Novel Rivastigmine Derivatives as Promising Multi-Target Compounds for Potential Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Methods and Materials
2.1.2. Preparation of Hydroxyphenylbenzimidazole Carboxylic Acids (BIMa, BIMb)
2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-6-carboxylic Acid (BIMa)
2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-7-carboxylic Acid (BIMb)
2.1.3. General Procedure for the Synthesis of the Carbamates (2a–d)
3-Nitrophenyl Ethylmethylcarbamate (2a)
3-Cyanophenyl Ethylmethylcarbamate (2b)
3-(Cyanomethyl)phenyl Ethylmethylcarbamate (2c)
4-(2-Cyanoethyl)phenyl Ethylmethylcarbamate (2d)
2.1.4. General Procedure for the Synthesis of the Amino-Carbamates (3a–d)
3-Aminophenyl Ethylmethylcarbamate (3a)
3-(Aminomethyl)phenyl Ethylmethylcarbamate (3b)
3-(2-Aminoethyl)phenyl Ethylmethylcarbamate (3c)
4-(3-Aminopropyl)phenyl Ethylmethylcarbamate (3d)
2.1.5. General Synthetic Procedure for the RIV–BIMa Hybrids (4a–d)
3-(2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-6-carboxamido)phenyl Ethylmethylcarbamate, RIV–BIMa (4a)
3-((2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-6-carboxamido)methyl) Phenyl Ethylmethylcarbamate, RIV–BIMa (4b)
3-(2-(2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-6-carboxamido)ethyl) Phenylethylmethylcarbamate, RIV–BIMa (4c)
4-(3-(2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-6-carboxamido)propyl) Phenyl Ethylmethylcarbamate, RIV–BIMa (4d)
2.1.6. General Procedure for the RIV–BIMb Hybrids (5a, 5b, 5d)
3-(2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-4-carboxamido)phenyl Ethylmethylcarbamate, RIV–BIMb (5a)
3-((2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-4-carboxamido)methyl) Phenyl Ethylmethylcarbamate RIV–BIMb (5b)
4-(3-(2-(2-Hydroxyphenyl)-1H-benzo[d]imidazole-4-carboxamido) Propyl) Phenyl Ethyl(methyl)carbamate, RIV–BIMb (5d)
