The Clinical Utility of Soluble Serum Biomarkers in Autoimmune Pancreatitis: A Systematic Review
Abstract
:1. Introduction
Aim
2. Methods
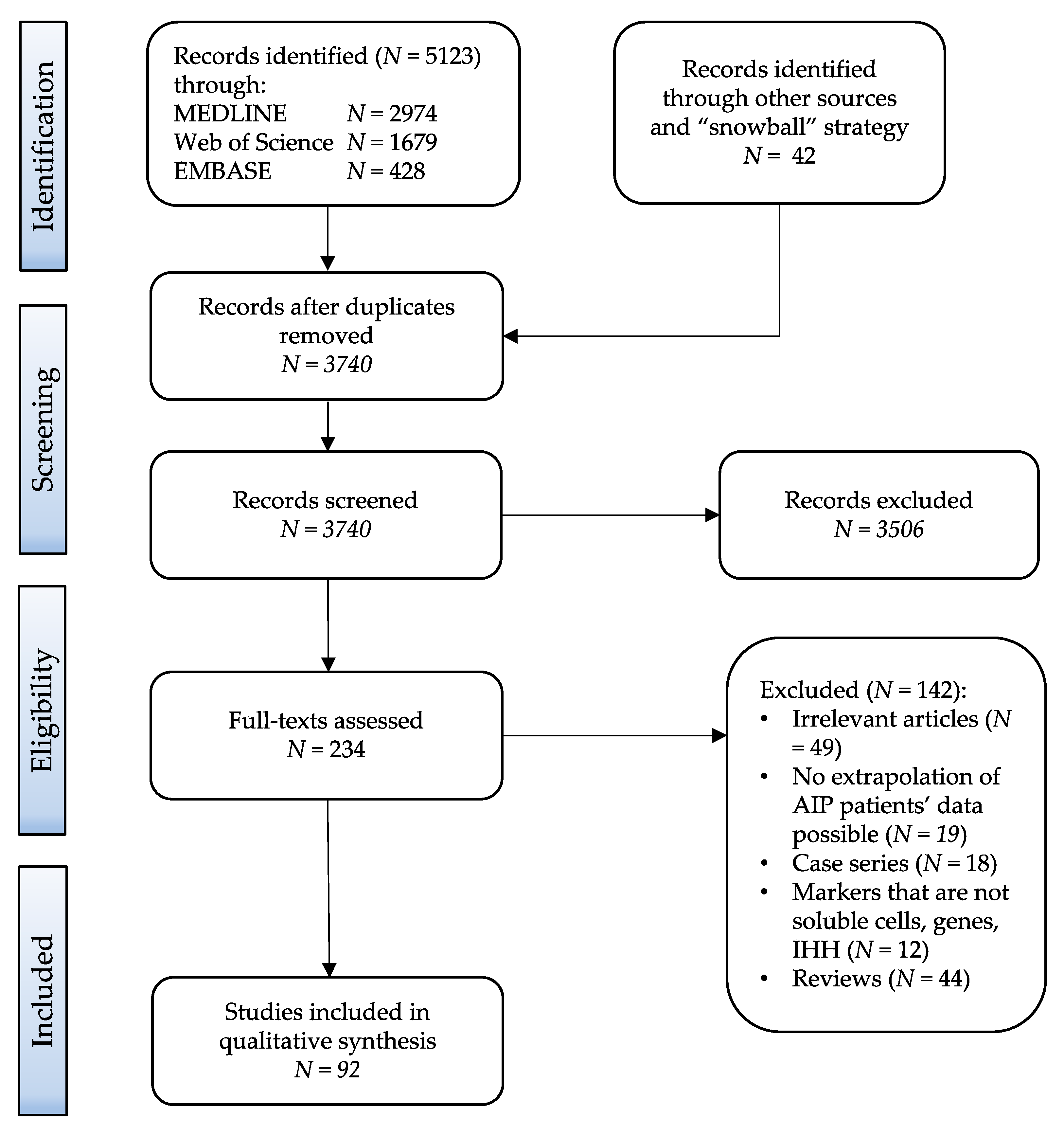
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Data Extraction
2.4. Reporting
3. Results
4. Discussion
4.1. IgG4 Properties and Their Role in AIP Pathogenesis
4.2. IgG4 as Diagnostic and Disease Severity Marker
4.3. Serological Distinction between Type 1 and Type 2 AIP
4.4. Serological Distinction between AIP and Pancreatic Cancer
4.5. Role of Biomarkers in Therapy Monitoring and Relapse Prediction
4.6. Autoantibodies
4.6.1. Antibody against Carboanhydrase II (anti-CA II)
4.6.2. Antibody against PBP
4.6.3. Antibody against Lactoferrin (anti-LF)
4.6.4. Antibody against Alpha 2A Amylase (Anti-Amylase α-2A) and HSP–10
4.6.5. Antibodies against Cationic (PRSS1) and Anionic (PRSS2) Trypsinogens and Pancreatic Secretory Trypsin Inhibitor (PSTI/SPINK1) Antibodies
4.6.6. Novel Candidate Antigens
4.6.7. Other Antibodies
4.7. Miscellaneous Markers
4.7.1. Changes in Serum N-Glycan Profile
4.7.2. Complement
4.7.3. Serum Apolipoprotein Isoforms
4.8. T Helper Lymphocyte Response in AIP and the Role of Cytokines as Biomarkers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIP | Autoimmune pancreatitis |
| LPSP | Lymphoplasmacytic sclerosing pancreatitis |
| IDCP | Idiopathic duct centric pancreatitis (IDCP) |
| ICDC | International Consensus Diagnostic Criteria |
| IgG4 | Immunoglobulin G 4 |
| IgG4-RD | IgG4 related disease |
| GEL | Granulocytic epithelial lesion |
| CP | Chronic pancreatitis |
| CST | Corticosteroid therapy |
| CIC | Circulating immune complexes |
| PDAC | Pancreatic ductal adenocarcinoma |
References
- Löhr, J.M.; Beuers, U.; Vujasinovic, M.; Alvaro, D.; Frokjaer, J.B.; Buttgereit, F.; Capurso, G.; Culver, E.L.; de Madaria, E.; Della-Torre, E.; et al. European Guideline on IgG4-related digestive disease—UEG and SGF evidence-based recommendations. United Eur. Gastroenterol. J. 2020, 8, 637–666. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Toki, F.; Takeuchi, T.; Watanabe, S.-I.; Shiratori, K.; Hayashi, N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Am. J. Dig. Dis. 1995, 40, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Kawa, S.; Horiuchi, A.; Unno, H.; Furuya, N.; Akamatsu, T.; Fukushima, M.; Nikaido, T.; Nakayama, K.; Usuda, N.; et al. High Serum IgG4 Concentrations in Patients with Sclerosing Pancreatitis. N. Engl. J. Med. 2001, 344, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Shimosegawa, T.; Chari, S.T.; Frulloni, L.; Kamisawa, T.; Kawa, S.; Mino-Kenudson, M.; Kim, M.-H.; Klöppel, G.; Lerch, M.M.; Löhr, M.; et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011, 40, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Löhr, J.-M.; Vujasinovic, M.; Rosendahl, J.; Stone, J.H.; Beuers, U. IgG4-related diseases of the digestive tract. Nat. Rev. Gastroenterol. Hepatol. 2021, 19, 185–197. [Google Scholar] [CrossRef]
