn, C.; Nakanishi, M.; Marino, J.N.; Ried, T.; Rosenberg, D.W. Carcinogen-induced colon tumors in mice are chromosomally stable and are characterized by low-level microsatellite instability. Oncogene 2004, 23, 3813–3821. [Google Scholar] [CrossRef][Green Version]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, M.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; Li, J. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin. Immunol. 2008, 126, 67–80. [Google Scholar] [CrossRef]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Ishikawa, T.O.; Herschman, H.R. Tumor formation in a mouse model of colitis-associated colon cancer does not require COX-1 or COX-2 expression. Carcinogenesis 2010, 31, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Burtin, F.; Mullins, C.S.; Linnebacher, M. Mouse models of colorectal cancer: Past, present and future perspectives. World J. Gastroenterol. 2020, 26, 1394–1426. [Google Scholar] [CrossRef]
- Xu, R.; Jin, J.; Hu, W.; Sun, W.; Bielawski, J.; Szulc, Z.; Taha, T.; Obeid, L.M.; Mao, C. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1813–1825. [Google Scholar] [CrossRef]
- Liang, J.; Nagahashi, M.; Kim, E.Y.; Harikumar, K.B.; Yamada, A.; Huang, W.C.; Hait, N.C.; Allegood, J.C.; Price, M.M.; Avni, D.; et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013, 23, 107–120. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Maltzman, T.; Whittington, J.; Driggers, L.; Stephens, J.; Ahnen, D. AOM-induced mouse colon tumors do not express full-length APC protein. Carcinogenesis 1997, 18, 2435–2439. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Takata, N.; Jinnai, T.; Morisawa, T.; Shiota, G.; Kawasaki, H.; Hasegawa, J. Sulindac and a cyclooxygenase-2 inhibitor, etodolac, increase APC mRNA in the colon of rats treated with azoxymethane. Gut 2000, 47, 812–819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kishimoto, Y.; Yashima, K.; Morisawa, T.; Ohishi, T.; Marumoto, A.; Sano, A.; Idobe-Fujii, Y.; Miura, N.; Shiota, G.; Murawaki, Y.; et al. Effects of long-term administration of sulindac on APC mRNA and apoptosis in colons of rats treated with azoxymethane. J. Cancer Res. Clin. Oncol. 2002, 128, 589–595. [Google Scholar] [PubMed]
- Mladenova, D.; Daniel, J.J.; Dahlstrom, J.E.; Bean, E.; Gupta, R.; Pickford, R.; Currey, N.; Musgrove, E.A.; Kohonen-Corish, M.R. The NSAID sulindac is chemopreventive in the mouse distal colon but carcinogenic in the proximal colon. Gut 2011, 60, 350–360. [Google Scholar] [CrossRef]
- Nambiar, P.R.; Giardina, C.; Guda, K.; Aizu, W.; Raja, R.; Rosenberg, D.W. Role of the alternating reading frame (P19)-p53 pathway in an in vivo murine colon tumor model. Cancer Res. 2002, 62, 3667–3674. [Google Scholar]
- Entezari Heravi, R.; Hadizadeh, F.; Sankian, M.; Tavakol Afshari, J.; Taghdisi, S.M.; Jafarian, H.; Behravan, J. Novel selective Cox-2 inhibitors induce apoptosis in Caco-2 colorectal carcinoma cell line. Eur. J. Pharm. Sci. 2011, 44, 479–486. [Google Scholar] [CrossRef]
- Shao, J.; Fujiwara, T.; Kadowaki, Y.; Fukazawa, T.; Waku, T.; Itoshima, T.; Yamatsuji, T.; Nishizaki, M.; Roth, J.A.; Tanaka, N. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene 2000, 19, 726–736. [Google Scholar] [CrossRef]
- Liang, X.; Li, H.; Tian, G.; Li, S. Dynamic microbe and molecule networks in a mouse model of colitis-associated colorectal cancer. Sci. Rep. 2014, 4, 4985. [Google Scholar] [CrossRef]
- Uronis, J.M.; Muhlbauer, M.; Herfarth, H.H.; Rubinas, T.C.; Jones, G.S.; Jobin, C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE 2009, 4, e6026. [Google Scholar] [CrossRef]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef] [PubMed]
- Coleman, O.I.; Lobner, E.M.; Bierwirth, S.; Sorbie, A.; Waldschmitt, N.; Rath, E.; Berger, E.; Lagkouvardos, I.; Clavel, T.; McCoy, K.D.; et al. Activated ATF6 Induces Intestinal Dysbiosis and Innate Immune Response to Promote Colorectal Tumorigenesis. Gastroenterology 2018, 155, 1539–1552.e12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X.; Xu, H.; Li, S.; Lau, H.C.; Chen, Q.; Zhang, B.; Zhao, L.; Chen, H.; Sung, J.J.; et al. Microbial Community Heterogeneity Within Colorectal Neoplasia and its Correlation With Colorectal Carcinogenesis. Gastroenterology 2021, 160, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Roerink, S.F.; Sasaki, N.; Lee-Six, H.; Young, M.D.; Alexandrov, L.B.; Behjati, S.; Mitchell, T.J.; Grossmann, S.; Lightfoot, H.; Egan, D.A.; et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 2018, 556, 457–462. [Google Scholar] [CrossRef]