2.2. Molecular Modelling
2.3. Acetylcholinesterase and Butyrylcholinesterase Activity
2.4. Inhibition of Aβ42 Aggregation
2.4.1. Fluorescence Assays
2.4.2. TEM Assays
2.5. Radical Scavenging Activity
2.6. Cell Viability and Neuroprotection of RIV–BIM Compounds in a SH-SY5Y Cell Line
2.7. Prediction of Pharmacokinetic Properties
2.8. Statistical Analysis
3. Results and Discussion
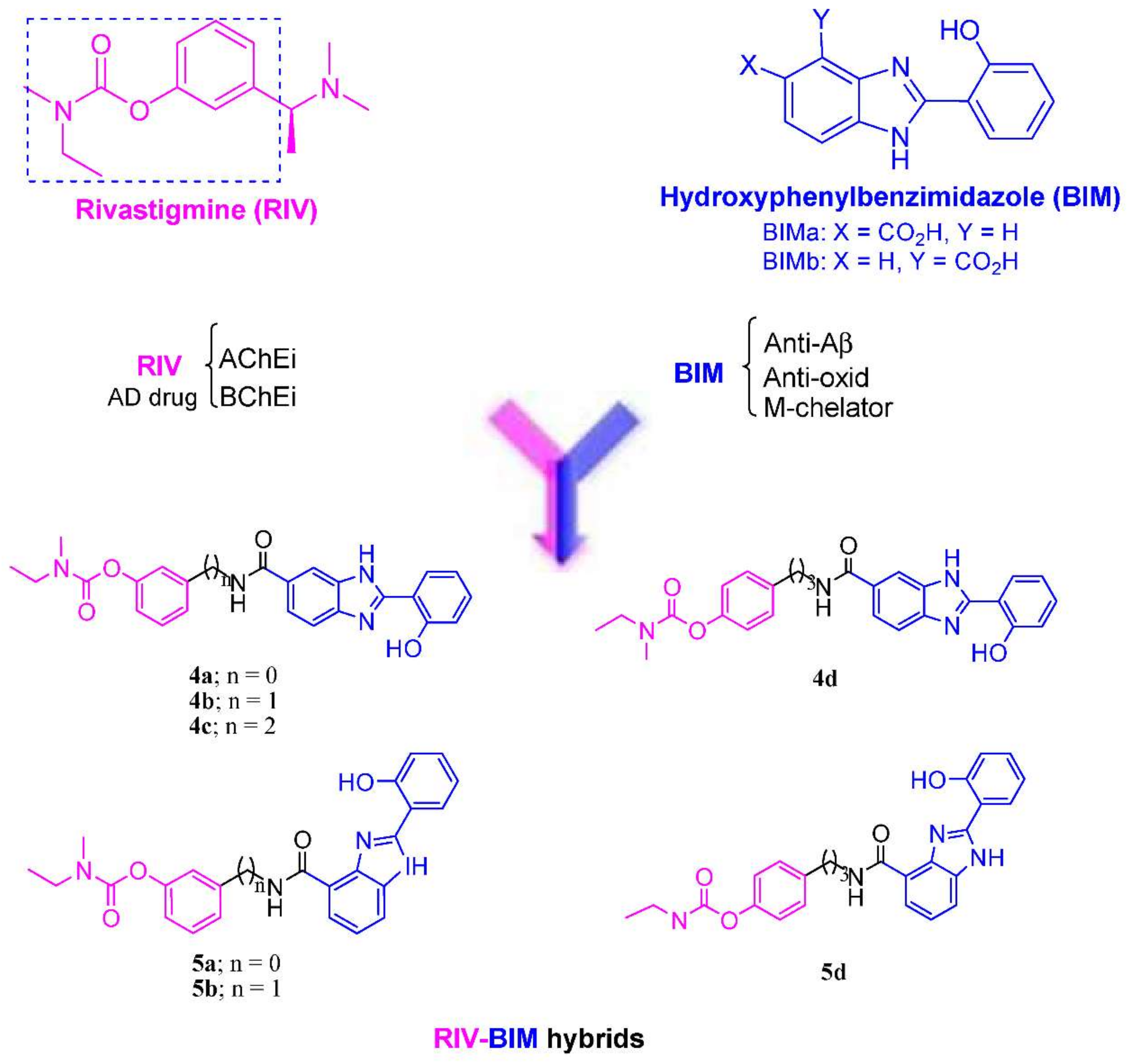
3.1. Molecular Design
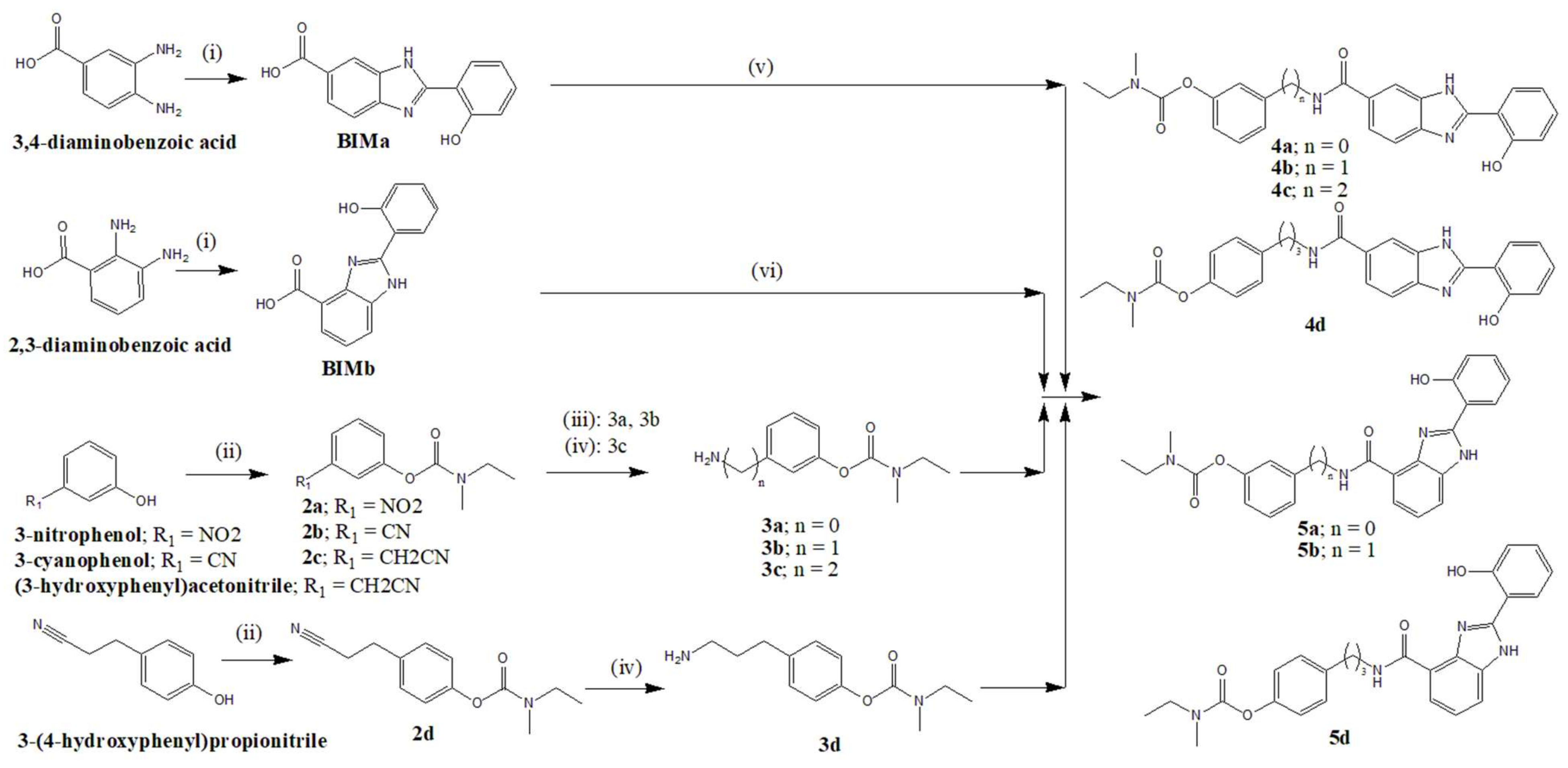
3.2. Synthesis of the Compounds
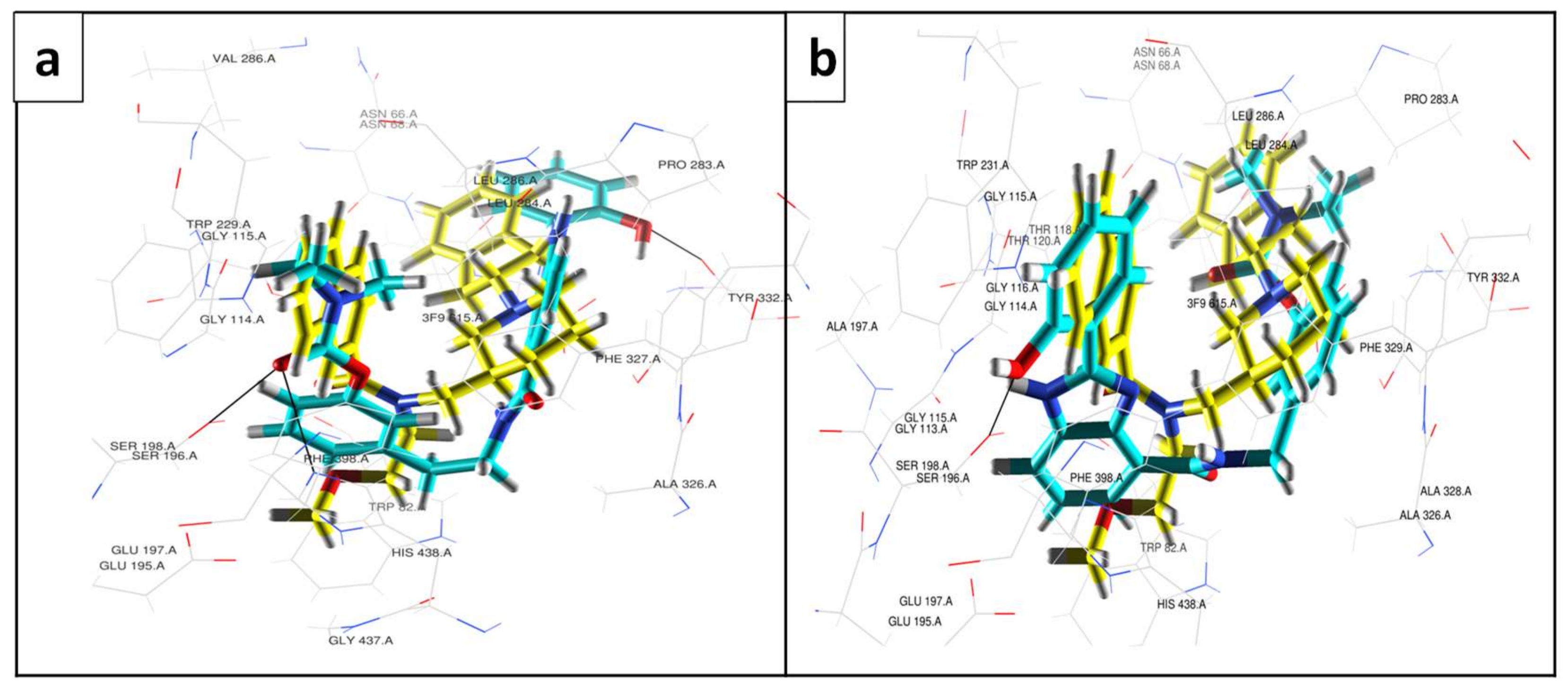
3.3. Molecular Modelling
3.4. Enzyme Inhibitory Activity of the Compounds
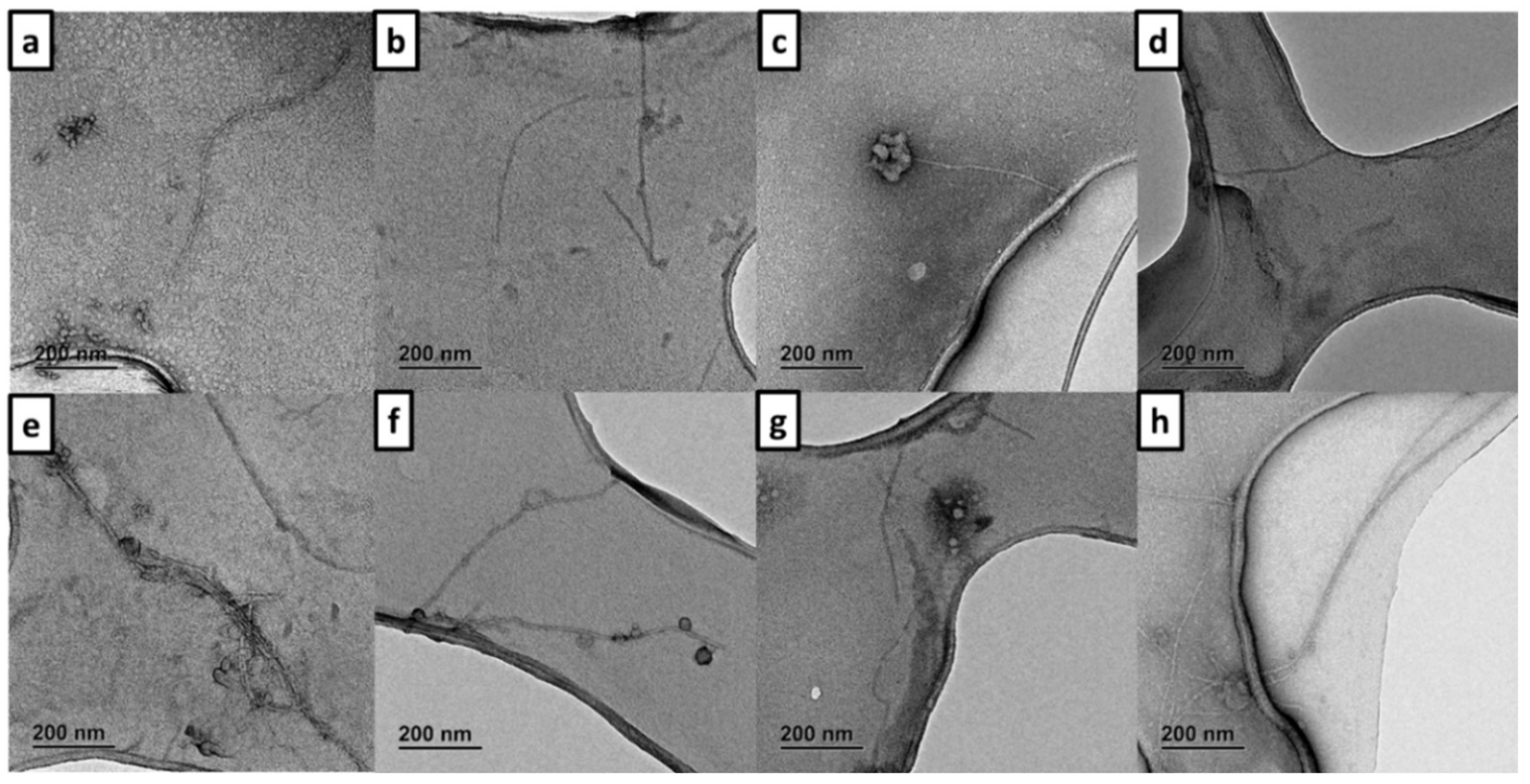
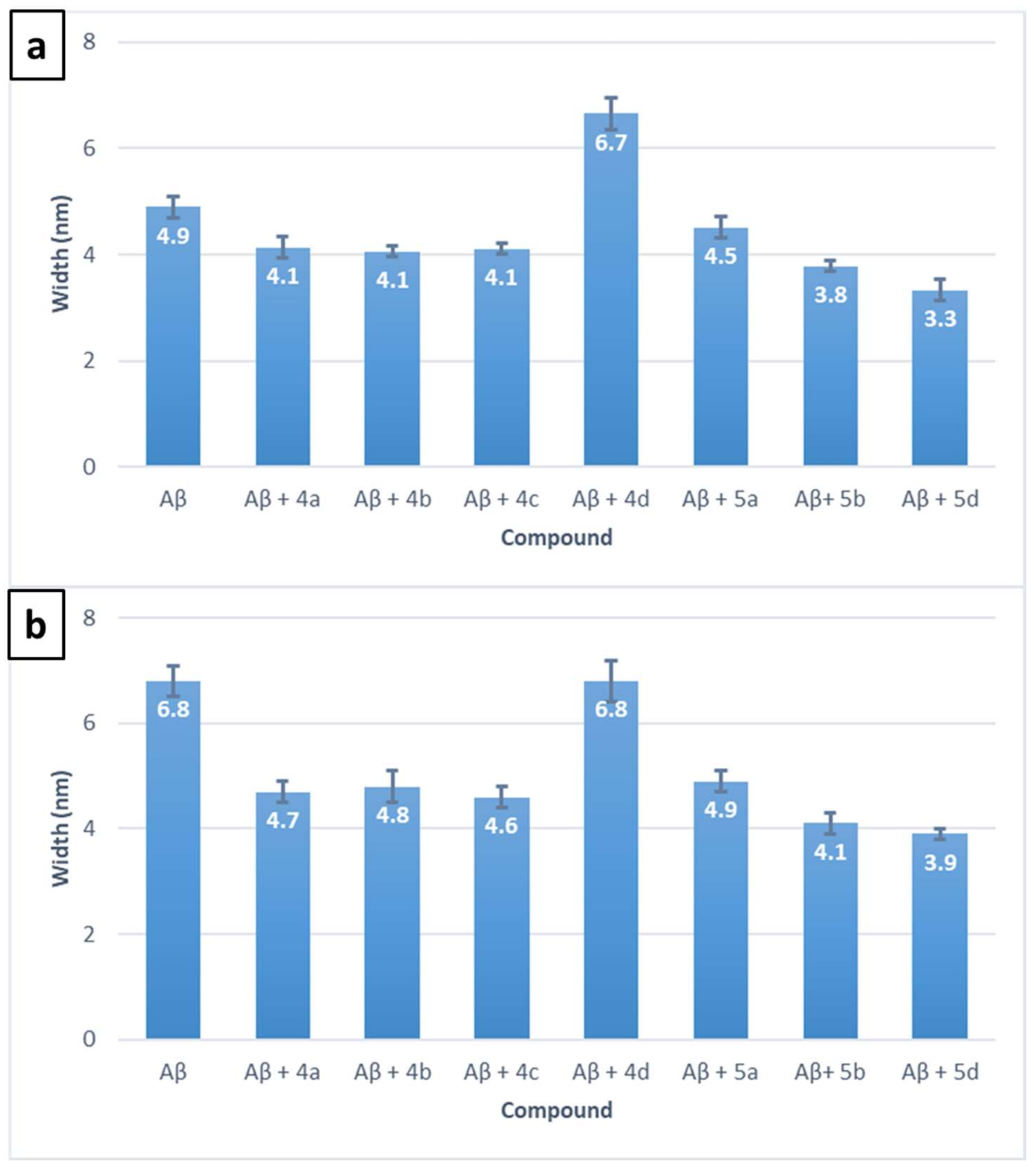
3.5. Inhibition of Self- and Cu(II)-Induced Aβ42 Aggregation
3.6. Radical Scavenging Activity
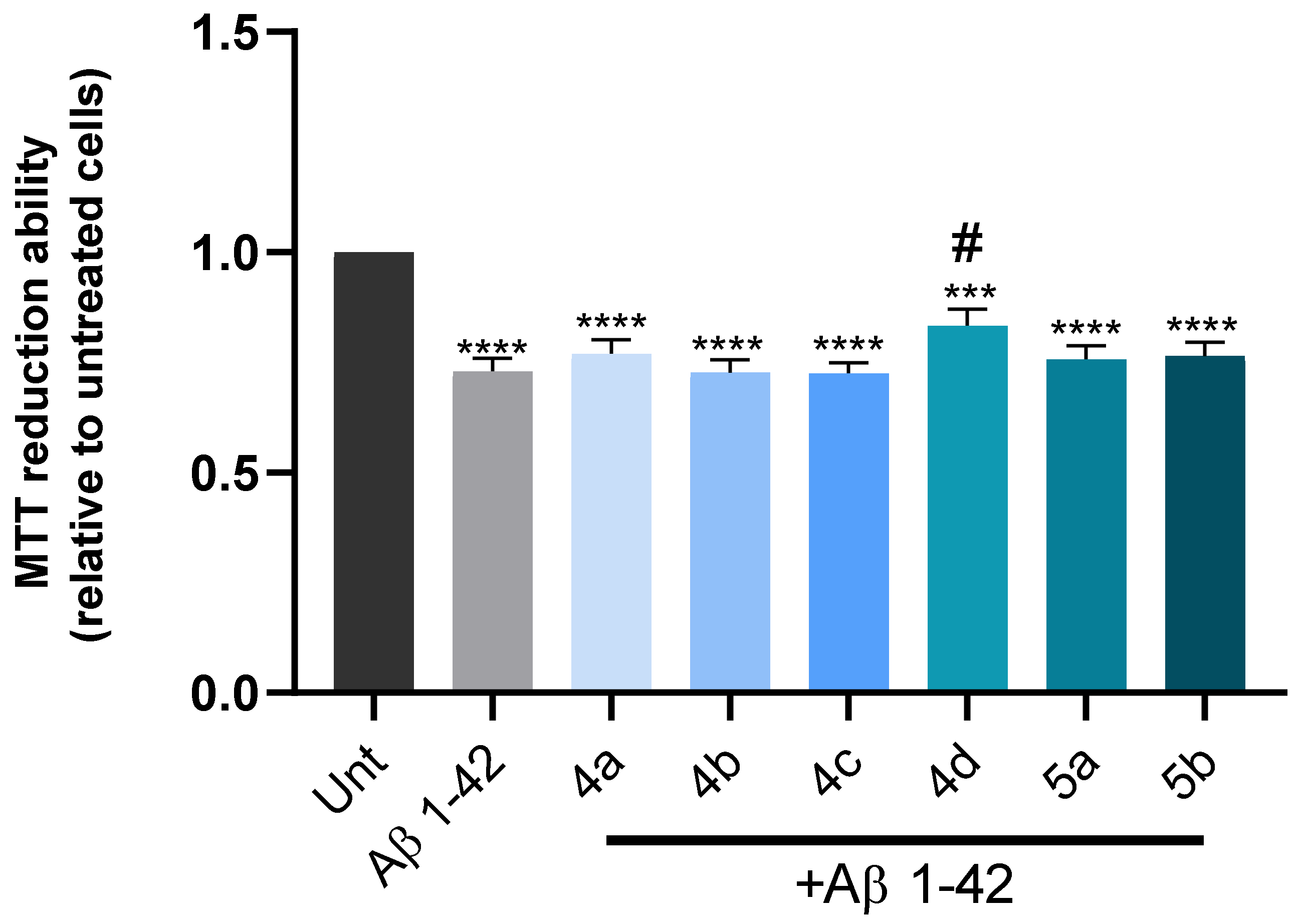
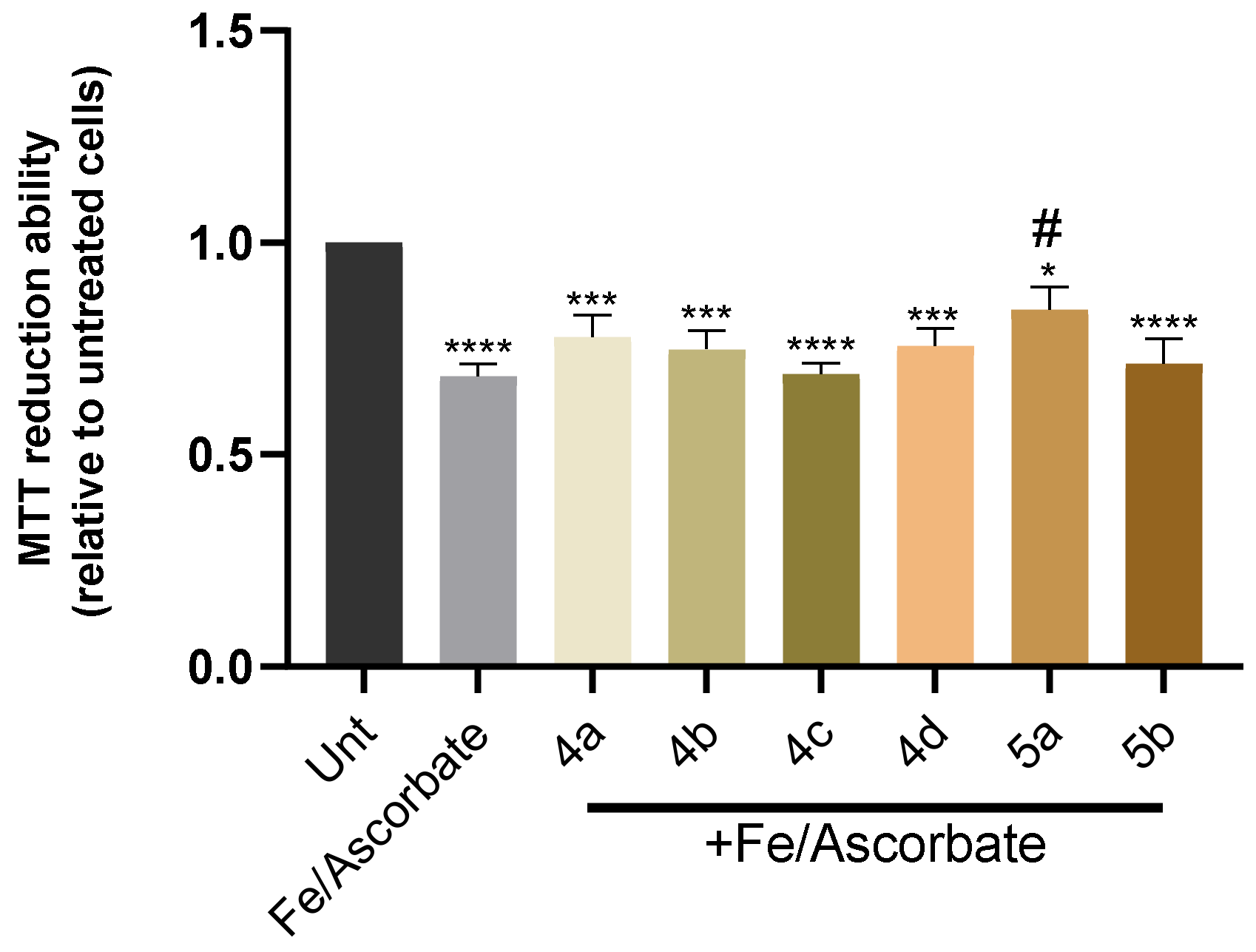