- Balasubramanian, G.; Sugumar, A.; Smyrk, T.C.; Takahashi, N.; Clain, J.E.; Gleeson, F.C.; Hart, P.A.; Levy, M.J.; Pearson, R.K.; Petersen, B.T.; et al. Demystifying seronegative autoimmune pancreatitis. Pancreatology 2012, 12, 289–294. [Google Scholar] [CrossRef]
- Paik, W.H.; Ryu, J.K.; Park, J.M.; Song, B.J.; Park, J.K.; Kim, Y.T.; Lee, K. Clinical and pathological differences between serum immunoglobulin G4-positive and -negative type 1 autoimmune pancreatitis. World J. Gastroenterol. 2013, 19, 4031–4038. [Google Scholar] [CrossRef]
- Hart, P.A.; Kamisawa, T.; Brugge, W.R.; Chung, J.B.; Culver, E.L.; Czakó, L.; Frulloni, L.; Go, V.L.W.; Gress, T.M.; Kim, M.-H.; et al. Long-term outcomes of autoimmune pancreatitis: A multicentre, international analysis. Gut 2012, 62, 1771–1776. [Google Scholar] [CrossRef] [Green Version]
- Van Heerde, M.J.; Biermann, K.; Zondervan, P.E.; Kazemier, G.; van Eijck, C.H.; Pek, C.; Kuipers, E.J.; van Buuren, H.R. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. Dig. Dis. Sci. 2012, 57, 2458–2465. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Akamatsu, M.; Makino, N.; Ikeda, Y.; Matsuda, A.; Ito, M.; Kakizaki, Y.; Saito, Y.; Ishizawa, T.; Kobayashi, T.; Furukawa, T.; et al. Specific MAPK-Associated MicroRNAs in Serum Differentiate Pancreatic Cancer from Autoimmune Pancreatitis. PLoS ONE 2016, 11, e0158669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, S.; Nakazawa, T.; Ohara, H.; Sano, H.; Nakao, H.; Joh, T.; Murase, T.; Eimoto, T.; Itoh, M. Immunohistochemical study of autoimmune pancreatitis using anti-IgG4 antibody and patients’ sera. Histopathology 2005, 47, 147–158. [Google Scholar] [CrossRef]
- Aparisi, L.; Farre, A.; Gomez-Cambronero, L.; Martinez, J.; Heras, G.D.L.; Corts, J.; Navarro, S.; Mora, J.; López-Hoyos, M.; Sabater, L.; et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: Relevance for diagnosis of autoimmune pancreatitis. Gut 2005, 54, 703–709. [Google Scholar] [CrossRef]
- Asada, M.; Nishio, A.; Uchida, K.; Kido, M.; Ueno, S.; Uza, N.; Kiriya, K.; Inoue, S.; Kitamura, H.; Ohashi, S.; et al. Identification of a Novel Autoantibody Against Pancreatic Secretory Trypsin Inhibitor in Patients with Autoimmune Pancreatitis. Pancreas 2006, 33, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buijs, J.; Cahen, D.L.; van Heerde, M.J.; Hansen, B.E.; van Buuren, H.R.; Peppelenbosch, M.; Fuhler, G.M.; Bruno, M.J. Testing for Anti-PBP Antibody Is Not Useful in Diagnosing Autoimmune Pancreatitis. Am. J. Gastroenterol. 2016, 111, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Liang, P.-C.; Jan, S.; Yang, C.-Y.; Tien, Y.-W.; Wei, S.-C.; Wong, J.-M.; Chang, Y.-T. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 levels. Pancreatology 2014, 14, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Takahashi, N.; Levy, M.J.; Smyrk, T.C.; Clain, J.E.; Pearson, R.K.; Petersen, B.T.; Topazian, M.A.; Vege, S.S. A Diagnostic Strategy to Distinguish Autoimmune Pancreatitis from Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2009, 7, 1097–1103. [Google Scholar] [CrossRef]
