Mutation of Proteolipid Protein 1 Gene: From Severe Hypomyelinating Leukodystrophy to Inherited Spastic Paraplegia
Abstract
1. Introduction
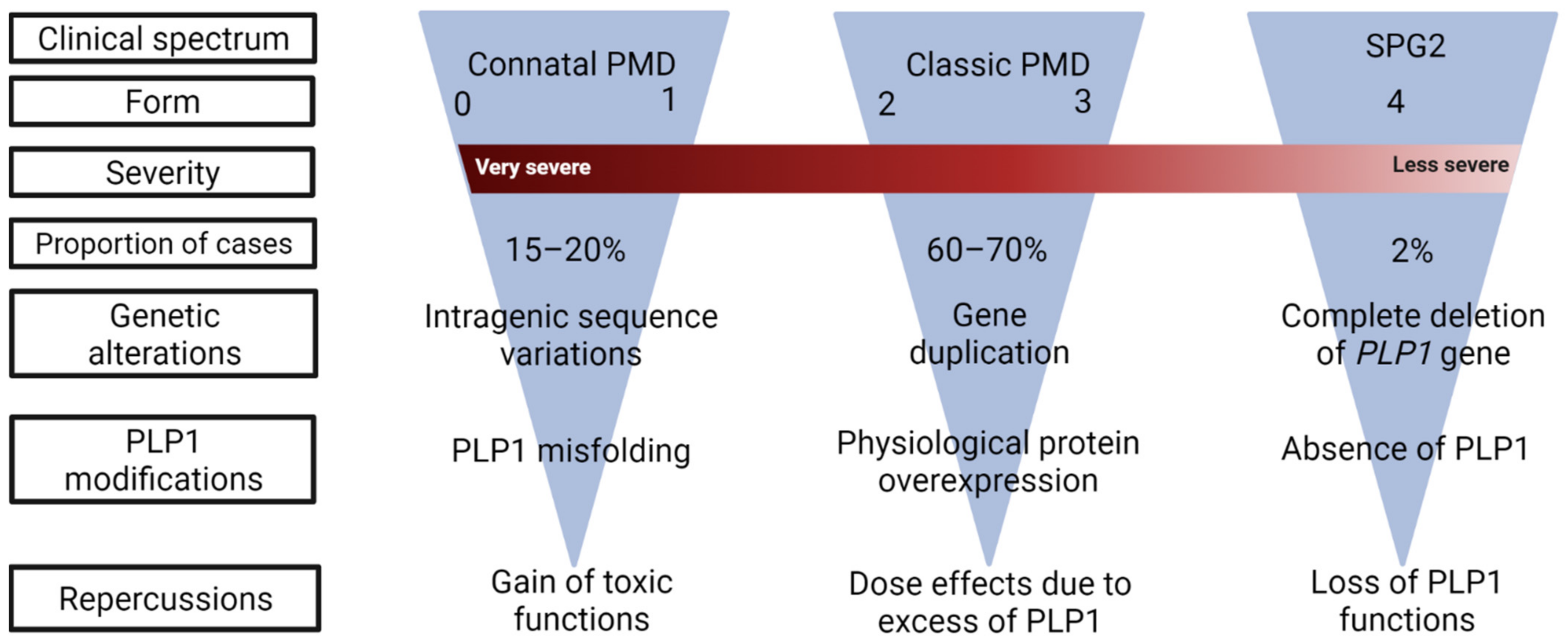
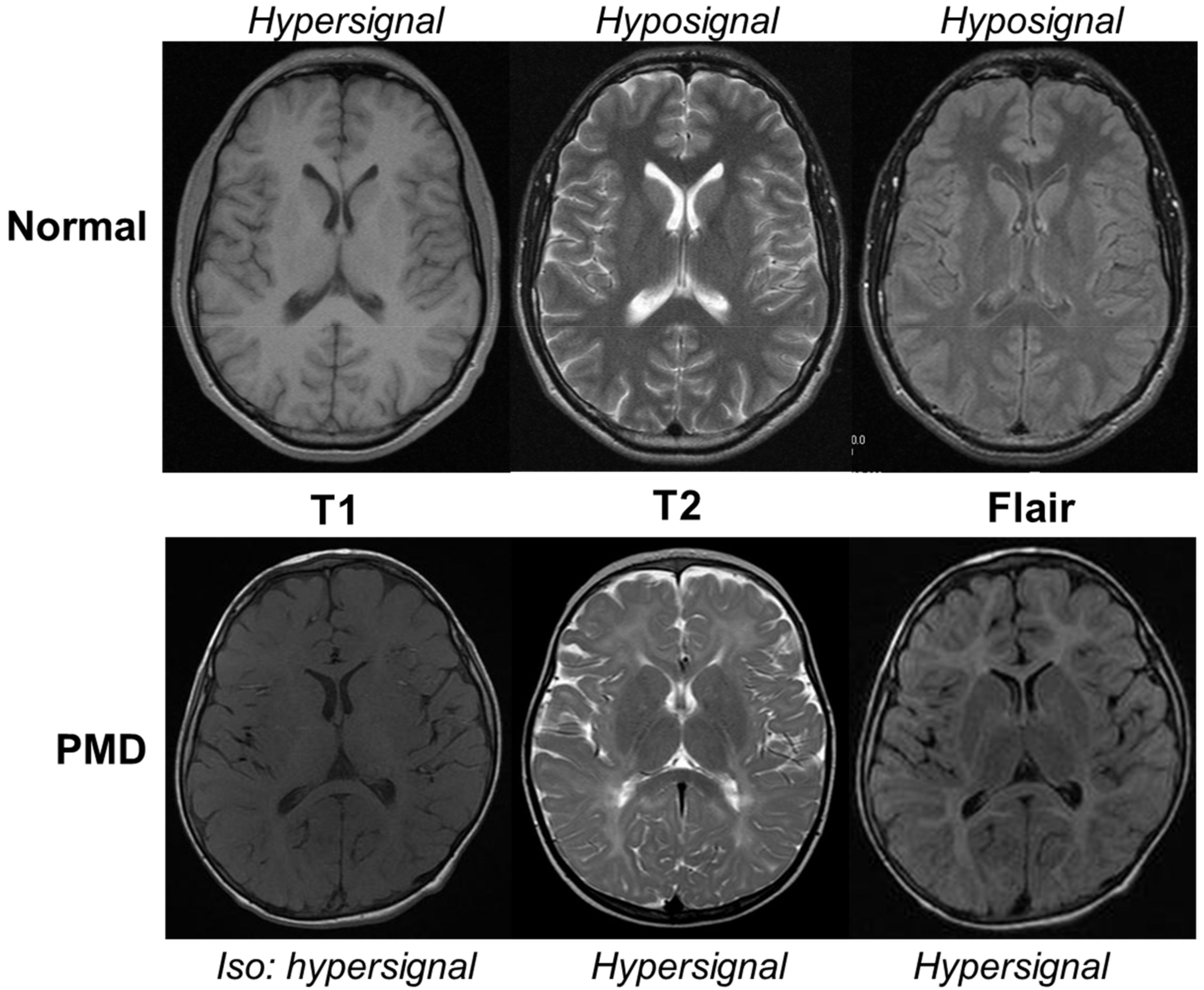
2. Clinical Presentation of PLP1-Related Disorders
3. Molecular Investigations
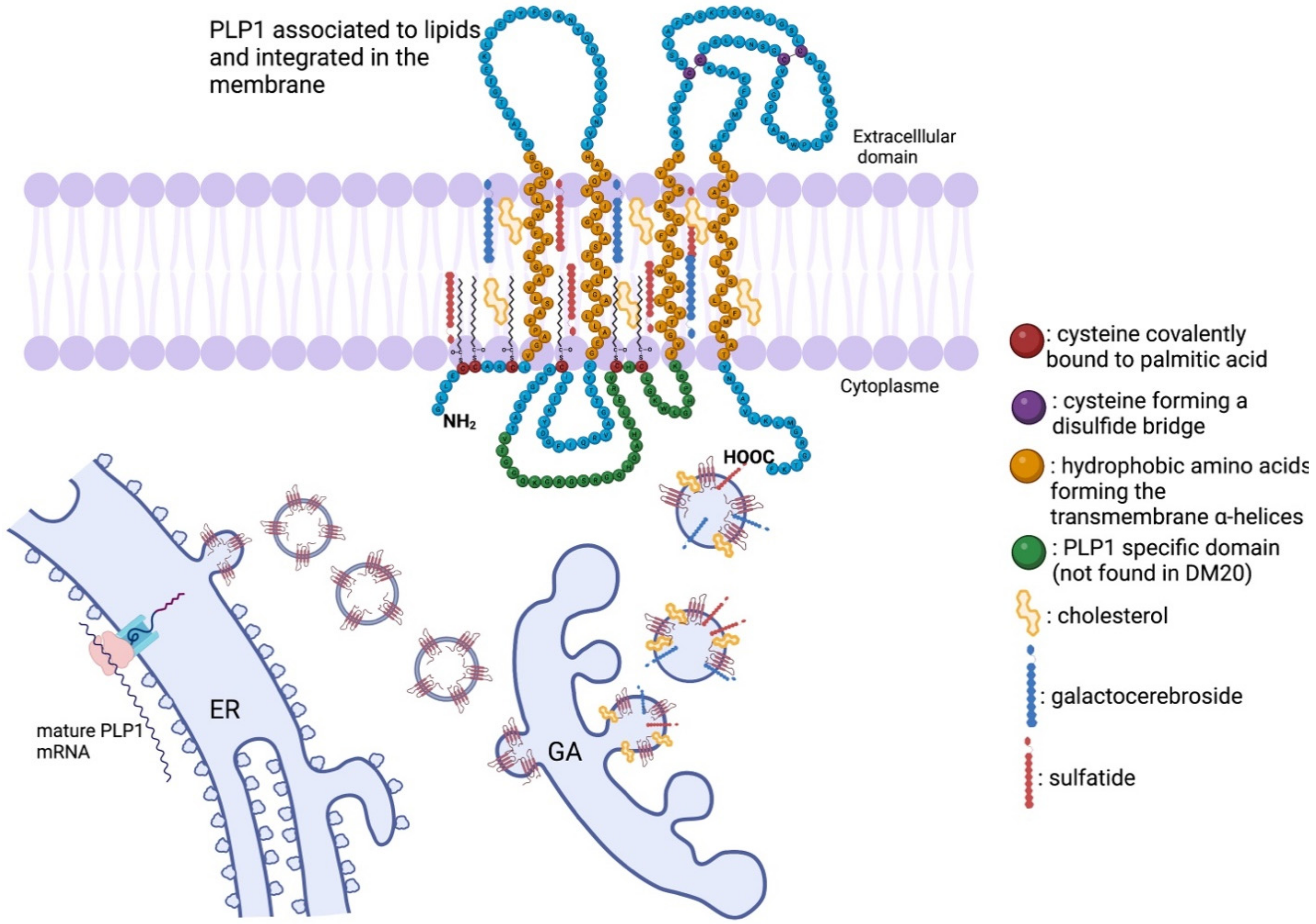
4. Proteolipid Protein 1: Substantial Physiological Functions
5. Genetic Intricacy and Phenotypic Repercussions
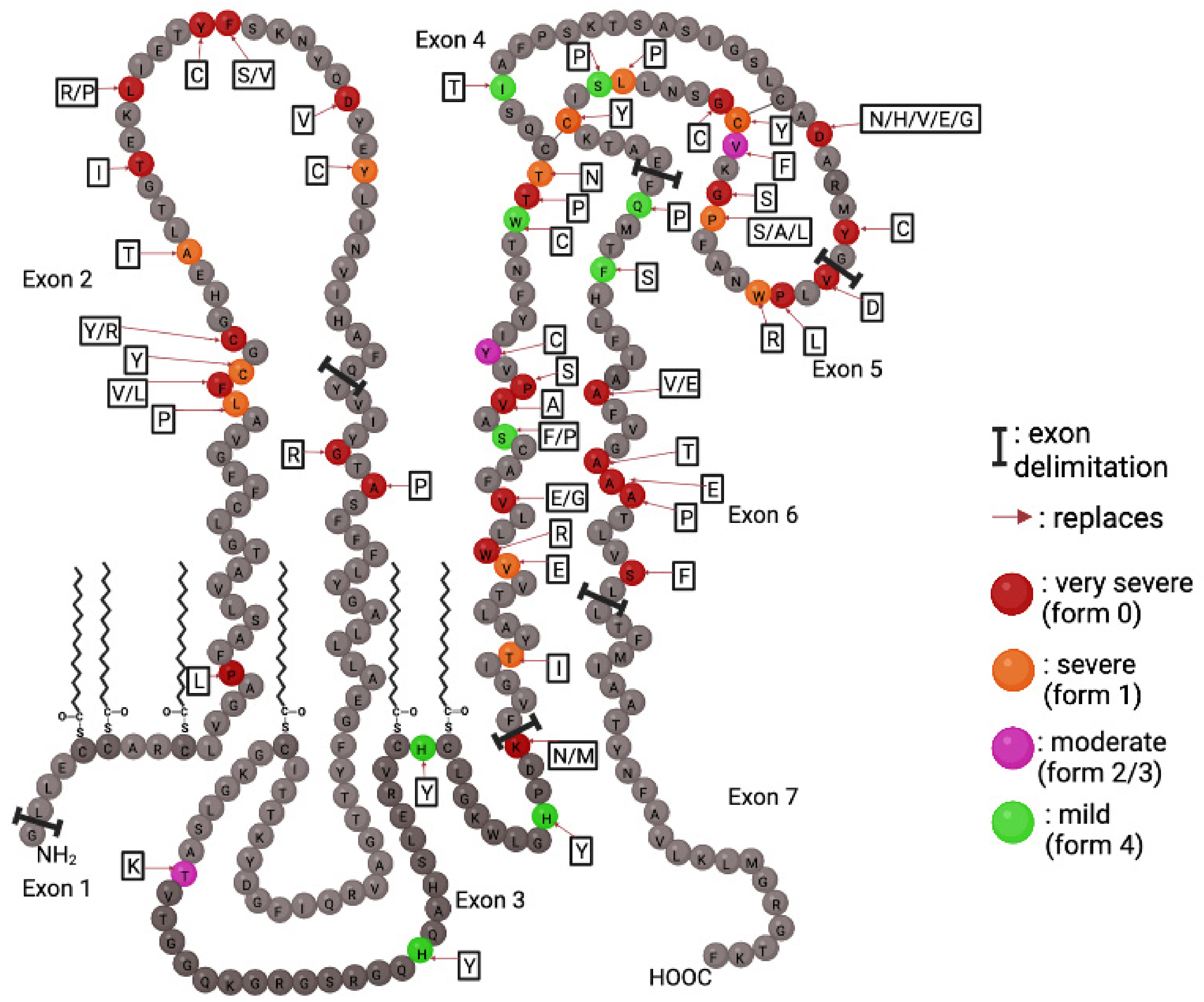
5.1. Point Mutations: The Most Diverse Genetic Alterations
5.2. Gene Duplication: The Most Common Genetic Alteration
5.3. Null Mutation: The Most Uncommon Genetic Alteration
5.4. Mecanism of Neurological Manifestations in Heterozygotes Female Carriers
6. Animal Models
7. Therapies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raine, C.S. Morphology of myelin and myelination. In Myelin; Springer: Berlin/Heidelberg, Germany, 1984; pp. 1–50. [Google Scholar]
- Lemke, G. Unwrapping the genes of myelin. Neuron 1988, 1, 535–543. [Google Scholar] [CrossRef]
- Nave, K.A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010, 11, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Lappe-Siefke, C.; Goebbels, S.; Gravel, M.; Nicksch, E.; Lee, J.; Braun, P.E.; Griffiths, I.R.; Nave, K.-A. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003, 33, 366–374. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, M.S.; Bugiani, M. Leukodystrophies: A proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol. 2017, 134, 351–382. [Google Scholar] [CrossRef] [PubMed]
- Vanderver, A.; Prust, M.; Tonduti, D.; Mochel, F.; Hussey, H.M.; Helman, G.; Garbern, J.; Eichler, F.; Labauge, P.; Aubourg, P. Case definition and classification of leukodystrophies and leukoencephalopathies. Mol. Genet. Metab. 2015, 114, 494–500. [Google Scholar] [CrossRef]
- Pelizaeus, F. Pelizaeus: Über eine eigenartige familiäre Entwicklungs. Arch. Psychiat. Nervenkr. 1899, 31, 100–104. [Google Scholar]
- Merzbacher, L. Eine eigenarige familiare Erkrankungsform (Aplasia axialis extracorticalis). Zeitschrift für die gesamte Neurologie und Psychiatrie 1910, 3, 1–138. [Google Scholar] [CrossRef]
- Willard, H.F.; Riordan, J.R. Assignment of the gene for myelin proteolipid protein to the X chromosome: Implications for X-linked myelin disorders. Science 1985, 230, 940–942. [Google Scholar] [CrossRef]
- Nave, K.A.; Lai, C.; Bloom, F.E.; Milner, R.J. Jimpy mutant mouse: A 74-base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect in RNA splicing. Proc. Natl. Acad. Sci. USA 1986, 83, 9264–9268. [Google Scholar] [CrossRef]
- Nave, K.-A.; Boespflug-Tanguy, O. X-Linked Developmental Defects of Myelination: From Mouse Mutants to Human Genetic Diseases. Neuroscientist 1996, 2, 33–43. [Google Scholar] [CrossRef]
- Boulloche, J.; Aicardi, J. Pelizaeus-Merzbacher Disease: Clinical and Nosological Study. J. Child Neurol. 1986, 1, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Boespflug-Tanguy, O.; Mimault, C.; Melki, J.; Cavagna, A.; Giraud, G.; Dinh, D.P.; Dastugue, B.; Dautigny, A. Genetic homogeneity of Pelizaeus-Merzbacher disease: Tight linkage to the proteolipoprotein locus in 16 affected families. Am. J. Hum. Genet. 1994, 55, 461. [Google Scholar] [PubMed]
- Mimault, C.; Giraud, G.; Courtois, V.; Cailloux, F.; Boire, J.Y.; Dastugue, B.; Boespflug-Tanguy, O.; Disease, C.E.N.o.B.D. Proteolipoprotein gene analysis in 82 patients with sporadic Pelizaeus-Merzbacher disease: Duplications, the major cause of the disease, originate more frequently in male germ cells, but point mutations do not. Am. J. Hum. Genet. 1999, 65, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Saugier-Veber, P.; Munnich, A.; Bonneau, D.; Rozet, J.-M.; Le Merrer, M.; Gil, R.; Boespflug-Tanguy, O. X–linked spastic paraplegia and Pelizaeus–Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat. Genet. 1994, 6, 257–262. [Google Scholar] [CrossRef]
- Meyyazhagan, A.; Orlacchio, A. Hereditary spastic paraplegia: An update. Int. J. Mol. Sci. 2022, 23, 1697. [Google Scholar] [CrossRef]
- Woodward, K.J. The molecular and cellular defects underlying Pelizaeus–Merzbacher disease. Expert Rev. Mol. Med. 2008, 10, e14. [Google Scholar] [CrossRef]
- Inoue, K. PLP1-related inherited dysmyelinating disorders: Pelizaeus-Merzbacher disease and spastic paraplegia type 2. Neurogenetics 2004, 6, 1–16. [Google Scholar] [CrossRef]
- Garbern, J.Y. Pelizaeus-Merzbacher disease: Genetic and cellular pathogenesis. Cell Mol. Life Sci. 2006, 64, 50–65. [Google Scholar] [CrossRef]
