Unexpected Motherhood-Triggered Hearing Loss in the Two-Pore Channel (TPC) Mutant Mouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Pure-Tone Audiogram
2.3. Statistics
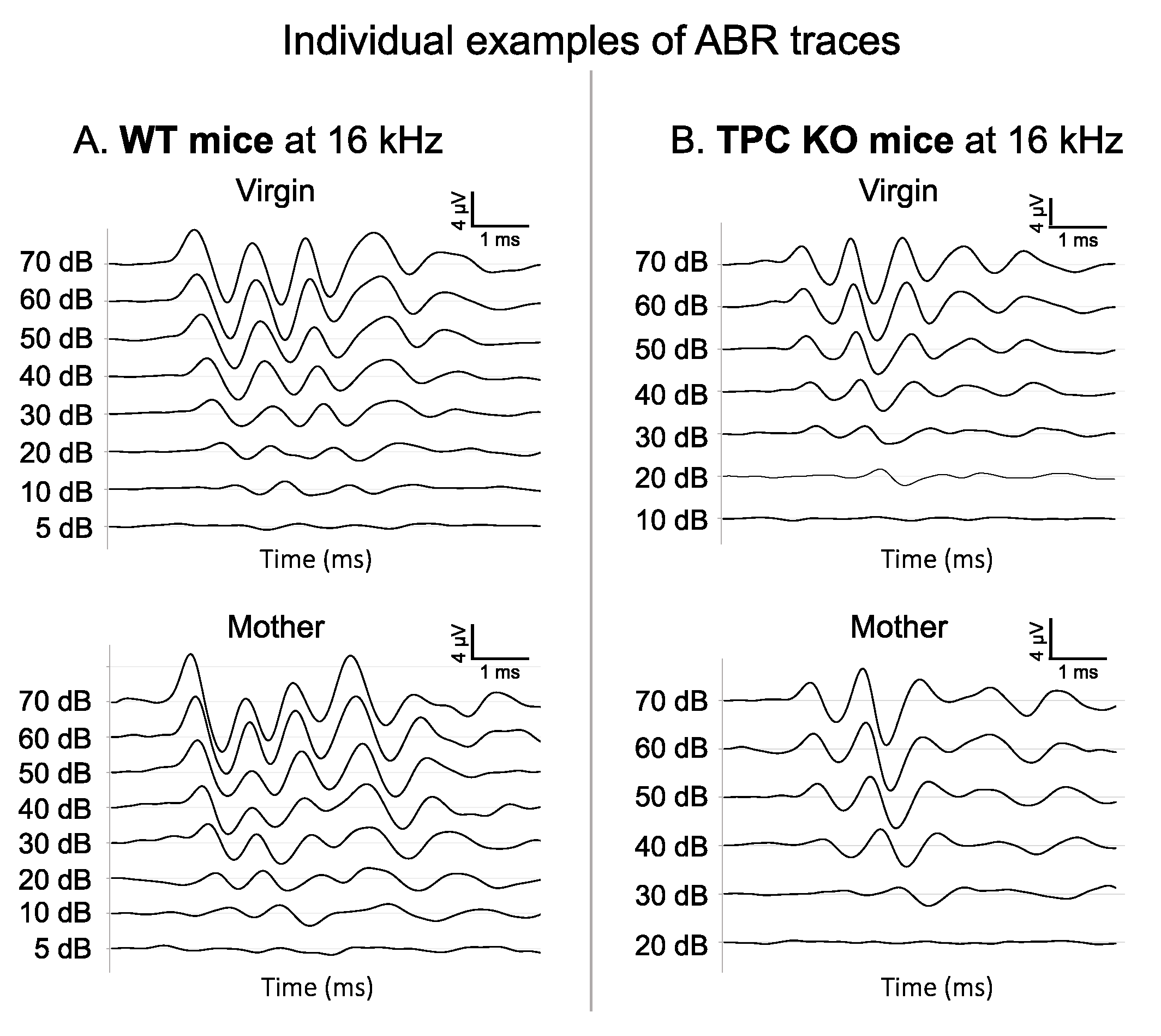
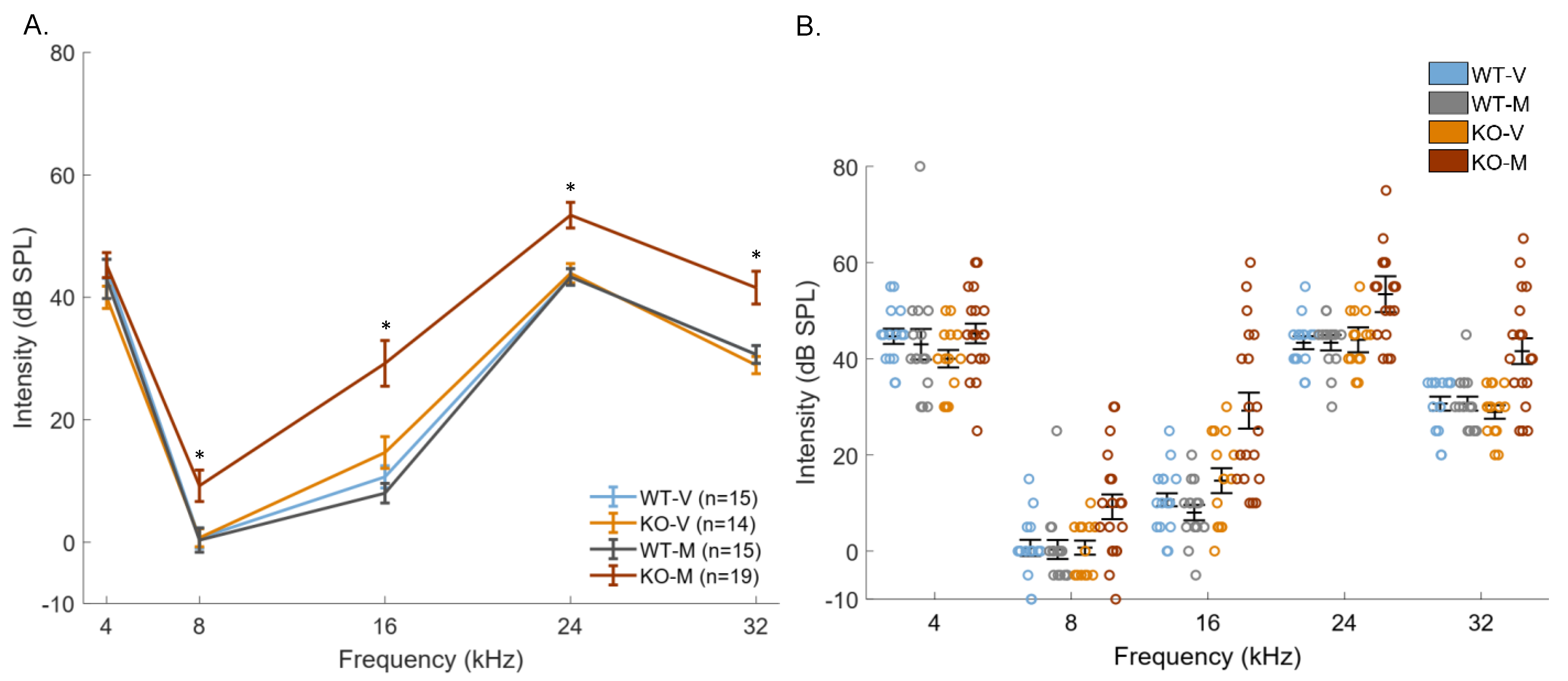
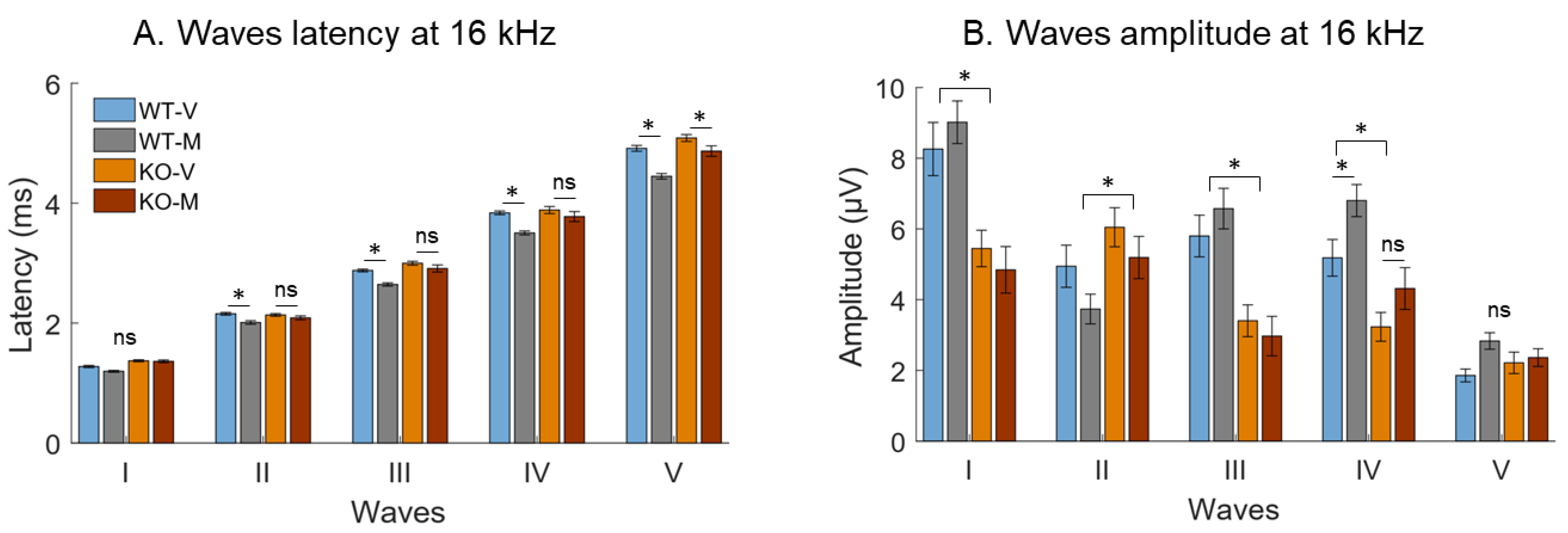
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowl, M.R.; Simon, M.M.; Ingham, N.J.; Greenaway, S.; Santos, L.; Cater, H.; Taylor, S.; Mason, J.; Kurbatova, N.; Pearson, S.; et al. A Large Scale Hearing Loss Screen Reveals an Extensive Unexplored Genetic Landscape for Auditory Dysfunction. Nat. Commun. 2017, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalski, N.; Petit, C. Genes Involved in the Development and Physiology of Both the Peripheral and Central Auditory Systems. Annu. Rev. Neurosci. 2019, 42, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Deffenbacher, K.; Marres, H.A.M.; Cremers, C.W.R.J.; Kimberling, W.J. Genomewide Search and Genetic Localization of a Second Gene Associated with Autosomal Dominant Branchio-Oto-Renal Syndrome: Clinical and Genetic Implications. Am. J. Hum. Genet. 2000, 66, 1715–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyroud, N.; Tesson, F.; Denjoy, I.; Leibovici, M.; Donger, C.; Barhanin, J.; Fauré, S.; Gary, F.; Coumel, P.; Petit, C.; et al. A Novel Mutation in the Potassium Channel Gene KVLQT1 Causes the Jervell and Lange-Nielsen Cardioauditory Syndrome. Nat. Genet. 1997, 15, 186–189. [Google Scholar] [CrossRef]
- Bondurand, N.; Moal, F.D.-L.; Stanchina, L.; Collot, N.; Baral, V.; Marlin, S.; Attie-Bitach, T.; Giurgea, I.; Skopinski, L.; Reardon, W.; et al. Deletions at the SOX10 Gene Locus Cause Waardenburg Syndrome Types 2 and 4. Am. J. Hum. Genet. 2007, 81, 1169–1185. [Google Scholar] [CrossRef] [Green Version]
- Elliott, K.L.; Fritzsch, B.; Yamoah, E.N.; Zine, A. Age-Related Hearing Loss: Sensory and Neural Etiology and Their Interdependence. Front. Aging Neurosci. 2022, 14, 814528. [Google Scholar] [CrossRef]