- Chatterjee, S.; Oppong, K.W.; Scott, J.S.; Jones, D.E.; Charnley, R.M.; Manas, D.M.; Jaques, B.C.; White, S.A.; French, J.J.; Sen, G.S.; et al. Autoimmune pancreatitis—diagnosis, management and longterm follow-up. J. Gastrointestin. Liver Dis. 2014, 23, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.K.; Kim, M.H.; Lee, T.Y.; Kwon, S.; Oh, H.C.; Hwang, C.Y.; Seo, D.V.; Lee, S.S.; Lee, S.K. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas 2007, 35, 56–61. [Google Scholar] [CrossRef]
- De Vries, E.; Tielbeke, F.; Hubers, L.; Helder, J.; Mostafavi, N.; Verheij, J.; van Hooft, J.; Besselink, M.; Fockens, P.; de Vries, N.; et al. IgG4/IgG RNA ratio does not accurately discriminate IgG4-related disease from pancreatobiliary cancer. JHEP Rep. 2020, 2, 100116. [Google Scholar] [CrossRef]
- Detlefsen, S.; de Vos, J.D.; Tanassi, J.T.; Heegaard, N.H.H.; Fristrup, C.; de Muckadell, O.B.S. Value of anti-plasminogen binding peptide, anti-carbonic anhydrase II, immunoglobulin G4, and other serological markers for the differentiation of autoimmune pancreatitis and pancreatic cancer. Medicine 2018, 97, e11641. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Shi, L.; Chen, P.; Yang, W.; Xun, Y.; Yang, C.; Zhao, L.; Zhou, Y.; Chen, G. Prohibitin Is Involved in Patients with IgG4 Related Disease. PLoS ONE 2015, 10, e0125331. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Takizawa, S.; Tanaka, S.; Takahashi, M.; Fujii, H.; Kamisawa, T.; Kobayashi, T. Amylase alpha-2A autoantibodies: Novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes 2009, 58, 732–737. [Google Scholar] [CrossRef] [Green Version]
- Felix, K.; Hauck, O.; Fritz, S.; Hinz, U.; Schnölzer, M.; Kempf, T.; Warnken, U.; Michel, A.; Pawlita, M.; Werner, J. Serum Protein Signatures Differentiating Autoimmune Pancreatitis versus Pancreatic Cancer. PLoS ONE 2013, 8, e82755. [Google Scholar] [CrossRef] [Green Version]
- Felix, K.; Hauck, O.; Schnölzer, M.; Kempf, T.; Warnken, U.; Schneider, K.; Bergmann, F.; Fritz, S.; Werner, J. Identification of Novel Serum Autoantibodies for Differential Diagnosis of Autoimmune Pancreatitis and Pancreatic Ductal Adenocarcinoma. Pancreas 2016, 45, 1309–1319. [Google Scholar] [CrossRef]
- Frulloni, L.; Lunardi, C.; Simone, R.; Dolcino, M.; Scattolini, C.; Falconi, M.; Benini, L.; Vantini, I.; Corrocher, R.; Puccetti, A. Identification of a Novel Antibody Associated with Autoimmune Pancreatitis. N. Engl. J. Med. 2009, 361, 2135–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frulloni, L.; Scattolini, C.; Falconi, M.; Zamboni, G.; Capelli, P.; Manfredi, R.; Graziani, R.; D’Onofrio, M.; Katsotourchi, A.M.; Amodio, A.; et al. Autoimmune Pancreatitis: Differences Between the Focal and Diffuse Forms in 87 Patients. Am. J. Gastroenterol. 2009, 104, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Fukiage, A.; Fujino, H.; Miki, D.; Ishii, Y.; Serikawa, M.; Tsuge, M.; Imamura, M.; Aikata, H.; Hayes, C.N.; Chayama, K. Clinical Usefulness of Serum Autotaxin for Early Prediction of Relapse in Male Patients with Type 1 Autoimmune Pancreatitis. Am. J. Dig. Dis. 2020, 66, 1268–1275. [Google Scholar] [CrossRef]