- Raskind, W.H.; A Williams, C.; Hudson, L.D.; Bird, T.D. Complete deletion of the proteolipid protein gene (PLP) in a family with X-linked Pelizaeus-Merzbacher disease. Am. J. Hum. Genet. 1991, 49, 1355–1360. [Google Scholar]
- Inoue, K.; Osaka, H.; Thurston, V.C.; Clarke, J.T.; Yoneyama, A.; Rosenbarker, L.; Bird, T.D.; Hodes, M.; Shaffer, L.G.; Lupski, J.R. Genomic Rearrangements Resulting in PLP1 Deletion Occur by Nonhomologous End Joining and Cause Different Dysmyelinating Phenotypes in Males and Females. Am. J. Hum. Genet. 2002, 71, 838–853. [Google Scholar] [CrossRef]
- Bonkowsky, J.L.; Nelson, C.; Kingston, J.L.; Filloux, F.M.; Mundorff, M.B.; Srivastava, R. The burden of inherited leukodystrophies in children. Neurology 2010, 75, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Numata, Y.; Gotoh, L.; Iwaki, A.; Kurosawa, K.; Takanashi, J.-I.; Deguchi, K.; Yamamoto, T.; Osaka, H.; Inoue, K. Epidemiological, clinical, and genetic landscapes of hypomyelinating leukodystrophies. J. Neurol. 2014, 261, 752–758. [Google Scholar] [CrossRef]
- Cailloux, F.; Disease, C.E.N.O.B.D.; Gauthier-Barichard, F.; Mimault, C.; Isabelle, V.; Courtois, V.; Giraud, G.; Dastugue, B.; Boespflug-Tanguy, O. Genotype–phenotype correlation in inherited brain myelination defects due to proteolipid protein gene mutations. Eur. J. Hum. Genet. 2000, 8, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Sarret, C.; Lemaire, J.-J.; Sontheimer, A.; Coste, J.; Savy, N.; Pereira, B.; Roche, B.; Boespflug-Tanguy, O. Brain Diffusion Imaging and Tractography to Distinguish Clinical Severity of Human PLP1-Related Disorders. Dev. Neurosci. 2018, 40, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Confort-Gouny, S.; Schneider, J.; Barberet, M.; Chapon, F.; Viola, A.; Pineau, S.; Combaz, X.; Cozzone, P. MR imaging of brain maturation. J. Neuroradiol. 2007, 34, 290–310. [Google Scholar] [CrossRef] [PubMed]
- Hobson, G.; Kamholz, J. PLP1-Related Disorders; GeneReviews; University of Washington: Seattle, WA, USA, 2013. [Google Scholar]
- Garbern, J.; Cambi, F.; Shy, M.; Kamholz, J. The molecular pathogenesis of Pelizaeus-Merzbacher disease. Arch. Neurol. 1999, 56, 1210. [Google Scholar] [CrossRef]
- Kevelam, S.H.; Taube, J.R.; van Spaendonk, R.M.L.; Bertini, E.; Sperle, K.; Tarnopolsky, M.; Tonduti, D.; Valente, E.M.; Travaglini, L.; Sistermans, E.A.; et al. AlteredPLP1splicing causes hypomyelination of early myelinating structures. Ann. Clin. Transl. Neurol. 2015, 2, 648–661. [Google Scholar] [CrossRef]
- Inoue, K.; Tanaka, H.; Scaglia, F.; Araki, A.; Shaffer, L.G.; Lupski, J.R. Compensating for central nervous system dysmyelination: Females with a proteolipid protein gene duplication and sustained clinical improvement. Ann. Neurol. 2001, 50, 747–754. [Google Scholar] [CrossRef]
- Hurst, S.; Garbern, J.; Trepanier, A.; Gow, A. Quantifying the carrier female phenotype in Pelizaeus-Merzbacher disease. Genet. Med. 2006, 8, 371–378. [Google Scholar] [CrossRef][Green Version]
- Wolf, N.I.; Ffrench-Constant, C.; van der Knaap, M.S. Hypomyelinating leukodystrophies—Unravelling myelin biology. Nat. Rev. Neurol. 2020, 17, 88–103. [Google Scholar] [CrossRef]
- Bonnet-Dupeyron, M.-N.; Combes, P.; Santander, P.; Cailloux, F.; Boespflug-Tanguy, O.; Vaurs-Barrière, C. PLP1splicing abnormalities identified in Pelizaeus-Merzbacher disease and SPG2 fibroblasts are associated with different types of mutations. Hum. Mutat. 2008, 29, 1028–1036. [Google Scholar] [CrossRef]
- Wang, E.; Dimova, N.; Sperle, K.; Huang, Z.; Lock, L.; McCulloch, M.C.; Edgar, J.M.; Hobson, G.M.; Cambi, F. Deletion of a splicing enhancer disrupts PLP1/DM20 ratio and myelin stability. Exp. Neurol. 2008, 214, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Lupski, J.R. Molecular mechanisms for genomic disorders. Annu. Rev. Genom. Hum. Genet. 2002, 3, 199–242. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J. Biol. Chem. 1951, 191, 807–817. [Google Scholar] [CrossRef]
- Greer, J.M.; Lees, M.B. Myelin proteolipid protein—the first 50 years. Int. J. Biochem. Cell Biol. 2002, 34, 211–215. [Google Scholar] [CrossRef]
- Stoffel, W.; Hillen, H.; Giersiefen, H. Structure and molecular arrangement of proteolipid protein of central nervous system myelin. Proc. Natl. Acad. Sci. USA 1984, 81, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Diehl, H.J.; Schaich, M.; Budzinski, R.M.; Stoffel, W. Individual exons encode the integral membrane domains of human myelin proteolipid protein. Proc. Natl. Acad. Sci. USA 1986, 83, 9807–9811. [Google Scholar] [CrossRef]