- Moser, T.; Predoehl, F.; Starr, A. Review of Hair Cell Synapse Defects in Sensorineural Hearing Impairment. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2013, 34, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Someya, S. Mouse Models of Age-Related Mitochondrial Neurosensory Hearing Loss. Mol. Cell. Neurosci. 2013, 55, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Keithley, E.M. Pathology and Mechanisms of Cochlear Aging. J. Neurosci. Res. 2020, 98, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, K.O.; Klepper, K.; Saliba, J.; Friedman, R.A. Advances in Understanding of Presbycusis. J. Neurosci. Res. 2020, 98, 1685–1697. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Acceleration of Age-Related Hearing Loss by Early Noise Exposure: Evidence of a Misspent Youth. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuknecht, H.F.; Gacek, M.R. Cochlear Pathology in Presbycusis. Ann. Otol. Rhinol. Laryngol. 1993, 102, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kurata, N.; Schachern, P.A.; Paparella, M.M.; Cureoglu, S. Histopathologic Evaluation of Vascular Findings in the Cochlea in Patients With Presbycusis. JAMA Otolaryngol. Neck Surg. 2016, 142, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Schulte, B.A.; Schmiedt, R.A. Lateral Wall Na, K-ATPase and Endocochlear Potentials Decline with Age in Quiet-Reared Gerbils. Hear. Res. 1992, 61, 35–46. [Google Scholar] [CrossRef]
- Gratton, M.A.; Schmiedt, R.A.; Schulte, B.A. Age-Related Decreases in Endocochlear Potential Are Associated with Vascular Abnormalities in the Stria Vascularis. Hear. Res. 1996, 102, 181–190. [Google Scholar] [CrossRef]
- Gratton, M.A.; Schulte, B.A.; Smythe, N.M. Quantification of the Stria Vascularis and Strial Capillary Areas in Quiet-Reared Young and Aged Gerbils. Hear. Res. 1997, 114, 1–9. [Google Scholar] [CrossRef]
- Diaz, R.C.; Vazquez, A.E.; Dou, H.; Wei, D.; Cardell, E.L.; Lingrel, J.; Shull, G.E.; Doyle, K.J.; Yamoah, E.N. Conservation of Hearing by Simultaneous Mutation of Na,K-ATPase and NKCC1. J. Assoc. Res. Otolaryngol. 2007, 8, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Lang, H.; Jyothi, V.; Smythe, N.M.; Dubno, J.R.; Schulte, B.A.; Schmiedt, R.A. Chronic Reduction of Endocochlear Potential Reduces Auditory Nerve Activity: Further Confirmation of an Animal Model of Metabolic Presbyacusis. J. Assoc. Res. Otolaryngol. 2010, 11, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Schmiedt, R.A. The Physiology of Cochlear Presbycusis. In The Aging Auditory System; Gordon-Salant, S., Frisina, R.D., Popper, A.N., Fay, R.R., Eds.; Springer Handbook of Auditory Research; Springer: New York, NY, USA, 2010; pp. 9–38. ISBN 978-1-4419-0993-0. [Google Scholar]