3.7. Cell Viability and Neuroprotection in a SH-SY5Y Cell Line
3.8. Predicted Pharmacokinetic Properties
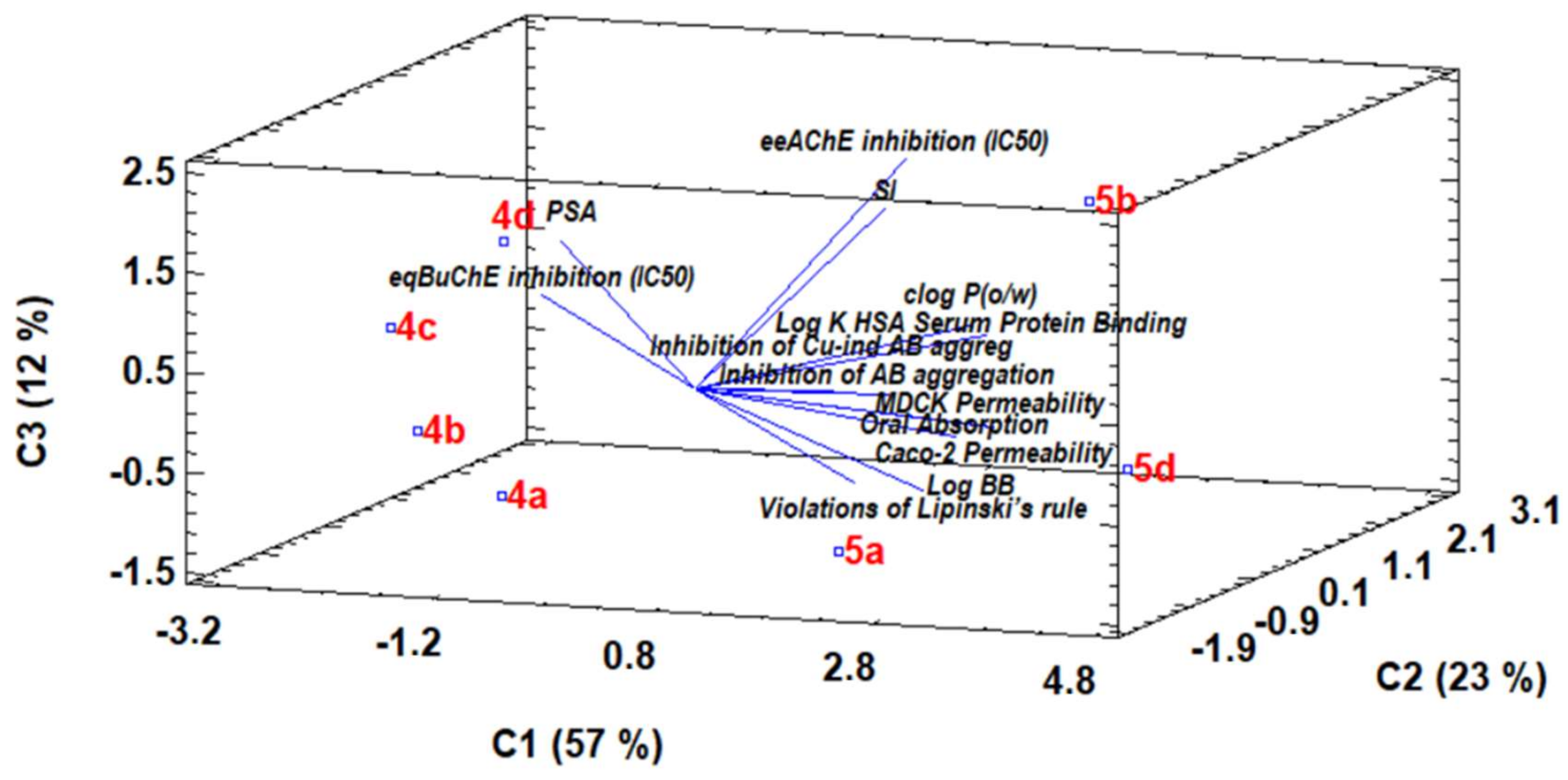
3.9. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylthiocholine |
| AChI | Acetylthiocholine iodide |
| AD | Alzheimer’s Disease |
| ADMET | Absorption, Distribution, Metabolism, Elimination, Toxicity |
| APP | Amyloid Precursor Protein |
| Aβ42 | Beta-amyloid protein, fragment 1-42 |
| BBB | Blood–brain Barrier |
| BIM | Hydroxyphenylbenzimidazole acid |
| BCh | Butyrylthiocholine |
| BChI | S-butyrylthiocholine iodide |
| Caco | Human colon carcinoma cell line |
| CNS | Central nervous system |
| DMA | N,N-dimethylacetamide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMF | Dimethylformamide |
| DMHP | 1,2-Dimethyl-3-hydroxy-4-pyridinone |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl free radical |
| DTNB | 5,5′-dithiobis-(2-nitrobenzoic acid) |