- Hobson, G.M.; Huang, Z.; Sperle, K.; Sistermans, E.; Rogan, P.K.; Garbern, J.Y.; Kolodny, E.; Naidu, S.; Cambi, F. Splice-site contribution in alternative splicing ofPLP1 andDM20: Molecular studies in oligodendrocytes. Hum. Mutat. 2005, 27, 69–77. [Google Scholar] [CrossRef]
- Agrawal, H.C.; Burton, R.M.; Fishman, M.A.; Mitchell, R.F.; Prensky, A.L. Partial characterization of a new myelin protein component. J. Neurochem. 1972, 19, 2083–2089. [Google Scholar] [CrossRef]
- Nave, K.A.; Lai, C.; Bloom, F.E.; Milner, R.J. Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proc. Natl. Acad. Sci. USA 1987, 84, 5665–5669. [Google Scholar] [CrossRef]
- Vouyiouklis, D.A.; Barrie, J.A.; Griffiths, I.R.; Thomson, C.E. A proteolipid protein-specific pre-mRNA (Ppm-1) contains intron 3 and is up-regulated during myelination in the CNS. J. Neurochem. 2000, 74, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Pham-Dinh, D.; Birling, M.-C.; Roussel, G.; Dautigny, A.; Nussbaum, J.-L. Proteolipid DM-20 predominates over PLP in peripheral nervous system. NeuroReport 1991, 2, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Southwood, C.M.; Gragerov, A.; Kelley, K.A.; Friedrich, V.L.; Gow, A. The Evolution of Lipophilin Genes from Invertebrates to Tetrapods: DM-20 Cannot Replace Proteolipid Protein in CNS Myelin. J. Neurosci. 2000, 20, 4002–4010. [Google Scholar] [CrossRef] [PubMed]
- Timsit, S.G.; Bally-Cuif, L.; Colman, D.R.; Zalc, B. DM-20 mRNA Is Expressed During the Embryonic Development of the Nervous System of the Mouse. J. Neurochem. 1992, 58, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Spörkel, O.; Uschkureit, T.; Büssow, H.; Stoffel, W. Oligodendrocytes expressing exclusively the DM20 isoform of the proteolipid protein gene: Myelination and development. Glia 2001, 37, 19–30. [Google Scholar] [CrossRef]
- A Bizzozero, O.; McGarry, J.F.; Lees, M.B. Autoacylation of myelin proteolipid protein with acyl coenzyme A. J. Biol. Chem. 1987, 262, 13550–13557. [Google Scholar] [CrossRef]
- Stoffel, W.; Bosio, A. Myelin glycolipids and their functions. Curr. Opin. Neurobiol. 1997, 7, 654–661. [Google Scholar] [CrossRef]
- Simons, M.; Krämer-Albers, E.-M.; Thiele, C.; Stoffel, W.; Trotter, J. Assembly of Myelin by Association of Proteolipid Protein with Cholesterol- and Galactosylceramide-Rich Membrane Domains. J. Cell Biol. 2000, 151, 143–154. [Google Scholar] [CrossRef]
- Gow, A.; Gragerov, A.; Gard, A.; Colman, D.R.; Lazzarini, R.A. Conservation of Topology, But Not Conformation, of the Proteolipid Proteins of the Myelin Sheath. J. Neurosci. 1997, 17, 181–189. [Google Scholar] [CrossRef]
- Lee, A.G. Myelin: Delivery by raft. Curr. Biol. 2001, 11, R60–R62. [Google Scholar] [CrossRef][Green Version]
- Garbern, J.Y. Pelizaeus–Merzbacher disease: Pathogenic mechanisms and insights into the roles of proteolipid protein 1 in the nervous system. J. Neurol. Sci. 2005, 228, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Helynck, G.; Luu, B.; Nussbaum, J.-L.; Picken, D.; Skalidis, G.; Trifilieff, E.; Van Dorsselaer, A.; Seta, P.; Sandeaux, R.; Gavach, C.; et al. Brain Proteolipids. Isolation, Purification and Effect on Ionic Permeability of Membranes. JBIC J. Biol. Inorg. Chem. 1983, 133, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, G.; Trifilieff, E.; Luu, B. Selective Extraction of the DM-20 Brain Proteolipid. J. Neurochem. 1986, 46, 297–299. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Hunter, D.; Thomson, C.; Yool, D.; Kirkham, D.; Freer, A.; Griffiths, I. Evidence for possible interactions between PLP and DM20 within the myelin sheath. Glia 2002, 39, 31–36. [Google Scholar] [CrossRef]
- Griffiths, I.; Klugmann, M.; Anderson, C.T.T.; Vouyiouklis, D.; Nave, K.-A. Current concepts of PLP and its role in the nervous system. Microsc. Res. Tech. 1998, 41, 344–358. [Google Scholar] [CrossRef]
- Yool, D.A.; Klugmann, M.; McLaughlin, M.; Vouyiouklis, D.A.; Dimou, L.; Barrie, J.A.; McCulloch, M.C.; Nave, K.A.; Griffiths, I.R. Myelin proteolipid proteins promote the interaction of oligodendrocytes and axons. J. Neurosci. Res. 2001, 63, 151–164. [Google Scholar] [CrossRef]
- Griffiths, I.; Klugmann, M.; Anderson, T.; Yool, D.; Thomson, C.; Schwab, M.H.; Schneider, A.; Zimmermann, F.; McCulloch, M.; Nadon, N.; et al. Axonal Swellings and Degeneration in Mice Lacking the Major Proteolipid of Myelin. Science 1998, 280, 1610–1613. [Google Scholar] [CrossRef]