- Smith, H.W. Effect of Pregnancy on Otosclerosis. Arch. Otolaryngol. 1948, 48, 159–170. [Google Scholar] [CrossRef]
- Fabbris, C.; Molteni, G.; Tommasi, N.; Marchioni, D. Does Pregnancy Have an Influence on Otosclerosis? J. Laryngol. Otol. 2022, 136, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Frosolini, A.; Marioni, G.; Gallo, C.; de Filippis, C.; Lovato, A. Audio-Vestibular Disorders and Pregnancy: A Systematic Review. Am. J. Otolaryngol. 2021, 42, 103136. [Google Scholar] [CrossRef] [PubMed]
- Wiwatpanit, T.; Remis, N.N.; Ahmad, A.; Zhou, Y.; Clancy, J.C.; Cheatham, M.A.; García-Añoveros, J. Codeficiency of Lysosomal Mucolipins 3 and 1 in Cochlear Hair Cells Diminishes Outer Hair Cell Longevity and Accelerates Age-Related Hearing Loss. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 3177–3189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magariños, M.; Pulido, S.; Aburto, M.R.; de Iriarte Rodríguez, R.; Varela-Nieto, I. Autophagy in the Vertebrate Inner Ear. Front. Cell Dev. Biol. 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Giffen, K.P.; Li, Y.; Liu, H.; Zhao, X.-C.; Zhang, C.-J.; Shen, R.-J.; Wang, T.; Janesick, A.; Chen, B.-B.; Gong, S.-S.; et al. Mutation of SLC7A14 Causes Auditory Neuropathy and Retinitis Pigmentosa Mediated by Lysosomal Dysfunction. Sci. Adv. 2022, 8, eabk0942. [Google Scholar] [CrossRef]
- Martucci, L.L.; Cancela, J.-M. Neurophysiological Functions and Pharmacological Tools of Acidic and Non-Acidic Ca2+ Stores. Cell Calcium 2022, 104, 102582. [Google Scholar] [CrossRef]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.-T.; et al. NAADP Mobilizes Calcium from Acidic Organelles through Two-Pore Channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Brailoiu, E.; Hooper, R.; Cai, X.; Brailoiu, G.C.; Keebler, M.V.; Dun, N.J.; Marchant, J.S.; Patel, S. An Ancestral Deuterostome Family of Two-Pore Channels Mediates Nicotinic Acid Adenine Dinucleotide Phosphate-Dependent Calcium Release from Acidic Organelles. J. Biol. Chem. 2010, 285, 2897–2901. