2-Arachidonoylglycerol Reduces the Production of Interferon-Gamma in T Lymphocytes from Patients with Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants’ Characteristics
2.2. Peripheral Blood Mononuclear Cells (PBMCs) Isolation and Culture Conditions
2.3. Intracellular Cytokine Assay, CB Receptors Inhibition, and Flow Cytometry
2.4. Cell Sorting
2.5. Total RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) Analysis
2.6. Statistical Analysis
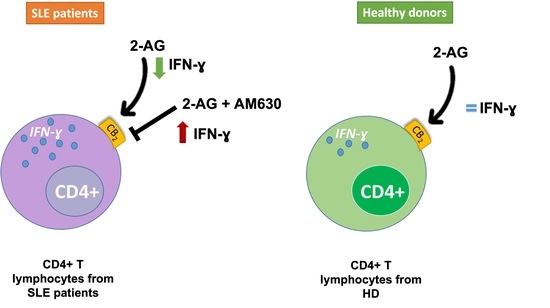
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, A.P.; Raghuram, J. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, 313–319. [Google Scholar] [CrossRef]
- Dörner, T.; Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 2019, 393, 2344–2358. [Google Scholar] [CrossRef]
- Van Vollenhoven, R.F.; Mosca, M.; Bertsias, G.; Isenberg, D.; Kuhn, A.; Lerstrøm, K.; Aringer, M.; Bootsma, H.; Boumpas, D.; Bruce, I.N.; et al. Treat-to-target in systemic lupus erythematosus: Recommendations from an international task force. Ann. Rheum. Dis. 2014, 73, 958–967. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, A.R.P.; Voskuyl, A.E.; van Vollenhoven, R.F. Treat-to-target in systemic lupus erythematosus: Advancing towards its implementation. Nat. Rev. Rheumatol. 2022, 18, 146–157. [Google Scholar] [CrossRef]
- Tsokos, G.C. Autoimmunity and organ damage in systemic lupus erythematosus. Nat. Immunol. 2020, 21, 605–614. [Google Scholar] [CrossRef]
- Luo, S.; Long, H.; Lu, Q. Recent advances in understanding pathogenesis and therapeutic strategies of Systemic Lupus Erythematosus. Int. Immunopharmacol. 2020, 89, 107028. [Google Scholar] [CrossRef]
- Robinson, G.A.; Wilkinson, M.G.L.; Wincup, C. The Role of Immunometabolism in the Pathogenesis of Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 806560. [Google Scholar] [CrossRef]
- Fasano, S.; Margiotta, D.P.E.; Pierro, L.; Navarini, L.; Riccardi, A.; Afeltra, A.; Valentini, G. Prolonged remission is associated with a reduced risk of cardiovascular disease in patients with systemic lupus erythematosus: A GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Clin. Rheumatol. 2019, 38, 457–463. [Google Scholar] [CrossRef]
- Margiotta, D.P.E.; Basta, F.; Dolcini, G.; Batani, V.; Lo Vullo, M.; Vernuccio, A.; Navarini, L.; Afeltra, A. Physical activity and sedentary behavior in patients with Systemic Lupus Erythematosus. PLoS ONE 2018, 13, e0193728. [Google Scholar] [CrossRef]
- Szabó, M.Z.; Szodoray, P.; Kiss, E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 2017, 65, 543–550. [Google Scholar] [CrossRef]
- Nuttall, S.L.; Heaton, S.; Piper, M.K.; Martin, U.; Gordon, C. Cardiovascular risk in systemic lupus erythematosus--evidence of increased oxidative stress and dyslipidaemia. Rheumatology 2003, 42, 758–762. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, H.M.; Sarhan, E.M.; Komber, U. Higher circulating levels of OxLDL % of LDL are associated with subclinical atherosclerosis in female patients with systemic lupus erythematosus. Rheumatol. Int. 2014, 34, 617–623. [Google Scholar] [CrossRef]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef]