- Knapp, P.E. Developm ental Neuroscience Short Review Proteolipid Protein: Is It More than Just a Structural Component of Myelin? Dev. Neurosci. 1996, 18, 297–308. [Google Scholar] [CrossRef]
- Timsit, S.; Martinez, S.; Allinquant, B.; Peyron, F.; Puelles, L.; Zalc, B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J. Neurosci. 1995, 15, 1012–1024. [Google Scholar] [CrossRef]
- Wight, P.A. Effects of Intron 1 Sequences on Human PLP1 Expression: Implications for PLP1-Related Disorders. ASN Neuro 2017, 9, 1759091417720583. [Google Scholar] [CrossRef]
- Miller, M.J.; Haxhiu, M.A.; Georgiadis, P.; Gudz, T.I.; Kangas, C.D.; Macklin, W.B. Proteolipid Protein Gene Mutation Induces Altered Ventilatory Response to Hypoxia in the Myelin-Deficient Rat. J. Neurosci. 2003, 23, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Bongarzone, E.R.; Campagnoni, C.W.; Kampf, K.; Jacobs, E.C.; Handley, V.W.; Schonmann, V.; Campagnoni, A.T. Identification of a New Exon in the Myelin Proteolipid Protein Gene Encoding Novel Protein Isoforms That Are Restricted to the Somata of Oligodendrocytes and Neurons. J. Neurosci. 1999, 19, 8349–8357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griffiths, I.R.; Dickinson, P.; Montague, P. Expression of the proteolipid protein gene in glial cells of the post-natal peripheral nervous system of rodents. Neuropathol. Appl. Neurobiol. 1995, 21, 97–110. [Google Scholar] [CrossRef]
- Sarret, C.; Combes, P.; Micheau, P.; Gelot, A.; Boespflug-Tanguy, O.; Vaurs-Barriere, C. Novel neuronal proteolipid protein isoforms encoded by the human myelin proteolipid protein 1 gene. Neuroscience 2010, 166, 522–538. [Google Scholar] [CrossRef]
- Campagnoni, C.W.; Garbay, B.; Micevych, P.; Pribyl, T.; Kampf, K.; Handley, V.W.; Campagnoni, A.T. DM20 mRNA splice product of the myelin proteolipid protein gene is expressed in the murine heart. J. Neurosci. Res. 1992, 33, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Pribyl, T.M.; Campagnoni, C.; Kampf, K.; Handley, V.W.; Campagnoni, A.T. The major myelin protein genes are expressed in the human thymus. J. Neurosci. Res. 1996, 45, 812–819. [Google Scholar] [CrossRef]
- Skoff, R.P.; A Bessert, D.; Cerghet, M.; Franklin, M.J.; Rout, U.K.; Nave, K.-A.; Carlock, L.; Ghandour, M.S.; Armant, D.R. The myelin proteolipid protein gene modulates apoptosis in neural and non-neural tissues. Cell Death Differ. 2004, 11, 1247–1257. [Google Scholar] [CrossRef] [PubMed][Green Version]
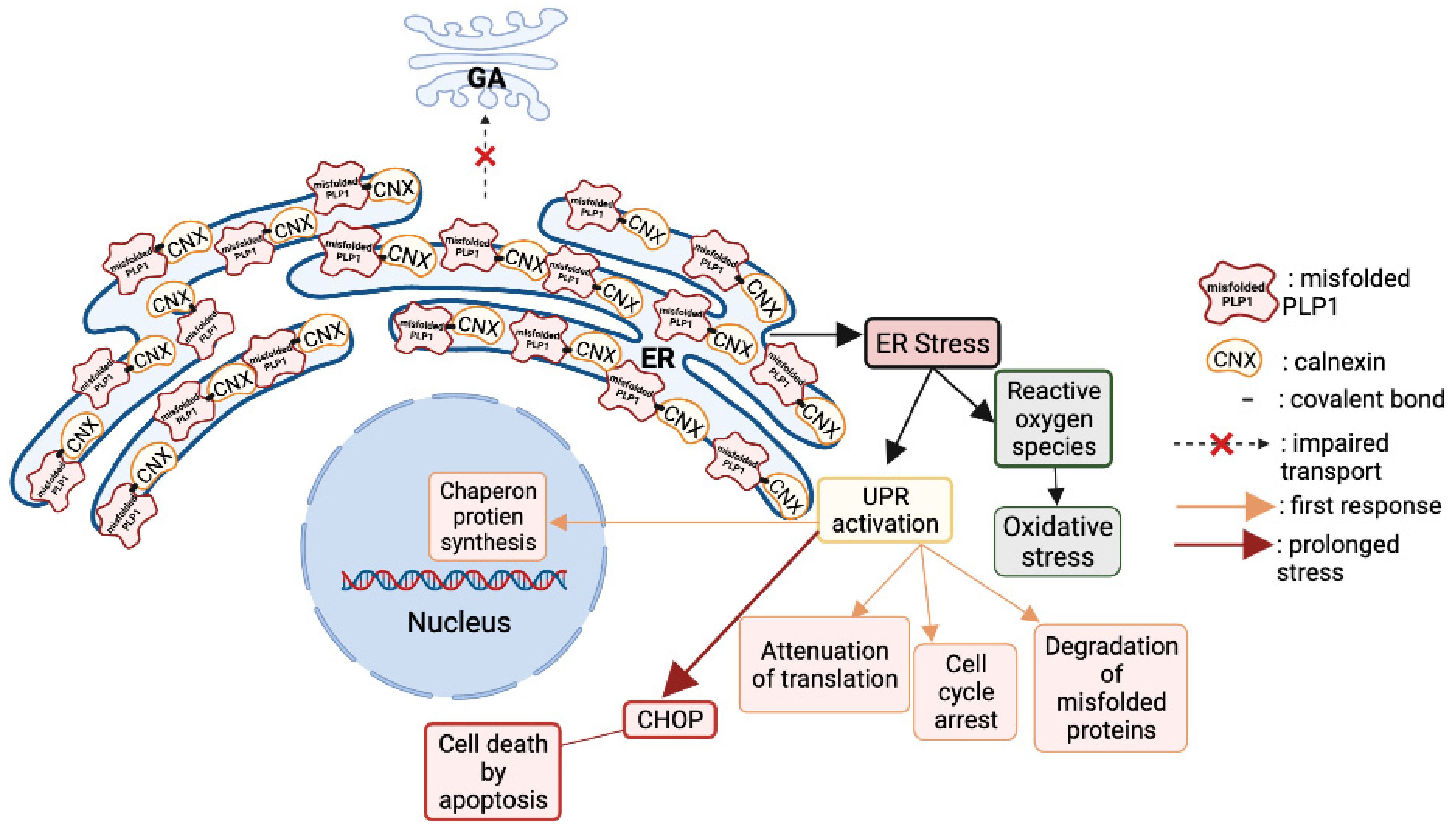
- Southwood, C.M.; Garbern, J.; Jiang, W.; Gow, A. The Unfolded Protein Response Modulates Disease Severity in Pelizaeus-Merzbacher Disease. Neuron 2002, 36, 585–596. [Google Scholar] [CrossRef]
- Forman, M.S.; Lee, V.M.; Trojanowski, J.Q. ‘Unfolding’pathways in neurodegenerative disease. Trends Neurosci. 2003, 26, 407–410. [Google Scholar] [CrossRef]
- Bond, C.; Si, X.; Crisp, M.; Wong, P.; Paulson, G.W.; Boesel, C.P.; Dlouhy, S.R.; Hodes, M. Family with Pelizaeus-Merzbacher disease/X-linked spastic paraplegia and a nonsense mutation in exon 6 of the proteolipid protein gene. Am. J. Med. Genet. 1997, 71, 357–360. [Google Scholar] [CrossRef]
- Inoue, K.; Khajavi, M.; Ohyama, T.; Hirabayashi, S.-I.; Wilson, J.H.; Reggin, J.D.; Mancias, P.; Butler, I.J.; Wilkinson, M.F.; Wegner, M.; et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat. Genet. 2004, 36, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gow, A.; Lazzarini, R.A. A cellular mechanism governing the severity of Pelizaeus–Merzbacher disease. Nat. Genet. 1996, 13, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Shimojima, K.; Akagawa, H.; Yanagi, K.; Kaname, T.; Okamoto, N.; Yamamoto, T. Deep intronic deletion in intron 3 of PLP1 is associated with a severe phenotype of Pelizaeus-Merzbacher disease. Hum. Genome Var. 2021, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Southwood, C.; Gow, A. Molecular pathways of oligodendrocyte apoptosis revealed by mutations in the proteolipid protein gene. Microsc. Res. Tech. 2001, 52, 700–708. [Google Scholar] [CrossRef]
- Gow, A.; Southwood, C.M.; Lazzarini, R.A. Disrupted Proteolipid Protein Trafficking Results in Oligodendrocyte Apoptosis in an Animal Model of Pelizaeus-Merzbacher Disease. J. Cell Biol. 1998, 140, 925–934. [Google Scholar] [CrossRef]
- Swanton, E.; High, S.; Woodman, P. Role of calnexin in the glycan-independent quality control of proteolipid protein. EMBO J. 2003, 22, 2948–2958. [Google Scholar] [CrossRef]