- Ghassem-Zadeh, S.; Gaida, M.M.; Szanyi, S.; Acha-Orbea, H.; Frossard, J.-L.; Hinz, U.; Hackert, T.; Strobel, O.; Felix, K. Distinct pathophysiological cytokine profiles for discrimination between autoimmune pancreatitis, chronic pancreatitis, and pancreatic ductal adenocarcinoma. J. Transl. Med. 2017, 15, 126. [Google Scholar] [CrossRef] [Green Version]
- Ghazale, A.; Chari, S.T.; Smyrk, T.C.; Levy, M.J.; Topazian, M.D.; Takahashi, N.; Clain, J.E.; Pearson, R.K.; Pelaez-Luna, M.; Petersen, B.T.; et al. Value of Serum IgG4 in the Diagnosis of Autoimmune Pancreatitis and in Distinguishing It From Pancreatic Cancer. Am. J. Gastroenterol. 2007, 102, 1646–1653. [Google Scholar] [CrossRef]
- Hamada, S.; Masamune, A.; Kanno, A.; Shimosegawa, T. Comprehensive Analysis of Serum microRNAs in Autoimmune Pancreatitis. Digestion 2015, 91, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Arakura, N.; Muraki, T.; Ozaki, Y.; Kiyosawa, K.; Kawa, S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J. Gastroenterol. 2007, 41, 1197–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, K.; Kawabe, T.; Yamamoto, N.; Nakai, Y.; Sasahira, N.; Tsujino, T.; Toda, N.; Isayama, H.; Tada, M.; Omata, M. Serum IgG4 concentrations in pancreatic and biliary diseases. Clin. Chim. Acta 2006, 367, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Tada, M.; Isayama, H.; Kawakubo, K.; Yagioka, H.; Sasaki, T.; Kogure, H.; Nakai, Y.; Sasahira, N.; Tsujino, T.; et al. Clinical analysis of high serum IgE in autoimmune pancreatitis. World J. Gastroenterol. 2010, 16, 5241–5246. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Tada, M.; Isayama, H.; Sasahira, N.; Koike, K. Significance of Measuring IgG and IgG4 During Follow-Up of Autoimmune Pancreatitis. Pancreas 2011, 40, 788–791. [Google Scholar] [CrossRef]
- Hirano, K.; Tada, M.; Isayama, H.; Sasahira, N.; Umefune, G.; Akiyama, D.; Watanabe, T.; Saito, T.; Takagi, K.; Takaharaet, N.; et al. Outcome of Long-term Maintenance Steroid Therapy Cessation in Patients with Autoimmune Pancreatitis: A Prospective Study. J. Clin. Gastroenterol. 2016, 50, 331–337. [Google Scholar] [CrossRef]
- Hirano, K.; Tada, M.; Isayama, H.; Yagioka, H.; Sasaki, T.; Kogure, H.; Nakai, Y.; Sasahira, N.; Tsujino, T.; Yoshida, H.; et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut 2007, 56, 1719–1724. [Google Scholar] [CrossRef] [Green Version]
- Hosoda, H.; Okawa-Takatsuji, M.; Shinmura, W.; Hasimoto, N.; Ozaki, Y.; Ikeda, Y. Potential for Differential Diagnosis of Autoimmune Pancreatitis and Pancreatic Cancer Using Carbonic Anhydrase II Antibody. Pancreas 2008, 37, e1–e7. [Google Scholar] [CrossRef]