- Jackstadt, R.; Sansom, O.J. Mouse models of intestinal cancer. J. Pathol. 2016, 238, 141–151. [Google Scholar] [CrossRef]
- Moser, A.R.; Luongo, C.; Gould, K.A.; McNeley, M.K.; Shoemaker, A.R.; Dove, W.F. ApcMin: A mouse model for intestinal and mammary tumorigenesis. Eur. J. Cancer 1995, 31A, 1061–1064. [Google Scholar] [CrossRef]
- Kwong, L.N.; Dove, W.F. APC and its modifiers in colon cancer. Adv. Exp. Med. Biol. 2009, 656, 85–106. [Google Scholar]
- Zhou, X.; Geng, L.; Wang, D.; Yi, H.; Talmon, G.; Wang, J. R-Spondin1/LGR5 Activates TGFbeta Signaling and Suppresses Colon Cancer Metastasis. Cancer Res. 2017, 77, 6589–6602. [Google Scholar] [CrossRef]
- Takaku, K.; Oshima, M.; Miyoshi, H.; Matsui, M.; Seldin, M.F.; Taketo, M.M. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell 1998, 92, 645–656. [Google Scholar] [CrossRef]
- Sodir, N.M.; Chen, X.; Park, R.; Nickel, A.E.; Conti, P.S.; Moats, R.; Bading, J.R.; Shibata, D.; Laird, P.W. Smad3 deficiency promotes tumorigenesis in the distal colon of ApcMin/+ mice. Cancer Res. 2006, 66, 8430–8438. [Google Scholar] [CrossRef]
- Muller, P.A.; Caswell, P.T.; Doyle, B.; Iwanicki, M.P.; Tan, E.H.; Karim, S.; Lukashchuk, N.; Gillespie, D.A.; Ludwig, R.L.; Gosselin, P.; et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009, 139, 1327–1341. [Google Scholar] [CrossRef]
- Sansom, O.J.; Meniel, V.; Wilkins, J.A.; Cole, A.M.; Oien, K.A.; Marsh, V.; Jamieson, T.J.; Guerra, C.; Ashton, G.H.; Barbacid, M.; et al. Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 14122–14127. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Tomkovich, S.; Yang, Y.; Winglee, K.; Gauthier, J.; Muhlbauer, M.; Sun, X.; Mohamadzadeh, M.; Liu, X.; Martin, P.; Wang, G.P.; et al. Locoregional Effects of Microbiota in a Preclinical Model of Colon Carcinogenesis. Cancer Res. 2017, 77, 2620–2632. [Google Scholar] [CrossRef]
- Qian, J.; Sarnaik, A.A.; Bonney, T.M.; Keirsey, J.; Combs, K.A.; Steigerwald, K.; Acharya, S.; Behbehani, G.K.; Barton, M.C.; Lowy, A.M.; et al. The APC tumor suppressor inhibits DNA replication by directly binding to DNA via its carboxyl terminus. Gastroenterology 2008, 135, 152–162. [Google Scholar] [CrossRef]
- Kimelman, D.; Xu, W. beta-catenin destruction complex: Insights and questions from a structural perspective. Oncogene 2006, 25, 7482–7491. [Google Scholar] [CrossRef]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Nakamura, M.; Zhou, X.Z.; Lu, K.P. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr. Biol. 2001, 11, 1062–1067. [Google Scholar] [CrossRef]
- Stypula-Cyrus, Y.; Mutyal, N.N.; Dela Cruz, M.; Kunte, D.P.; Radosevich, A.J.; Wali, R.; Roy, H.K.; Backman, V. End-binding protein 1 (EB1) up-regulation is an early event in colorectal carcinogenesis. FEBS Lett. 2014, 588, 829–835. [Google Scholar] [CrossRef]
- Ishidate, T.; Matsumine, A.; Toyoshima, K.; Akiyama, T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene 2000, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, V.K.; Narayan, N.; Massimi, P.; Banks, L. Regulation of the DLG tumor suppressor by beta-catenin. Int. J. Cancer 2012, 131, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lee, J.H.; Shu, L.; Huang, Y.; Li, W.; Zhang, C.; Yang, A.Y.; Boyanapalli, S.S.; Perekatt, A.; Hart, R.P.; et al. Association of aberrant DNA methylation in Apc(min/+) mice with the epithelial-mesenchymal transition and Wnt/beta-catenin pathways: Genome-wide analysis using MeDIP-seq. Cell Biosci. 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rai, K.; Sarkar, S.; Broadbent, T.J.; Voas, M.; Grossmann, K.F.; Nadauld, L.D.; Dehghanizadeh, S.; Hagos, F.T.; Li, Y.; Toth, R.K.; et al. DNA demethylase activity maintains intestinal cells in an undifferentiated state following loss of APC. Cell 2010, 142, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Schafer, A.; Marhold, J.; Stach, D.; Swaminathan, S.K.; Handa, V.; Doderlein, G.; Maltry, N.; Wu, W.; Lyko, F.; et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007, 445, 671–675. [Google Scholar] [CrossRef]