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Schieder, M.; Cuny, H.; Fenske, S.; Gruner, C.; Rötzer, K.; Griesbeck, O.; Harz, H.; Biel, M.; Wahl-Schott, C. The Two-Pore Channel TPCN2 Mediates NAADP-Dependent Ca(2+)-Release from Lysosomal Stores. Pflugers Arch. 2009, 458, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Pitt, S.J.; Lam, A.K.M.; Rietdorf, K.; Galione, A.; Sitsapesan, R. Reconstituted Human TPC1 Is a Proton-Permeable Ion Channel and Is Activated by NAADP or Ca2+. Sci. Signal. 2014, 7, ra46. [Google Scholar] [CrossRef]
- Gerndt, S.; Chen, C.-C.; Chao, Y.-K.; Yuan, Y.; Burgstaller, S.; Scotto Rosato, A.; Krogsaeter, E.; Urban, N.; Jacob, K.; Nguyen, O.N.P.; et al. Agonist-Mediated Switching of Ion Selectivity in TPC2 Differentially Promotes Lysosomal Function. eLife 2020, 9, e54712. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Zhou, Y.; Navarro, B.; Seo, Y.-J.; Aranda, K.; Shi, L.; Battaglia-Hsu, S.; Nissim, I.; Clapham, D.E.; Ren, D. MTOR Regulates Lysosomal ATP-Sensitive Two-Pore Na(+) Channels to Adapt to Metabolic State. Cell 2013, 152, 778–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.-H.; Duann, P.; Komazaki, S.; Park, K.H.; Li, H.; Sun, M.; Sermersheim, M.; Gumpper, K.; Parrington, J.; Galione, A.; et al. Lysosomal Two-Pore Channel Subtype 2 (TPC2) Regulates Skeletal Muscle Autophagic Signaling. J. Biol. Chem. 2015, 290, 3377–3389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Rúa, V.; Feijóo-Bandín, S.; Rodríguez-Penas, D.; Mosquera-Leal, A.; Abu-Assi, E.; Beiras, A.; María Seoane, L.; Lear, P.; Parrington, J.; Portolés, M.; et al. Endolysosomal Two-Pore Channels Regulate Autophagy in Cardiomyocytes. J. Physiol. 2016, 594, 3061–3077. [Google Scholar] [CrossRef] [Green Version]
- Gerasimenko, J.V.; Charlesworth, R.M.; Sherwood, M.W.; Ferdek, P.E.; Mikoshiba, K.; Parrington, J.; Petersen, O.H.; Gerasimenko, O.V. Both RyRs and TPCs Are Required for NAADP-Induced Intracellular Ca2+ Release. Cell Calcium 2015, 58, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Foster, W.J.; Taylor, H.B.C.; Padamsey, Z.; Jeans, A.F.; Galione, A.; Emptage, N.J. Hippocampal MGluR1-Dependent Long-Term Potentiation Requires NAADP-Mediated Acidic Store Ca2+ Signaling. Sci. Signal. 2018, 11, eaat9093. [Google Scholar] [CrossRef] [Green Version]