- Igarashi, H.; Ito, T.; Oono, T.; Nakamura, T.; Fujimori, N.; Niina, Y.; Hijioka, M.; Uchida, M.; Lee, R.; Iwao, R.; et al. Relationship between pancreatic and/or extrapancreatic lesions and serum IgG and IgG4 levels in IgG4-related diseases. J. Dig. Dis. 2012, 13, 274–279. [Google Scholar] [CrossRef]
- Ikemune, M.; Uchida, K.; Tsukuda, S.; Ito, T.; Nakamaru, K.; Tomiyama, T.; Ikeura, T.; Naganuma, M.; Okazaki, K. Serum free light chain assessment in type 1 autoimmune pancreatitis. Pancreatology 2021, 21, 658–665. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kawashima, H.; Ohno, E.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yamada, K.; Yashika, J.; Yoshikawa, M.; Gibo, N.; et al. Risks and characteristics of pancreatic cancer and pancreatic relapse in autoimmune pancreatitis patients. J. Gastroenterol. Hepatol. 2020, 35, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kawashima, H.; Ohno, E.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yashika, J.; Yamada, K.; Yoshikawa, M.; Gibo, N.; et al. Clinical characteristics and long-term prognosis of autoimmune pancreatitis with renal lesions. Sci. Rep. 2021, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, T.; Nakamaru, K.; Tomiyama, T.; Yamaguchi, T.; Ando, Y.; Ikeura, T.; Fukui, T.; Uchida, K.; Nishio, A.; et al. Interleukin-35 promotes the differentiation of regulatory T cells and suppresses Th2 response in IgG4-related type 1 autoimmune pancreatitis. J. Gastroenterol. 2020, 55, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Takedatsu, H.; Okabe, Y.; Ishida, Y.; Sugiyama, G.; Yonemoto, K.; Mitsuyama, K.; Tsuruta, O.; Sata, M. Serum immunoglobulin G4 associated with number and distribution of extrapancreatic lesions in type 1 autoimmune pancreatitis patients. J. Gastroenterol. Hepatol. 2011, 27, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Anjiki, H.; Egawa, N.; Kubota, N. Allergic manifestations in autoimmune pancreatitis. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Imai, M.; Egawa, N.; Tsuruta, K.; Okamoto, A. Serum IgG4 levels and extrapancreatic lesions in autoimmune pancreatitis. Eur. J. Gastroenterol. Hepatol. 2008, 20, 1167–1170. [Google Scholar] [CrossRef]
- Kamisawa, T.; Imai, M.; Chen, P.Y.; Tu, Y.; Egawa, N.; Tsuruta, K.; Okamoto, A.; Suzuki, M.; Kamata, N. Strategy for Differentiating Autoimmune Pancreatitis from Pancreatic Cancer. Pancreas 2008, 37, e62–e67. [Google Scholar] [CrossRef]
- Kamisawa, T.; Kim, M.-H.; Liao, W.-C.; Liu, Q.; Balakrishnan, V.; Okazaki, K.; Shimosegawa, T.; Chung, J.B.; Lee, K.T.; Wang, H.-P.; et al. Clinical Characteristics of 327 Asian Patients with Autoimmune Pancreatitis Based on Asian Diagnostic Criteria. Pancreas 2011, 40, 200–205. [Google Scholar] [CrossRef]
- Kamisawa, T.; Okamoto, A.; Funata, N. Clinicopathological Features of Autoimmune Pancreatitis in Relation to Elevation of Serum IgG4. Pancreas 2005, 31, 28–31. [Google Scholar] [CrossRef]