| EC50 | Half maximal effective concentration |
| EDCI | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| eeAChE | Electric eel acetylcholinesterase |
| eqBChE | Equine butyrylcholinesterase |
| FBS | Fetal bovine serum |
| HD | Huntington’s disease |
| HEPES | 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid |
| HFIP | 1,1,1,3,3,3-Hexafluoro-2-propanol |
| HSA | Human serum albumin |
| IC50 | Half maximal inhibitory concentration |
| LC | Liquid chromatography |
| MDCK | Madin–Darby canine kidney cell line |
| M.P | Melting points |
| MS-ESI | Electrospray ionization mass spectrometry |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NMM | N-Methylmorpholine |
| NMR | Nuclear magnetic resonance |
| PCA | Principal component analysis |
| PD | Parkinson’s disease |
| PSA | Polar surface area (Van der Waals surface area of polar nitrogen and oxygen atoms) |
| RF | Retention factor |
| RIV | Rivastigmine |
| ROS | Reactive oxygen species |
| SEM | Standard error of the mean |
| SD | Standard deviation |
| SI | Selectivity index (IC50(AChE)/IC50(BChE) |
| TBTU | 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate |
| TEA | Triethylamine |
| TEM | Transmission electron microscopy |
| ThT | Thioflavin T |
| TLC | Thin-layer chromatography |
| TMS | Tetramethylsilane |
| TRIS | Tris(hydroxymethyl)aminomethane |
| UV-Vis | Ultraviolet–visible spectroscopy |
References
- Onyango, I.G.; Bennett, J.P.; Stokin, G.B. Regulation of neuronal bioenergetics as a therapeutic strategy in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-Y.; Fu, W.-M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Heron, M. Deaths: Leading causes for 2014. In Natl. Vital Stat. Rep.; 2016; 65, p. 5. Available online: https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf (accessed on 1 March 2022).