- Numasawa-Kuroiwa, Y.; Okada, Y.; Shibata, S.; Kishi, N.; Akamatsu, W.; Shoji, M.; Nakanishi, A.; Oyama, M.; Osaka, H.; Inoue, K.; et al. Involvement of ER Stress in Dysmyelination of Pelizaeus-Merzbacher Disease with PLP1 Missense Mutations Shown by iPSC-Derived Oligodendrocytes. Stem Cell Rep. 2014, 2, 648–661. [Google Scholar] [CrossRef]
- Gow, A.; Sharma, R. The Unfolded Protein Response in Protein Aggregating Diseases. NeuroMolecular Med. 2003, 4, 73–94. [Google Scholar] [CrossRef]
- Ellis, D.; Malcolm, S. Proteolipid protein gene dosage effect in Pelizaeus–Merzbacher disease. Nat. Genet. 1994, 6, 333–334. [Google Scholar] [CrossRef]
- Inoue, K.; Osaka, H.; Sugiyama, N.; Kawanishi, C.; Onishi, H.; Nezu, A.; Kimura, K.; Yamada, Y.; Kosaka, K. A duplicated PLP gene causing Pelizaeus-Merzbacher disease detected by comparative multiplex PCR. Am. J. Hum. Genet. 1996, 59, 32–39. [Google Scholar]
- Inoue, K.; Osaka, H.; Imaizumi, K.; Nezu, A.; Takanashi, J.-I.; Arii, J.; Murayama, K.; Ono, J.; Kikawa, Y.; Mito, T.; et al. Proteolipid protein gene duplications causing Pelizaeus-Merzbacher disease: Molecular mechanism and phenotypic manifestations. Ann. Neurol. 1999, 45, 624–632. [Google Scholar] [CrossRef]
- Sistermans, E.A.; de Coo, R.F.; De Wijs, I.J.; Van Oost, B.A. Duplication of the proteolipid protein gene is the major cause of Pelizaeus-Merzbacher disease. Neurology 1998, 50, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Woodward, K.; Kendall, E.; Vetrie, D.; Malcolm, S. Pelizaeus-Merzbacher Disease: Identification of Xq22 Proteolipid-Protein Duplications and Characterization of Breakpoints by Interphase FISH. Am. J. Hum. Genet. 1998, 63, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, P.; Lupski, J.R. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002, 18, 74–82. [Google Scholar] [CrossRef]
- Woodward, K.J.; Cundall, M.; Sperle, K.; Sistermans, E.A.; Ross, M.; Howell, G.; Gribble, S.M.; Burford, D.C.; Carter, N.P.; Hobson, D.L.; et al. Heterogeneous Duplications in Patients with Pelizaeus-Merzbacher Disease Suggest a Mechanism of Coupled Homologous and Nonhomologous Recombination. Am. J. Hum. Genet. 2005, 77, 966–987. [Google Scholar] [CrossRef]
- Reiter, L.; Murakami, T.; Koeuth, T.; Pentao, L.; Muzny, D.M.; Gibbs, R.A.; Lupski, J.R. A recombination hotspot responsible for two inherited peripheral neuropathies is located near a mariner transposon-like element. Nat. Genet. 1996, 12, 288–297. [Google Scholar] [CrossRef]
- Lupski, J.R.; de Oca-Luna, R.M.; Slaugenhaupt, S.; Pentao, L.; Guzzetta, V.; Trask, B.J.; Saucedo-Cardenas, O.; Barker, D.F.; Killian, J.M.; Garcia, C.A.; et al. DNA Duplication Associated with Charcot-Marie-Tooth Disease Type 1 A. Cell 1991, 66, 219–232. [Google Scholar] [CrossRef]
- Wolf, N.I.; Sistermans, E.A.; Cundall, M.; Hobson, G.M.; Davis-Williams, A.P.; Palmer, R.; Stubbs, P.; Davies, S.; Endziniene, M.; Wu, Y.; et al. Three or more copies of the proteolipid protein gene PLP1 cause severe Pelizaeus-Merzbacher disease. Brain 2005, 128, 743–751. [Google Scholar] [CrossRef]
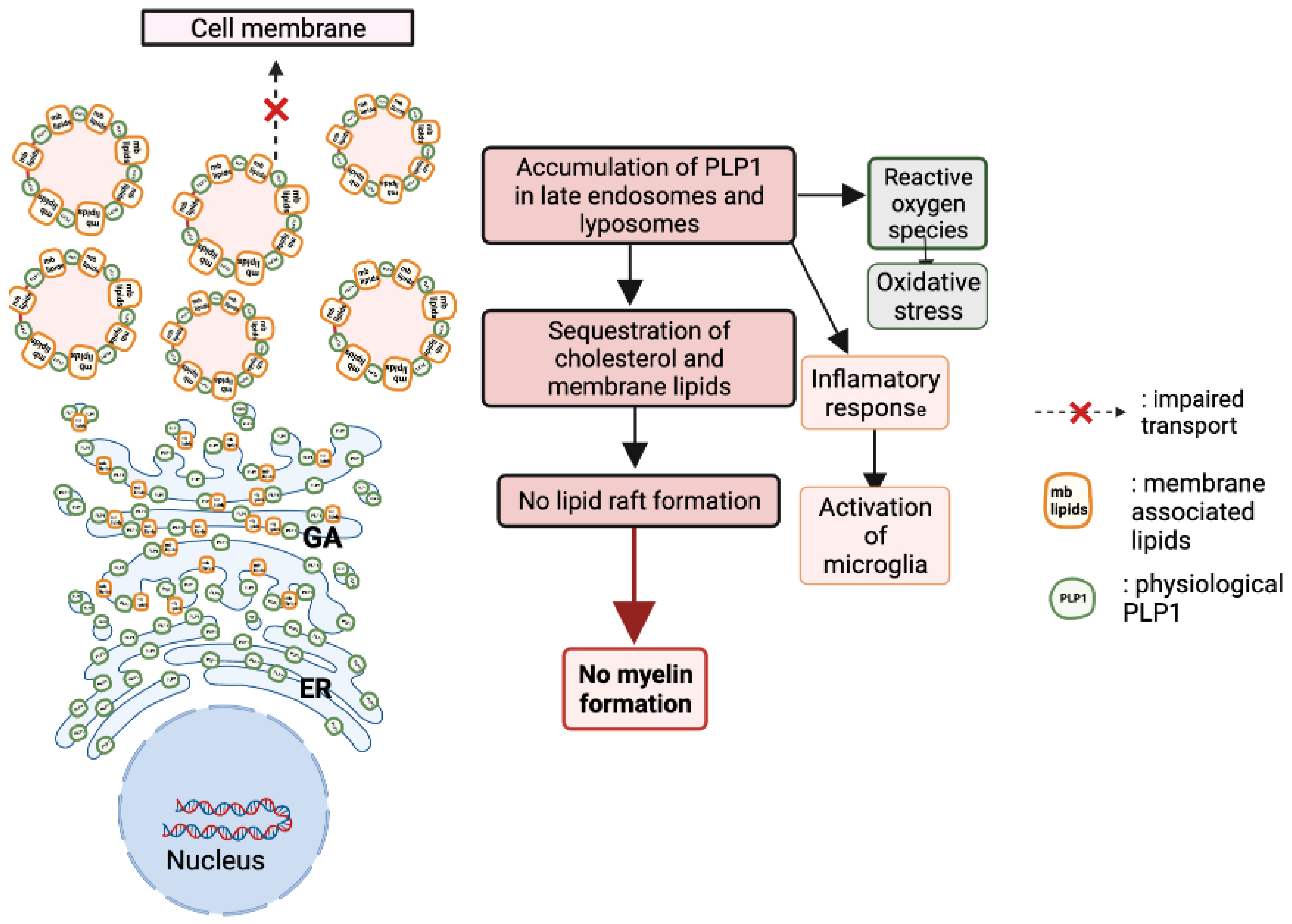
- Simons, M.; Krämer, E.-M.; Macchi, P.; Rathke-Hartlieb, S.; Trotter, J.; Nave, K.-A.; Schulz, B., Jr. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes implications for Pelizaeus-Merzbacher disease. J. Cell Biol. 2002, 157, 327–336. [Google Scholar] [CrossRef]
- Tatar, C.L.; Appikatla, S.; A Bessert, D.; Paintlia, A.S.; Singh, I.; Skoff, R.P. Increased Plp1 Gene Expression Leads to Massive Microglial Cell Activation and Inflammation Throughout the Brain. ASN Neuro 2010, 2, AN20100016. [Google Scholar] [CrossRef]
- Ruiz, M.; Bégou, M.; Launay, N.; Ranea-Robles, P.; Bianchi, P.; López-Erauskin, J.; Morató, L.; Guilera, C.; Petit, B.; Vaurs-Barriere, C. Oxidative stress and mitochondrial dynamics malfunction are linked in P elizaeus-M erzbacher disease. Brain Pathol. 2018, 28, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Li, L.; Yan, H.; He, M.; Gao, K.; Xing, S.; Ji, H.; Wang, J.; Cao, B.; Li, D.; et al. Novel Insight into the Potential Pathogenicity of Mitochondrial Dysfunction Resulting from PLP1 Duplication Mutations in Patients with Pelizaeus–Merzbacher Disease. Neuroscience 2021, 476, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.Y.; Cambi, F.; Tang, X.-M.; Sima, A.A.; Vallat, J.M.; Bosch, E.; Lewis, R.; Shy, M.; Sohi, J.; Kraft, G.; et al. Proteolipid Protein Is Necessary in Peripheral as Well as Central Myelin. Neuron 1997, 19, 205–218. [Google Scholar] [CrossRef]