- Kamisawa, T.; Takuma, K.; Tabata, T.; Inaba, Y.; Egawa, N.; Tsuruta, K.; Hishima, T.; Sasaki, T.; Itoi, T. Serum IgG4-negative autoimmune pancreatitis. J. Gastroenterol. 2010, 46, 108–116. [Google Scholar] [CrossRef]
- Kanno, A.; Masamune, A.; Okazaki, K.; Kamisawa, T.; Kawa, S.; Nishimori, I.; Tsuji, I.; Shimosegawa, T. Nationwide Epidemiological Survey of Autoimmune Pancreatitis in Japan in 2011. Pancreas 2015, 44, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Kitahara, K.; Hamano, H.; Ozaki, Y.; Arakura, N.; Yoshizawa, K.; Umemura, T.; Ota, M.; Mizoguchi, S.; Shimozuru, Y.; et al. A Novel Immunoglobulin-Immunoglobulin Interaction in Autoimmunity. PLoS ONE 2008, 3, e1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Sato, Y.; Nishiumi, S.; Yagi, Y.; Sakai, A.; Shiomi, H.; Masuda, A.; Okaya, S.; Kutsumi, H.; Yoshida, M.; et al. Serum apolipoprotein A2 isoforms in autoimmune pancreatitis. Biochem. Biophys. Res. Commun. 2018, 497, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Watanabe, S.; Uchiyama, T.; Kato, S.; Sekino, Y.; Suzuki, K.; Mawatari, H.; Iida, H.; Endo, H.; Fujita, K.; et al. Factors predictive of relapse and spontaneous remission of autoimmune pancreatitis patients treated/not treated with corticosteroids. J. Gastroenterol. 2011, 46, 834–842. [Google Scholar] [CrossRef]
- Kuruma, S.; Kamisawa, T.; Tabata, T.; Chiba, K.; Iwasaki, S.; Fujiwara, T.; Kuwata, G.; Egarashira, H.; Koizumi, K.; Koizumi, S.; et al. Allergen-specific IgE Antibody Serologic Assays in Patients with Autoimmune Pancreatitis. Intern. Med. 2014, 53, 541–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Moon, S.-H.; Kim, M.-H.; Cho, D.H.; Jun, J.H.; Nam, K.; Song, T.J.; Park, D.H.; Lee, S.S.; Seo, D.-W.; et al. Relapse rate and predictors of relapse in a large single center cohort of type 1 autoimmune pancreatitis: Long-term follow-up results after steroid therapy with short-duration maintenance treatment. J. Gastroenterol. 2018, 53, 967–977. [Google Scholar] [CrossRef]
- Liu, Q.-C.; Dong, F.; Pan, J.-F.; Zhuang, Z.-H.; Gao, F.; Liu, G.-Z.; Chen, Q.-Q.; Chen, S.; Weng, S.-H.; Lin, L.-Q.; et al. Antibodies to Type IV Collagen Induce Type 1 Autoimmune Pancreatitis. Inflammation 2015, 39, 592–600. [Google Scholar] [CrossRef]
- Löhr, J.-M.; Faissner, R.; Koczan, D.; Bewerunge, P.; Bassi, C.; Brors, B.; Eils, R.; Frulloni, L.; Funk, A.; Halangk, W.; et al. Autoantibodies Against the Exocrine Pancreas in Autoimmune Pancreatitis: Gene and Protein Expression Profiling and Immunoassays Identify Pancreatic Enzymes as a Major Target of the Inflammatory Process. Am. J. Gastroenterol. 2010, 105, 2060–2071. [Google Scholar] [CrossRef] [Green Version]