- Favia, A.; Desideri, M.; Gambara, G.; D’Alessio, A.; Ruas, M.; Esposito, B.; Del Bufalo, D.; Parrington, J.; Ziparo, E.; Palombi, F.; et al. VEGF-Induced Neoangiogenesis Is Mediated by NAADP and Two-Pore Channel-2-Dependent Ca2+ Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E4706–E4715. [Google Scholar] [CrossRef] [Green Version]
- Ruas, M.; Galione, A.; Parrington, J. Two-Pore Channels: Lessons from Mutant Mouse Models. Messenger 2015, 4, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Chabout, J.; Cressant, A.; Hu, X.; Edeline, J.-M.; Granon, S. Making Choice between Competing Rewards in Uncertain vs. Safe Social Environment: Role of Neuronal Nicotinic Receptors of Acetylcholine. Front. Hum. Neurosci. 2013, 7, 468. [Google Scholar] [CrossRef] [Green Version]
- Chaussenot, R.; Edeline, J.-M.; Le Bec, B.; El Massioui, N.; Laroche, S.; Vaillend, C. Cognitive Dysfunction in the Dystrophin-Deficient Mouse Model of Duchenne Muscular Dystrophy: A Reappraisal from Sensory to Executive Processes. Neurobiol. Learn. Mem. 2015, 124, 111–122. [Google Scholar] [CrossRef]
- Martucci, L.L.; Amar, M.; Chaussenot, R.; Benet, G.; Bauer, O.; de Zélicourt, A.; Nosjean, A.; Launay, J.-M.; Callebert, J.; Sebrié, C.; et al. A Multiscale Analysis in CD38-/- Mice Unveils Major Prefrontal Cortex Dysfunctions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 5823–5835. [Google Scholar] [CrossRef] [PubMed]
- Royer, J.; Huetz, C.; Occelli, F.; Cancela, J.-M.; Edeline, J.-M. Enhanced Discriminative Abilities of Auditory Cortex Neurons for Pup Calls Despite Reduced Evoked Responses in C57BL/6 Mother Mice. Neuroscience 2021, 453, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.Y.; Johnson, K.R.; Erway, L.C. Assessment of Hearing in 80 Inbred Strains of Mice by ABR Threshold Analyses. Hear. Res. 1999, 130, 94–107. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.A.; Shepard, K.N.; McClintock, S.K.; Liu, R.C. Adult Plasticity in the Subcortical Auditory Pathway of the Maternal Mouse. PLoS ONE 2014, 9, e101630. [Google Scholar] [CrossRef] [PubMed]