- Sistermans, E.; de Wijs, S.; de Coo, I.; van Oost, B.A.; Smit, L.M.E.; Menko, F.H. A (G-to-A) mutation in the initiation codon of the proteolipid protein gene causing a relatively mild form of Pelizaeus-Merzbacher disease in a Dutch family. Qual. Life Res. 1996, 97, 337–339. [Google Scholar] [CrossRef]
- Taube, J.R.; Sperle, K.; Banser, L.; Seeman, P.; Cavan, B.C.V.; Garbern, J.Y.; Hobson, G.M. PMD patient mutations reveal a long-distance intronic interaction that regulates PLP1/DM20 alternative splicing. Hum. Mol. Genet. 2014, 23, 5464–5478. [Google Scholar] [CrossRef]
- Shy, M.E.; Hobson, G.; Jain, M.; Boespflug-Tanguy, O.; Garbern, J.; Sperle, K.; Li, W.; Gow, A.; Rodriguez, D.; Bertini, E.; et al. Schwann cell expression of PLP1 but not DM20 is necessary to prevent neuropathy. Ann. Neurol. 2003, 53, 354–365. [Google Scholar] [CrossRef]
- Lee, J.A.; Carvalho, C.M.; Lupski, J.R. A DNA Replication Mechanism for Generating Nonrecurrent Rearrangements Associated with Genomic Disorders. Cell 2007, 131, 1235–1247. [Google Scholar] [CrossRef]
- Sivakumar, K.; Sambuughin, N.; Selenge, B.; Nagle, J.W.; Baasanjav, D.; Hudson, L.D.; Goldfarb, L.G. Novel exon 3B proteolipid protein gene mutation causing late-onset spastic paraplegia type 2 with variable penetrance in female family members. Ann. Neurol. 1999, 45, 680–683. [Google Scholar] [CrossRef]
- Osório, M.J.; Goldman, S.A. Neurogenetics of Pelizaeus–Merzbacher disease. Handb. Clin. Neurol. 2018, 148, 701–722. [Google Scholar]
- Nance, M.; Boyadjiev, S.; Pratt, V.; Taylor, S.; Hodes, M.; Dlouhy, S. Adult-onset neurodegenerative disorder due to proteolipid protein gene mutation in the mother of a man with Pelizaeus-Merzbacher disease. Neurology 1996, 47, 1333–1335. [Google Scholar] [CrossRef]
- Schneider, A.; Montague, P.; Griffiths, I.; Fanarraga, M.; Kennedy, P.; Brophy, P.; Nave, K.-A. Uncoupling of hypomyelination and glial cell death by a mutation in the proteolipid protein gene. Nature 1992, 358, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Woodward, K.; Kirtland, K.; Dlouhy, S.; Raskind, W.; Bird, T.; Malcolm, S.; Abeliovich, D. X inactivation phenotype in carriers of Pelizaeus-Merzbacher disease: Skewed in carriers of a duplication and random in carriers of point mutations. Eur. J. Hum. Genet. 2000, 8, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.; Anderson, T.; Dickinson, P.; Barrie, J.; McCulloch, M.; Nave, K.-A.; Griffiths, I. Survival of, and competition between, oligodendrocytes expressing different alleles of the Plp gene. J. Cell Biol. 2002, 158, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.S. Jimpy, a new totally sex-linked gene in the house mouse. Z. Für Indukt. Abstamm. - Und Vererb. 1954, 86, 322–326. [Google Scholar] [CrossRef]
- Nave, K.-A.; Bloom, F.E.; Milner, R.J.; Bloom, E. A Single Nucleotide Difference in the Gene for Myelin Proteolipid Protein Defines the Jimpy Mutation in Mouse. J. Neurochem. 1987, 49, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.D.; A Berndt, J.; Puckett, C.; A Kozak, C.; A Lazzarini, R. Aberrant splicing of proteolipid protein mRNA in the dysmyelinating jimpy mutant mouse. Proc. Natl. Acad. Sci. USA 1987, 84, 1454–1458. [Google Scholar] [CrossRef]
- Moriguchi, A.; Ikenaka, K.; Furuichi, T.; Okano, H.; Iwasaki, Y.; Mikoshiba, K. The fifth exon of the myelin proteolipid protein-coding gene is not utilized in the brain of jimpy mutant mice. Gene 1987, 55, 333–337. [Google Scholar] [CrossRef]
- Dautigny, A.; Mattei, M.-G.; Morello, D.; Alliel, P.M.; Pham-Dinh, D.; Amar, L.; Arnaud, D.; Simon, D.; Mattei, J.-F.; Guenet, J.-L.; et al. The structural gene coding for myelin-associated proteolipid protein is mutated in jimpy mice. Nature 1986, 321, 867–869. [Google Scholar] [CrossRef]
- Gencic, S.; Hudson, L.D. Conservative amino acid substitution in the myelin proteolipid protein of jimpymsd mice. J. Neurosci. 1990, 10, 117–124. [Google Scholar] [CrossRef]
- Pearsall, G.B.; Nadon, N.L.; Wolf, M.K.; Billings-Gagliardi, S. Jimpy-4J Mouse Has a Missense Mutation in Exon 2 of the Plp Gene. Dev. Neurosci. 1997, 19, 337–341. [Google Scholar] [CrossRef]
- Griffiths, I.R.; Scott, I.; McCulloch, M.C.; Barrie, J.A.; McPhilemy, K.; Cattanach, B.M. Rumpshaker mouse: A new X-linked mutation affecting myelination: Evidence for a defect in PLP expression. J. Neurocytol. 1990, 19, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Hoffman, E.; Marks, H.G. The rumpshaker mutation in spastic paraplegia. Nat. Genet. 1994, 7, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Nadon, N.L.; Duncan, I.D. Gene expression and oligodendrocyte development in the myelin deficient rat. J. Neurosci. Res. 1995, 41, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nadon, N.; Duncan, I.; Hudson, L. A point mutation in the proteolipid protein gene of the ‘shaking pup’ interrupts oligodendrocyte development. Development 1990, 110, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Tosic, M.; Dolivo, M.; Domanska-Janik, K.; Matthieu, J.-M. Paralytic Tremor (pt): A New Allele of the Proteolipid Protein Gene in Rabbits. J. Neurochem. 2002, 63, 2210–2216. [Google Scholar] [CrossRef]