- Navarini, L.; Bisogno, T.; Margiotta, D.P.E.; Piccoli, A.; Angeletti, S.; Laudisio, A.; Ciccozzi, M.; Afeltra, A.; Maccarrone, M. Role of the Specialized Proresolving Mediator Resolvin D1 in Systemic Lupus Erythematosus: Preliminary Results. J. Immunol. Res. 2018, 2018, 5264195. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Ding, S.; Liu, S.; Li, X.; Tang, X.; Sun, L. Resolvin D1 Improves the Treg/Th17 Imbalance in Systemic Lupus Erythematosus Through miR-30e-5p. Front. Immunol. 2021, 12, 1926. [Google Scholar] [CrossRef]
- Abdoel, N.; Brun, S.; Bracho, C.; Rodríguez, M.A.; Blasini, A.M. Linker for activation of T cells is displaced from lipid rafts and decreases in lupus T cells after activation via the TCR/CD3 pathway. Clin. Immunol. 2012, 142, 243–251. [Google Scholar] [CrossRef]
- Jury, E.C.; Kabouridis, P.S.; Flores-Borja, F.; Mageed, R.A.; Isenberg, D.A. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J. Clin. Investig. 2004, 113, 1176–1187. [Google Scholar] [CrossRef]
- McDonald, G.; Deepak, S.; Miguel, L.; Hall, C.J.; Isenberg, D.A.; Magee, A.I.; Butters, T.; Jury, E.C. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. J. Clin. Investig. 2014, 124, 712–724. [Google Scholar] [CrossRef] [Green Version]
- Surls, J.; Nazarov-Stoica, C.; Kehl, M.; Olsen, C.; Casares, S.; Brumeanu, T.D. Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. PLoS ONE 2012, 7, e38733. [Google Scholar] [CrossRef] [Green Version]
- Flores-Borja, F.; Kabouridis, P.S.; Jury, E.C.; Isenberg, D.A.; Mageed, R.A. Altered lipid raft-associated proximal signaling and translocation of CD45 tyrosine phosphatase in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 291–302. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Navarini, L.; Bisogno, T.; Mozetic, P.; Piscitelli, F.; Margiotta, D.P.E.; Basta, F.; Afeltra, A.; Maccarrone, M. Endocannabinoid system in systemic lupus erythematosus: First evidence for a deranged 2-arachidonoylglycerol metabolism. Int. J. Biochem. Cell Biol. 2018, 99, 161–168. [Google Scholar] [CrossRef]
- Rahaman, O.; Bhattacharya, R.; Liu, C.S.C.; Raychaudhuri, D.; Ghosh, A.R.; Bandopadhyay, P.; Pal, S.; Goswami, R.P.; Sircar, G.; Ghosh, P.; et al. Cutting Edge: Dysregulated Endocannabinoid-Rheostat for Plasmacytoid Dendritic Cell Activation in a Systemic Lupus Endophenotype. J. Immunol. 2019, 202, 1674–1679. [Google Scholar] [CrossRef] [Green Version]
- Petri, M.; Orbai, A.M.; Alarcõn, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Doria, A.; Gatto, M.; Zen, M.; Iaccarino, L.; Punzi, L. Optimizing outcome in SLE: Treating-to-target and definition of treatment goals. Autoimmun. Rev. 2014, 13, 770–777. [Google Scholar] [CrossRef]
- Romero-Diaz, J.; Isenberg, D.; Ramsey-Goldman, R. Measures of adult systemic lupus erythematosus: Updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res. (Hoboken) 2011, 63 (Suppl. 11), S37–S46. [Google Scholar] [CrossRef] [Green Version]
- Gasperi, V.; Evangelista, D.; Chiurchiù, V.; Florenzano, F.; Savini, I.; Oddi, S.; Avigliano, L.; Catani, M.V.; Maccarrone, M. 2-Arachidonoylglycerol modulates human endothelial cell/leukocyte interactions by controlling selectin expression through CB1 and CB2 receptors. Int. J. Biochem. Cell Biol. 2014, 51, 79–88. [Google Scholar] [CrossRef]
- Walter, M.R. The Role of Structure in the Biology of Interferon Signaling. Front. Immunol. 2020, 11, 606489. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019, 20, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvi, E.; Lorenzini, S.; Garcia-Gonzalez, E.; Maggio, R.; Lazzerini, P.E.; Capecchi, P.L.; Balistreri, E.; Spreafico, A.; Niccolini, S.; Pompella, G.; et al. Inhibitory effect of synthetic cannabinoids on cytokine production in rheumatoid fibroblast-like synoviocytes. Clin. Exp. Rheumatol. 2008, 26, 574–581. [Google Scholar] [PubMed]