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.-X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- de Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Karran, E.; de Strooper, B. The amyloid cascade hypothesis: Are we poised for success or failure? J. Neurochem. 2016, 139, 237–252. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K.; et al. Immunization with amyloid-β attenuates Alzheimer disease-like pathology in the PDAPP mouse. Nature 1999, 400, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Anderson, J.P.; Barbour, R.; Basi, G.S.; Caccavello, R.; Davis, D.; Doan, M.; Dovey, H.F.; Frigon, N.; Hong, J.; et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 1999, 402, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Xu, J.; Church, S.J.; Patassini, S.; Begley, P.; Waldvogel, H.J.; Curtis, M.A.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 2017, 9, 1106. [Google Scholar] [CrossRef]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross talk in alzheimer’s disease and parkinson’s disease. Drug Des. Dev. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef]
- Bush, A.I.; Tanzi, R.E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [CrossRef]
- White, A.R.; Kanninen, K.M.; Crouch, P.J. Editorial: Metals and neurodegeneration: Restoring the balance. Front. Aging Neurosci. 2015, 7, 127. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Romero-Sánchez, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Ability of selenium species to inhibit metal-induced Aβ aggregation involved in the development of Alzheimer’s disease. Anal. Bioanal. Chem. 2020, 412, 6485–6497. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, X.B.; Kong, L.Y. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 71, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in multifunctional metal chelators as potential drugs for Alzheimer’s disease. Coord. Chem. Rev. 2016, 327–328, 287–303. [Google Scholar] [CrossRef]
- Ismaili, L.; Refouvelet, B.; Benchekroun, M.; Brogi, S.; Brindisi, M.; Gemma, S.; Campiani, G.; Filipic, S.; Agbaba, D.; Esteban, G.; et al. Multitarget compounds bearing tacrine- and donepezil-like structural and functional motifs for the potential treatment of Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Chaves, S.; Várnagy, K.; Santos, M.A. Recent multi-target approaches on the development of anti-Alzheimer’s agents integrating metal chelation activity. Curr. Med. Chem. 2021, 28, 7247–7277. [Google Scholar] [CrossRef]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Terry, A.V., Jr.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stoffler, A.; Schmitt, F.; Ferris, S.; Mobius, H.J. Memantine in moderateto-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. Ther. 2021, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Wang, K.; Shi, J.; Liu, W.; Cheng, X.; Zhu, G.; Wang, Y.; Zhao, Y.; Qiao, Z.; Wu, A.; et al. The development of advanced structural framework as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112180. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in repositioning Alzheimer´s disease drugs based on a multitarget strategy. Fut. Med. Chem. 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Jeřábek, J.; Uliassi, E.; Guidotti, L.; Korábečný, J.; Soukup, O.; Sepsova, V.; Hrabinova, M.; Kuča, K.; Bartolini, M.; Peña-Altamira, L.E.; et al. Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 250–262. [Google Scholar] [CrossRef]
- Hiremathad, A.; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel Tacrine-hydroxyphenylbenzimidazole hybrids as potential multitarget drug candidates for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 148, 255–267. [Google Scholar] [CrossRef]
- Queda, F.; Calò, S.; Gwizdala, K.; Magalhães, J.D.; Cardoso, S.M.; Chaves, S.; Piemontese, L.; Santos, M.A. Novel Donepezil-Arylsulfonamide Hybrids as Multitarget-Directed Ligands for Potential Treatment of Alzheimer’s Disease. Molecules 2021, 26, 1658. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Bar-On, P.; Millard, C.B.; Harel, M.; Dvir, H.; Enz, A.; Sussman, J.L.; Silman, I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug Rivastigmine. Biochemistry 2002, 41, 3555–3564. [Google Scholar] [CrossRef]
- Chen, Z.; Digiacomo, M.; Tu, Y.; Gu, Q.; Wang, S.; Yang, X.; Chu, J.; Chen, Q.; Han, Y.; Chen, J.; et al. Discovery of novel rivastigmine-hydroxycinnamic acid hybrids as multi-targeted agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 125, 784–792. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Shi, J.; Cheng, X.; Zhu, G.; Wei, R.; Ma, Q.; Yu, L.; Zhao, Y.; Tan, Z.; et al. Apigenin-rivastigmine hybrids as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 187, 11958. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, C.; de Groot, N.S.; Rimola, A.; Álvarez-Larena, Á.; Lloveras, V.; Vidal-Gancedo, J.; Ventura, S.; Vendrell, J.; Sodupe, M.; González-Duarte, P. Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J. Am. Chem. Soc. 2009, 131, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Chaves, S.; Hiremathad, A.; Tomás, D.; Keri, R.S.; Piemontese, L.; Santos, M.A. Exploring the chelating capacity of 2-hydroxyphenyl-benzimidazole based hybrids with multi-target ability as anti-Alzheimer’s agents. New J. Chem. 2018, 42, 16503–16515. [Google Scholar] [CrossRef]
- Piemontese, L.; Tomás, D.; Hiremathad, A.; Capriati, V.; Candeias, E.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J. Enzym. Inhib. Med. Chem. 2018, 33, 1212–1224. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth Heinemann: Oxford, UK, 1999; pp. 1–544. [Google Scholar]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Brus, B.; Košak, U.; Turk, S.; Pišlar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.-P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef]
- Maestro, Version 9.3; Schrödinger Inc.: Portland, OR, USA, 2012.
- Acton, A.; Banck, M.; Bréfort, J.; Cruz, M.; Curtis, D.; Hassinen, T.; Heikkilä, V.; Hutchison, G.; Huuskonen, J.; Jensen, J.; et al. Ghemical, Version 3.0; GPL: Burbank, CA, USA, 2011. [Google Scholar]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Quintanova, C.; Keri, R.S.; Marques, S.M.; Fernandes, M.G.; Cardoso, S.M.; Serralheiro, M.L.; Santos, M.A. Design, synthesis and bioevaluation of tacrine hybrids with cinnamate and cinnamylidene acetate derivatives as potential anti-Alzheimer drugs. MedChemComm 2015, 6, 1969–1977. [Google Scholar] [CrossRef]
- Chao, X.; He, X.; Yang, Y.; Zhou, X.; Jin, M.; Liu, S.; Cheng, Z.; Liu, P.; Wang, Y.; Yu, J.; et al. Design, synthesis and pharmacological evaluation of novel tacrine-caffeic acid hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2012, 22, 6498–6502. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight into the kinetic of amyloid beta (1–42) peptide self-aggregation: Elucidation of inhibitors mechanism of action. ChemBioChem 2007, 8, 2152–2161. [Google Scholar] [CrossRef]
- Lioudyno, M.I.; Broccio, M.; Sokolov, Y.; Rasool, S.; Wu, J.; Alkire, M.T.; Liu, V.; Kozak, J.A.; Dennison, P.R.; Glabe, C.G.; et al. Effect of synthetic Aβ peptide oligomers and fluorinated solvents on Kv1.3 channel properties and membrane conductance. PLoS ONE 2012, 7, e35090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- QikProp, Version 2.5; Schrödinger, LLC.: New York, NY, USA, 2005.