- Sherman, L.S.; Su, W.; Johnson, A.L.; Peterson, S.M.; Cullin, C.; Lavinder, T.; Ferguson, B.; Lewis, A.D. A novel non-human primate model of Pelizaeus-Merzbacher disease. Neurobiol. Dis. 2021, 158, 105465. [Google Scholar] [CrossRef]
- Inoue, Y.; Kagawa, T.; Matsumura, Y.; Ikenaka, K.; Mikoshiba, K. Cell death of oligodendrocytes or demyelination induced by overexpression of proteolipid protein depending on expressed gene dosage. Neurosci. Res. 1996, 25, 161–172. [Google Scholar] [CrossRef]
- Readhead, C.; Schneider, A.; Griffiths, I.; Nave, K.-A. Premature arrest of myelin formation in transgenic mice with increased proteolipid protein gene dosage. Neuron 1994, 12, 583–595. [Google Scholar] [CrossRef]
- Anderson, T.J.; Klugmann, M.; Thomson, C.E.; Schneider, A.; Readhead, C.; Griffiths, I.R.; Nave, K.-A. Distinct Phenotypes Associated with Increasing Dosage of the PLP Gene: Implications for CMT1A Due toPMP22Gene Duplication. Ann. N. Y. Acad. Sci. 1999, 883, 234–246. [Google Scholar] [CrossRef]
- Clark, K.; Sakowski, L.; Sperle, K.; Banser, L.; Landel, C.P.; Bessert, D.A.; Skoff, R.; Hobson, G.M. Gait abnormalities and progressive myelin degeneration in a new murine model of Pelizaeus-Merzbacher disease with tandem genomic duplication. J. Neurosci. 2013, 33, 11788–11799. [Google Scholar] [CrossRef]
- Bradl, M.; Bauer, J.; Inomata, T.; Zielasek, J.; Nave, K.-A.; Toyka, K.; Lassmann, H.; Wekerle, H. Transgenic Lewis rats overexpressing the proteolipid protein gene: Myelin degeneration and its effect on T cell-mediated experimental autoimmune encephalomyelitis. Acta Neuropathol. 1999, 97, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.A.; Larsen, E.C.; Kondo, Y.; Duncan, I.D. Characterization of a PLP-overexpressing transgenic rat, a model for the connatal form of Pelizaeus–Merzbacher disease. Neurobiol. Dis. 2011, 44, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Stoffel, W. Disruption of the compacted myelin sheath of axons of the central nervous system in proteolipid protein-deficient mice. Proc. Natl. Acad. Sci. USA 1994, 91, 11709–11713. [Google Scholar] [CrossRef] [PubMed]
- Klugmann, M.; Schwab, M.H.; Pühlhofer, A.; Schneider, A.; Zimmermann, F.; Griffiths, I.R.; Nave, K.-A. Assembly of CNS Myelin in the Absence of Proteolipid Protein. Neuron 1997, 18, 59–70. [Google Scholar] [CrossRef]
- Garbern, J.Y.; Yool, D.A.; Moore, G.J.; Wilds, I.B.; Faulk, M.W.; Klugmann, M.; Nave, K.A.; Sistermans, E.A.; van der Knaap, M.S.; Bird, T.D. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 2002, 125, 551–561. [Google Scholar] [CrossRef]
- Duncan, I.D.; Kondo, Y.; Zhang, S.-C. The Myelin Mutants as Models to Study Myelin Repair in the Leukodystrophies. Neurotherapeutics 2011, 8, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Hobson, G.M.; Garbern, J.Y. Pelizaeus-Merzbacher Disease, Pelizaeus-Merzbacher-Like Disease 1, and Related Hypomyelinating Disorders. Skull Base 2012, 32, 062–067. [Google Scholar] [CrossRef]
- Kaga, K. Pelizaeus–Merzbacher Disease. In Landau-Kleffner Syndrome and Central Auditory Disorders in Children; Springer: Singapore, 2021; pp. 131–134. [Google Scholar]
- Prukop, T.; Epplen, D.B.; Nientiedt, T.; Wichert, S.P.; Fledrich, R.; Stassart, R.M.; Rossner, M.J.; Edgar, J.M.; Werner, H.B.; Nave, K.-A.; et al. Progesterone Antagonist Therapy in a Pelizaeus-Merzbacher Mouse Model. Am. J. Hum. Genet. 2014, 94, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Epplen, D.B.; Prukop, T.; Nientiedt, T.; Albrecht, P.; Arlt, F.A.; Stassart, R.M.; Kassmann, C.M.; Methner, A.; Nave, K.; Werner, H.B.; et al. Curcumin therapy in a Plp1 transgenic mouse model of Pelizaeus-Merzbacher disease. Ann. Clin. Transl. Neurol. 2015, 2, 787–796. [Google Scholar] [CrossRef]
- Saher, G.; Rudolphi, F.; Corthals, K.; Ruhwedel, T.; Schmidt, K.-F.; Löwel, S.; Dibaj, P.; Barrette, B.; Möbius, W.; Nave, K.-A. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat. Med. 2012, 18, 1130–1135. [Google Scholar] [CrossRef]