- Lourbopoulos, A.; Grigoriadis, N.; Lagoudaki, R.; Touloumi, O.; Polyzoidou, E.; Mavromatis, I.; Tascos, N.; Breuer, A.; Ovadia, H.; Karussis, D.; et al. Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res. 2011, 1390, 126–141. [Google Scholar] [CrossRef]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur. J. Immunol. 2016, 46, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Chiurchiù, V.; Leuti, A.; Smoum, R.; Mechoulam, R.; Maccarrone, M. Bioactive lipids ALIAmides differentially modulate inflammatory responses of distinct subsets of primary human T lymphocytes. FASEB J. 2018, 32, 5716–5723. [Google Scholar] [CrossRef]
- Muhammad Yusoff, F.; Wong, K.K.; Mohd Redzwan, N. Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity 2020, 53, 8–20. [Google Scholar] [CrossRef]
- Lee, S.K.; Silva, D.G.; Martin, J.L.; Pratama, A.; Hu, X.; Chang, P.P.; Walters, G.; Vinuesa, C.G. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 2012, 37, 880–892. [Google Scholar] [CrossRef] [Green Version]
- Domeier, P.P.; Chodisetti, S.B.; Soni, C.; Schell, S.L.; Elias, M.J.; Wong, E.B.; Cooper, T.K.; Kitamura, D.; Rahman, Z.S.M. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J. Exp. Med. 2016, 213, 715–732. [Google Scholar] [CrossRef] [Green Version]
- Kokic, V.; Martinovic Kaliterna, D.; Radic, M.; Perkovic, D.; Cvek, M.; Capkun, V. Relationship between vitamin D, IFN-γ, and E2 levels in systemic lupus erythematosus. Lupus 2016, 25, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E.; Lu, R.; Zhao, Y.D.; Fife, D.A.; Robertson, J.M.; Guthridge, J.M.; Niewold, T.B.; Tsokos, G.C.; Keith, M.P.; Harley, J.B.; et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 2016, 75, 2014–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harigai, M.; Kawamoto, M.; Hara, M.; Kubota, T.; Kamatani, N.; Miyasaka, N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J. Immunol. 2008, 181, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Li, Y.; Liu, Y.; Wang, K.; Pan, W. Association Between the Interferon-γ +874 T/A Polymorphism and the Risk and Clinical Manifestations of Systemic Lupus Erythematosus: A Preliminary Study. Pharmgenomics Pers. Med. 2021, 14, 1475–1482. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Wang, Z.; Wang, J. IFN-γ Mediates the Development of Systemic Lupus Erythematosus. BioMed Res. Int. 2020, 2020, 7176515. [Google Scholar] [CrossRef]
- Wen, S.; He, F.; Zhu, X.; Yuan, S.; Liu, H.; Sun, L. IFN-γ, CXCL16, uPAR: Potential biomarkers for systemic lupus erythematosus. Clin. Exp. Rheumatol. 2018, 36, 36–43. [Google Scholar]
- Fava, A.; Buyon, J.; Mohan, C.; Zhang, T.; Belmont, H.M.; Izmirly, P.; Clancy, R.; Trujillo, J.M.; Fine, D.; Zhang, Y.; et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight 2020, 5, e138345. [Google Scholar] [CrossRef]
- Katsuyama, T.; Li, H.; Krishfield, S.M.; Kyttaris, V.C.; Moulton, V.R. Splicing factor SRSF1 limits IFN-γ production via RhoH and ameliorates experimental nephritis. Rheumatology 2021, 60, 420–429. [Google Scholar] [CrossRef]
- Postal, M.; Ruocco, H.H.; Brandão, C.O.; Costallat, L.T.L.; Silva, L.; Cendes, F.; Appenzeller, S. Interferon-γ Is Associated with Cerebral Atrophy in Systemic Lupus Erythematosus. Neuroimmunomodulation 2017, 24, 100–105. [Google Scholar] [CrossRef]