- Kössl, M.; Vater, M. Noradrenaline Enhances Temporal Auditory Contrast and Neuronal Timing Precision in the Cochlear Nucleus of the Mustached Bat. J. Neurosci. Off. J. Soc. Neurosci. 1989, 9, 4169–4178. [Google Scholar] [CrossRef]
- Hurley, L.M.; Pollak, G.D. Serotonin Differentially Modulates Responses to Tones and Frequency-Modulated Sweeps in the Inferior Colliculus. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 8071–8082. [Google Scholar] [CrossRef] [Green Version]
- Hurley, L.M.; Pollak, G.D. Serotonin Shifts First-Spike Latencies of Inferior Colliculus Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 7876–7886. [Google Scholar] [CrossRef] [Green Version]
- Davis, L.C.; Morgan, A.J.; Galione, A. Acidic Ca2+ Stores and Immune-Cell Function. Cell Calcium 2022, 101, 102516. [Google Scholar] [CrossRef]
- Marchant, J.S.; Patel, S. Two-Pore Channels at the Intersection of Endolysosomal Membrane Traffic. Biochem. Soc. Trans. 2015, 43, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Occelli, F.; Hasselmann, F.; Bourien, J.; Puel, J.-L.; Desvignes, N.; Wiszniowski, B.; Edeline, J.-M.; Gourévitch, B. Temporal Alterations to Central Auditory Processing without Synaptopathy after Lifetime Exposure to Environmental Noise. Cereb. Cortex 2022, 32, 1737–1754. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Young, Y.-H. Sudden Deafness during Antepartum versus Postpartum Periods. ORL J. Oto-Rhino-Laryngol. Its Relat. Spec. 2017, 79, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jiang, Q.; Tang, H. Sudden Sensorineural Hearing Loss during Pregnancy: Clinical Characteristics, Management and Outcome. Acta Otolaryngol. 2019, 139, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.-L.; Zeng, F.-Q.; Zhou, Z.; Yan, M.; Zhang, W.; Liu, M.; Ke, Z.-Y. Intratympanic Dexamethasone Injection for Sudden Sensorineural Hearing Loss in Pregnancy. World J. Clin. Cases 2020, 8, 4051–4058. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M.; Cadge, B.A.; Ziff, J.L.; Mowat, A.J.; Nash, R.; Lavy, J.A.; Powell, H.R.F.; Aldren, C.P.; Saeed, S.R.; Dawson, S.J. The Epidemiology of Otosclerosis in a British Cohort. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2019, 40, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippy, W.H.; Berenholz, L.P.; Schuring, A.G.; Burkey, J.M. Does Pregnancy Affect Otosclerosis? Laryngoscope 2005, 115, 1833–1836. [Google Scholar] [CrossRef]