- Harel, M.; Sussman, J.L.; Krejci, E.; Bon, S.; Chanal, P.; Massoulie, J.; Silman, I. Conversion of acetylcholinesterase to butyrylcholinesterase: Modelling and mutagenesis. Proc. Natl. Acad. Sci. USA 1992, 89, 10827–10831. [Google Scholar] [CrossRef]
- Chatonnet, A.; Lockridge, O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef]
- Dighe, S.N.; Deora, G.S.; Mora, E.; Nachon, F.; Chan, S.; Parat, M.O.; Brazzolotto, X.; Ross, B.P. Discovery and structure-activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar] [CrossRef] [PubMed]
- Benek, O.; Korabecny, J.; Soukup, O. A perspective on multi-target drugs for Alzheimer´s disease. Trends Pharmacol. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Arora, S.; Attri, S.; Kaur, P.; Gulati, H.K.; Bhagat, K.; Kumar, N.; Singh, H.; Singh, J.V.; et al. New coumarin-benzotriazole based hybrid molecules as inhibitors of acetylcholinesterase and amyloid aggregation. Bioorganic Med. Chem. Lett. 2020, 30, 127477. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid Aβ peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef]
- Tahmasebinia, F.; Emadi, S. Effect of metal chelators on the aggregation of beta-amyloid peptides in the presence of copper and iron. Biometals 2017, 30, 285–293. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]


| Comp | Antioxid. a EC50 (mM) | AChE Inhib b IC50 (μM) | BChE Inhib b IC50 (μM) | SI c | Aβ42 Self-Aggreg. Inhib d (%) | Aβ42 Cu-Ind. Aggreg. Inhib d (%) |
|---|---|---|---|---|---|---|
| 4a | – | 17.6 ± 0.6 | 5.85 ± 0.03 | 3.03 | 39.0 | 41.2 |
| 4b | 0.9 ± 0.1 | 14 ± 2 | 6.5 ± 0.4 | 2.17 | 39.6 | 48.8 |
| 4c | 10.6 ± 0.3 | 17.7 ± 0.8 | 4.8 ± 0.1 | 3.69 | 21.2 | 22 |
| 4d | – | 24 ± 1 | 19.2 ± 0.7 | 1.25 | 20.1 | 21.3 |
| 5a | 8 ± 1 | 17.6 ± 0.4 | 0.9 ± 0.1 | 19.6 | 44.5 | 45.4 |
| 5b | – | 31.7 ± 0.1 | 0.30 ± 0.01 | 105.7 | 58.7 | 60.8 |
| 5d | – | 21.4 ± 0.4 | 1.7 ± 0.2 | 12.6 | 42.1 | 40.3 |
| DMHP | 0.157 ± 0.008 | – | – | – | – | – |
| Tacrine | – | 0.024 ± 0.007 | – | – | 28.1 | – |
| Curcumin | – | – | – | – | 65.7 | 62.7 |
| Donepezil | – | 0.0075 | 1.42 | 0.005 | – | – |
| Rivastigmine | – | 32.1 | 0.39 | 82.3 | – | – |
| Molecule | Mol. Weight a | PSA b | clog Po/w c | log K (HSA) Serum Protein Binding d | log BB e | Caco-2 Permeability (nm s−1) f | MDCK Permeability (nm s−1) g | Oral Absorption h |
|---|---|---|---|---|---|---|---|---|
| 4a | 430.462 | 114.416 | 3.884 | 0.519 | −1.449 | 401 | 184 | 91 |
| 4b | 444.489 | 116.724 | 4.045 | 0.563 | −1.58 | 365 | 166 | 89 |
| 4c | 458.516 | 117.689 | 4.412 | 0.679 | −1.758 | 333 | 151 | 88 |
| 4d | 472.543 | 116.719 | 4.695 | 0.743 | −1.724 | 382 | 175 | 89 |
| 5a | 430.462 | 113.662 | 4.318 | 0.673 | −1.238 | 535 | 251 | 94 |
| 5b | 444.489 | 115.249 | 4.737 | 0.803 | −1.345 | 539 | 253 | 94 |
| 5d | 472.543 | 115.284 | 5.398 | 0.998 | −1.5 | 548 | 258 | 93 |
| RIV | 250.340 | 40.321 | 2.488 | −0.133 | 0.475 | 1381 | 776 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Brunetti, L.; Piemontese, L.; Pereira-Santos, A.R.; Cardoso, S.M.; Madrid, Y.; Chaves, S.; Santos, M.A. Novel Rivastigmine Derivatives as Promising Multi-Target Compounds for Potential Treatment of Alzheimer’s Disease. Biomedicines 2022, 10, 1510. https://doi.org/10.3390/biomedicines10071510
Vicente-Zurdo D, Rosales-Conrado N, León-González ME, Brunetti L, Piemontese L, Pereira-Santos AR, Cardoso SM, Madrid Y, Chaves S, Santos MA. Novel Rivastigmine Derivatives as Promising Multi-Target Compounds for Potential Treatment of Alzheimer’s Disease. Biomedicines. 2022; 10(7):1510. https://doi.org/10.3390/biomedicines10071510
Chicago/Turabian StyleVicente-Zurdo, David, Noelia Rosales-Conrado, M. Eugenia León-González, Leonardo Brunetti, Luca Piemontese, A. Raquel Pereira-Santos, Sandra M. Cardoso, Yolanda Madrid, Sílvia Chaves, and M. Amélia Santos. 2022. "Novel Rivastigmine Derivatives as Promising Multi-Target Compounds for Potential Treatment of Alzheimer’s Disease" Biomedicines 10, no. 7: 1510. https://doi.org/10.3390/biomedicines10071510
APA StyleVicente-Zurdo, D., Rosales-Conrado, N., León-González, M. E., Brunetti, L., Piemontese, L., Pereira-Santos, A. R., Cardoso, S. M., Madrid, Y., Chaves, S., & Santos, M. A. (2022). Novel Rivastigmine Derivatives as Promising Multi-Target Compounds for Potential Treatment of Alzheimer’s Disease. Biomedicines, 10(7), 1510. https://doi.org/10.3390/biomedicines10071510