- Iwata, S.; Zhang, M.; Hao, H.; Trimova, G.; Hajime, M.; Miyazaki, Y.; Ohkubo, N.; Satoh Kanda, Y.; Todoroki, Y.; Miyata, H.; et al. Enhanced Fatty Acid Synthesis Leads to Subset Imbalance and IFN-γ Overproduction in T Helper 1 Cells. Front. Immunol. 2020, 11, 593103. [Google Scholar] [CrossRef]
- Kaplan, B.L.F.; Ouyang, Y.; Rockwell, C.E.; Rao, G.K.; Kaminski, N.E. 2-Arachidonoyl-glycerol suppresses interferon-gamma production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2. J. Leukoc. Biol. 2005, 77, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Battistini, L.; Maccarrone, M. Endocannabinoid signalling in innate and adaptive immunity. Immunology 2015, 144, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Ettaro, R.; Laudermilk, L.; Clark, S.D.; Maitra, R. Behavioral assessment of rimonabant under acute and chronic conditions. Behav. Brain Res. 2020, 390, 112697. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.; Kuwana, M.; Khanna, D.; Hummers, L.; Frech, T.; Stevens, W.; Gordon, J.; Kafaja, S.; Matucci-Cerinic, M.; Distler, O.; et al. OP0171 PHASE 3 Trial of Lenabasum, a CB2 Agonist, for the Treatment of Diffuse Cutaneous Systemic Sclerosis (DCSSC). Ann. Rheum. Dis. 2021, 80, 102–103. [Google Scholar] [CrossRef]



| Variable | SLE Participants (n = 12) | Healthy Donors (n = 12) |
|---|---|---|
| Age (years) | 42 (34.5–54.25) | 44 (33–57.5) |
| Disease duration (months) | 123 (38.25–144.5) | NA |
| SLEDAI-2k | 2 (2–2.75) | NA |
| Antinuclear antibodies positivity (n) | 12 | 0 |
| Anti-dsDNA antibodies positivity (n) | 6 | 0 |
| Anti-Sm antibodies positivity (n) | 6 | 0 |
| Anti-phospholipids antibodies positivity (n) | 4 | 0 |
| Anti-RNP antibodies positivity (n) | 4 | 0 |
| Low C3 or C4 levels (n) | 10 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarini, L.; Vomero, M.; Di Donato, S.; Currado, D.; Berardicurti, O.; Marino, A.; Bearzi, P.; Biaggi, A.; Ferrito, M.; Ruscitti, P.; et al. 2-Arachidonoylglycerol Reduces the Production of Interferon-Gamma in T Lymphocytes from Patients with Systemic Lupus Erythematosus. Biomedicines 2022, 10, 1675. https://doi.org/10.3390/biomedicines10071675
Navarini L, Vomero M, Di Donato S, Currado D, Berardicurti O, Marino A, Bearzi P, Biaggi A, Ferrito M, Ruscitti P, et al. 2-Arachidonoylglycerol Reduces the Production of Interferon-Gamma in T Lymphocytes from Patients with Systemic Lupus Erythematosus. Biomedicines. 2022; 10(7):1675. https://doi.org/10.3390/biomedicines10071675
Chicago/Turabian StyleNavarini, Luca, Marta Vomero, Stefano Di Donato, Damiano Currado, Onorina Berardicurti, Annalisa Marino, Pietro Bearzi, Alice Biaggi, Matteo Ferrito, Piero Ruscitti, and et al. 2022. "2-Arachidonoylglycerol Reduces the Production of Interferon-Gamma in T Lymphocytes from Patients with Systemic Lupus Erythematosus" Biomedicines 10, no. 7: 1675. https://doi.org/10.3390/biomedicines10071675
APA StyleNavarini, L., Vomero, M., Di Donato, S., Currado, D., Berardicurti, O., Marino, A., Bearzi, P., Biaggi, A., Ferrito, M., Ruscitti, P., Fava, M., Leuti, A., Cipriani, P., Maccarrone, M., & Giacomelli, R. (2022). 2-Arachidonoylglycerol Reduces the Production of Interferon-Gamma in T Lymphocytes from Patients with Systemic Lupus Erythematosus. Biomedicines, 10(7), 1675. https://doi.org/10.3390/biomedicines10071675