- Woo, V.; Eshleman, E.M.; Hashimoto-Hill, S.; Whitt, J.; Wu, S.E.; Engleman, L.; Rice, T.; Karns, R.; Qualls, J.E.; Haslam, D.B.; et al. Commensal segmented filamentous bacteria-derived retinoic acid primes host defense to intestinal infection. Cell Host Microbe 2021, 29, 1744–1756.e5. [Google Scholar] [CrossRef]
- Son, J.S.; Khair, S.; Pettet, D.W., 3rd; Ouyang, N.; Tian, X.; Zhang, Y.; Zhu, W.; Mackenzie, G.G.; Robertson, C.E.; Ir, D.; et al. Altered Interactions between the Gut Microbiome and Colonic Mucosa Precede Polyposis in APCMin/+ Mice. PLoS ONE 2015, 10, e0127985. [Google Scholar] [CrossRef]
- Jager, R.; Maurer, J.; Jacob, A.; Schorle, H. Cell type-specific conditional regulation of the c-myc proto-oncogene by combining Cre/loxP recombination and tamoxifen-mediated activation. Genesis 2004, 38, 145–150. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef]
- Hanaoka, M.; Ishikawa, T.; Ishiguro, M.; Tokura, M.; Yamauchi, S.; Kikuchi, A.; Uetake, H.; Yasuno, M.; Kawano, T. Expression of ATF6 as a marker of pre-cancerous atypical change in ulcerative colitis-associated colorectal cancer: A potential role in the management of dysplasia. J. Gastroenterol. 2018, 53, 631–641. [Google Scholar] [CrossRef]
- Trahey, M.; Weissman, I.L. Cyclophilin C-associated protein: A normal secreted glycoprotein that down-modulates endotoxin and proinflammatory responses in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Torlakovic, E.E.; Keeler, V.; Wang, C.; Lim, H.J.; Lining, L.A.; Laferte, S. Cyclophilin C-associated protein (CyCAP) knock-out mice spontaneously develop colonic mucosal hyperplasia and exaggerated tumorigenesis after treatment with carcinogen azoxymethane. BMC Cancer 2009, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Davis, C.; Ryan, J.; Janney, C.; Pena, M.M. Development and characterization of a reliable mouse model of colorectal cancer metastasis to the liver. Clin. Exp. Metastasis 2013, 30, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Wong, C.C.; Zhang, J.; Dong, Y.; Li, X.; Kang, W.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. RNF6 Promotes Colorectal Cancer by Activating the Wnt/beta-Catenin Pathway via Ubiquitination of TLE3. Cancer Res. 2018, 78, 1958–1971. [Google Scholar] [CrossRef]
- Moret, R.; Hellmers, L.; Zhang, X.; Gills, J.; Hite, N.; Klinger, A.; Maresh, G.A.; Canter, D.; Bardot, S.; Margolin, D.A.; et al. Patient-derived Orthotopic Xenograft Models for Human Urothelial Cell Carcinoma and Colorectal Cancer Tumor Growth and Spontaneous Metastasis. J. Vis. Exp. 2019, 147, e5922. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, M.; Han, Q.; Tan, Y.; Sun, Y.; Bouvet, M.; Clary, B.; Singh, S.R.; Ye, J.; Hoffman, R.M. Temozolomide and Pazopanib Combined with FOLFOX Regressed a Primary Colorectal Cancer in a Patient-derived Orthotopic Xenograft Mouse Model. Transl. Oncol. 2020, 13, 100739. [Google Scholar] [CrossRef]
- Kwon, J.; Oh, S.; Park, M.; Kong, J.S.; Lee, S.; Lee, H.; Kim, Y.; Kang, K.T.; Shin, U.S.; Jung, J. Advanced Xenograft Model with Cotransplantation of Patient-Derived Organoids and Endothelial Colony-Forming Cells for Precision Medicine. J. Oncol. 2021, 2021, 9994535. [Google Scholar] [CrossRef]
- Kang, K.T.; Lin, R.Z.; Kuppermann, D.; Melero-Martin, J.M.; Bischoff, J. Endothelial colony forming cells and mesenchymal progenitor cells form blood vessels and increase blood flow in ischemic muscle. Sci. Rep. 2017, 7, 770. [Google Scholar] [CrossRef]
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef]
- Rad, R.; Cadinanos, J.; Rad, L.; Varela, I.; Strong, A.; Kriegl, L.; Constantino-Casas, F.; Eser, S.; Hieber, M.; Seidler, B.; et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer Cell 2013, 24, 15–29. [Google Scholar] [CrossRef]
- Davies, E.J.; Marsh Durban, V.; Meniel, V.; Williams, G.T.; Clarke, A.R. PTEN loss and KRAS activation leads to the formation of serrated adenomas and metastatic carcinoma in the mouse intestine. J. Pathol. 2014, 233, 27–38. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).