- Xu, K.; Bai, X.; Chen, S.; Xie, L.; Qiu, Y.; Li, H.; Sun, Y. CCDC154 Mutant Caused Abnormal Remodeling of the Otic Capsule and Hearing Loss in Mice. Front. Cell Dev. Biol. 2021, 9, 637011. [Google Scholar] [CrossRef]
- Notomi, T.; Ezura, Y.; Noda, M. Identification of Two-Pore Channel 2 as a Novel Regulator of Osteoclastogenesis. J. Biol. Chem. 2012, 287, 35057–35064. [Google Scholar] [CrossRef] [Green Version]
- Capel, R.A.; Bolton, E.L.; Lin, W.K.; Aston, D.; Wang, Y.; Liu, W.; Wang, X.; Burton, R.-A.B.; Bloor-Young, D.; Shade, K.-T.; et al. Two-Pore Channels (TPC2s) and Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) at Lysosomal-Sarcoplasmic Reticular Junctions Contribute to Acute and Chronic β-Adrenoceptor Signaling in the Heart. J. Biol. Chem. 2015, 290, 30087–30098. [Google Scholar] [CrossRef] [Green Version]
- Favia, A.; Pafumi, I.; Desideri, M.; Padula, F.; Montesano, C.; Passeri, D.; Nicoletti, C.; Orlandi, A.; Del Bufalo, D.; Sergi, M.; et al. NAADP-Dependent Ca2+ Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016, 6, 18925. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Royer, J.; Cancela, J.-M.; Edeline, J.-M. Unexpected Motherhood-Triggered Hearing Loss in the Two-Pore Channel (TPC) Mutant Mouse. Biomedicines 2022, 10, 1708. https://doi.org/10.3390/biomedicines10071708
Royer J, Cancela J-M, Edeline J-M. Unexpected Motherhood-Triggered Hearing Loss in the Two-Pore Channel (TPC) Mutant Mouse. Biomedicines. 2022; 10(7):1708. https://doi.org/10.3390/biomedicines10071708
Chicago/Turabian StyleRoyer, Juliette, José-Manuel Cancela, and Jean-Marc Edeline. 2022. "Unexpected Motherhood-Triggered Hearing Loss in the Two-Pore Channel (TPC) Mutant Mouse" Biomedicines 10, no. 7: 1708. https://doi.org/10.3390/biomedicines10071708
APA StyleRoyer, J., Cancela, J.-M., & Edeline, J.-M. (2022). Unexpected Motherhood-Triggered Hearing Loss in the Two-Pore Channel (TPC) Mutant Mouse. Biomedicines, 10(7), 1708. https://doi.org/10.3390/biomedicines10071708