- Stumpf, S.K.; Berghoff, S.A.; Trevisiol, A.; Spieth, L.; Düking, T.; Schneider, L.V.; Schlaphoff, L.; Dreha-Kulaczewski, S.; Bley, A.; Burfeind, D.; et al. Correction to: Ketogenic diet ameliorates axonal defects and promotes myelination in Pelizaeus–Merzbacher disease. Acta Neuropathol. 2019, 138, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Marteyn, A.; Sarrazin, N.; Yan, J.; Bachelin, C.; Deboux, C.; Santin, M.D.; Gressens, P.; Zujovic, V.; Evercooren, A.B.-V. Modulation of the Innate Immune Response by Human Neural Precursors Prevails over Oligodendrocyte Progenitor Remyelination to Rescue a Severe Model of Pelizaeus-Merzbacher Disease. Stem Cells 2015, 34, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Henry, R.G.; Strober, J.; Kang, S.-M.; Lim, D.A.; Bucci, M.; Caverzasi, E.; Gaetano, L.; Mandelli, M.L.; Ryan, T.; et al. Neural Stem Cell Engraftment and Myelination in the Human Brain. Sci. Transl. Med. 2012, 4, 155ra137. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Buntz, S.; Scotland, P.; Xu, L.; Noeldner, P.; Patel, S.; Wollish, A.; Gunaratne, A.; Gentry, T.; Troy, J.; et al. A cord blood monocyte-derived cell therapy product accelerates brain remyelination. JCI Insight 2016, 1, e86667. [Google Scholar] [CrossRef]
- Wishnew, J.; Page, K.; Wood, S.; Galvin, L.; Provenzale, J.; Escolar, M.; Gustafson, K.; Kurtzberg, J. Umbilical Cord Blood Transplantation to Treat Pelizaeus-Merzbacher Disease in 2 Young Boys. Pediatrics 2014, 134, e1451–e1457. [Google Scholar] [CrossRef]
- Elitt, M.S.; Barbar, L.; Shick, H.E.; Powers, B.E.; Maeno-Hikichi, Y.; Madhavan, M.; Allan, K.C.; Nawash, B.S.; Gevorgyan, A.S.; Hung, S.; et al. Suppression of proteolipid protein rescues Pelizaeus–Merzbacher disease. Nature 2020, 585, 397–403. [Google Scholar] [CrossRef]
- Li, H.; Okada, H.; Suzuki, S.; Sakai, K.; Izumi, H.; Matsushima, Y.; Ichinohe, N.; Goto, Y.-I.; Okada, T.; Inoue, K. Gene suppressing therapy for Pelizaeus-Merzbacher disease using artificial microRNA. JCI Insight. 2019, 4, e125052. [Google Scholar] [CrossRef]
| Animal Model | Species | Genetic Alteration | Protein Alteration | Pathological Features | References |
|---|---|---|---|---|---|
| Jimpy | Mus musculus | A > G 3′ end | Del. Aa 208 > 232 | Dysmelination, gliosis, glial apoptosis | [110] |
| Jimpy msd | Mus musculus | C > T exon 6 | Val > Ala | Dysmelination, gliosis, glial apoptosis | [111] |
| Jimpy 4j | Mus musculus | Exon 2 mutation | Ala 38 > Ser | Dysmelination, gliosis, glial apoptosis | [112] |
| PlpTg66/66 | Mus musculus | 14 extra copies | Dose effect | hypomyelination, astrocytosis, seizures, | [120] |
| PlpTg72/72 | Mus musculus | 6 extra copies | Dose effect | Seizures, convulsions, demyelination, | [121] |
| Rumpshaker | Mus musculus | Point mutation | Ile 186 > Thr | Hypomyelination | [103] |
| PLP null | Mus musculus | Neo cassette exon 3 | - | Hypomyelination | [125] |
| md rats | Rattus norvegicus | A > C exon 3 | The 75 > His | Dysmelination, gliosis, glial, apoptosis | [115] |
| Shaking pups | C. lupus familiaris | Point mutation | Pro > His | Dysmelination, glial activation apoptosis | [116] |
| pt rabbits | O. cuniculs | T > A exon 2 | His 36 > Gln | Dysmyelination, gliosis | [117] |
| RM PMD | Macaca mulata | T 682 > C | Cys 228 > Arg | Dysmelination, glial activation, apoptosis | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, G.; Mattern, C.; Begou, M.; Boespflug-Tanguy, O.; Massaad, C.; Massaad-Massade, L. Mutation of Proteolipid Protein 1 Gene: From Severe Hypomyelinating Leukodystrophy to Inherited Spastic Paraplegia. Biomedicines 2022, 10, 1709. https://doi.org/10.3390/biomedicines10071709
Khalaf G, Mattern C, Begou M, Boespflug-Tanguy O, Massaad C, Massaad-Massade L. Mutation of Proteolipid Protein 1 Gene: From Severe Hypomyelinating Leukodystrophy to Inherited Spastic Paraplegia. Biomedicines. 2022; 10(7):1709. https://doi.org/10.3390/biomedicines10071709
Chicago/Turabian StyleKhalaf, Guy, Claudia Mattern, Mélina Begou, Odile Boespflug-Tanguy, Charbel Massaad, and Liliane Massaad-Massade. 2022. "Mutation of Proteolipid Protein 1 Gene: From Severe Hypomyelinating Leukodystrophy to Inherited Spastic Paraplegia" Biomedicines 10, no. 7: 1709. https://doi.org/10.3390/biomedicines10071709
APA StyleKhalaf, G., Mattern, C., Begou, M., Boespflug-Tanguy, O., Massaad, C., & Massaad-Massade, L. (2022). Mutation of Proteolipid Protein 1 Gene: From Severe Hypomyelinating Leukodystrophy to Inherited Spastic Paraplegia. Biomedicines, 10(7), 1709. https://doi.org/10.3390/biomedicines10071709