- Matsubayashi, H.; Sawai, H.; Kimura, H.; Yamaguchi, Y.; Tanaka, M.; Kakushima, N.; Takizawa, K.; Kadooka, M.; Takao, T.; Hebbar, S.; et al. Characteristics of autoimmune pancreatitis based on serum IgG4 level. Dig. Liver Dis. 2011, 43, 731–735. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Uesaka, K.; Kanemoto, H.; Asakura, K.; Kakushima, N.; Tanaka, M.; Kimura, H.; Ono, H. Soluble IL-2 Receptor, a New Marker for Autoimmune Pancreatitis. Pancreas 2012, 41, 493–496. [Google Scholar] [CrossRef]
- Miki, M.; Fujimori, N.; Oono, T.; Kawabe, K.; Ohno, A.; Matsumoto, K.; Teramatsu, K.; Tachibana, Y.; Ogawa, Y. Relapse patterns and predictors of IgG4-related diseases involved with autoimmune pancreatitis: A single-center retrospective study of 115 patients. J. Dig. Dis. 2019, 20, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Watanabe, T.; Hara, A.; Kamata, K.; Omoto, S.; Nakai, A.; Otsuka, Y.; Sekai, I.; Yoshikawa, T.; Yamao, K.; et al. Identification of serum IFN-α and IL-33 as novel biomarkers for type 1 autoimmune pancreatitis and IgG4-related disease. Sci. Rep. 2020, 10, 14879. [Google Scholar] [CrossRef] [PubMed]
- Muraki, T.; Hamano, H.; Ochi, Y.; Komatsu, K.; Komiyama, Y.; Arakura, N.; Yoshizawa, K.; Ota, M.; Kawa, S.; Kiyosawa, K. Autoimmune Pancreatitis and Complement Activation System. Pancreas 2006, 32, 16–21. [Google Scholar] [CrossRef]
- Naitoh, I.; Nakazawa, T.; Hayashi, K.; Okumura, F.; Miyabe, K.; Shimizu, S.; Kondo, H.; Yoshida, M.; Yamashita, H.; Ohara, H.; et al. Clinical differences between mass-forming autoimmune pancreatitis and pancreatic cancer. Scand. J. Gastroenterol. 2012, 47, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, I.; Nakazawa, T.; Ohara, H.; Ando, T.; Hayashi, K.; Tanaka, H.; Okumura, F.; Miyabe, K.; Yoshida, M.; Sano, H.; et al. Clinical Significance of Extrapancreatic Lesions in Autoimmune Pancreatitis. Pancreas 2010, 39, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, K.; Tomiyama, T.; Kobayashi, S.; Ikemune, M.; Tsukuda, S.; Ito, T.; Tanaka, T.; Yamaguchi, T.; Ando, Y.; Ikeura, T.; et al. Extracellular vesicles microRNA analysis in type 1 autoimmune pancreatitis: Increased expression of microRNA-21. Pancreatology 2020, 20, 318–324. [Google Scholar] [CrossRef]
- Nakazawa, T.; Ohara, H.; Sano, H.; Ando, T.; Aoki, S.; Kobayashi, S.; Okamoto, T.; Nomura, T.; Joh, T.; Itoh, M. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas 2005, 30, 20–25. [Google Scholar]
- Ngwa, T.; Law, R.; Hart, P.; Smyrk, T.C.; Chari, S.T. Serum IgG4 elevation in pancreatic cancer: Diagnostic and prognostic significance and association with autoimmune pancreatitis. Pancreas 2015, 44, 557–560. [Google Scholar] [CrossRef]
- Nishimori, I.; Miyaji, E.; Morimoto, K.; Nagao, K.; Kamada, M.; Onishi, S. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut 2005, 54, 274–281. [Google Scholar] [CrossRef]
- Nishino, T.; Oyama, H.; Hashimoto, E.; Toki, F.; Oi, I.; Kobayashi, M.; Shiratori, K. Clinicopathological differentiation between sclerosing cholangitis with autoimmune pancreatitis and primary sclerosing cholangitis. J. Gastroenterol. 2007, 42, 550–559. [Google Scholar] [CrossRef]
- Okazaki, K.; Uchida, K.; Ohana, M.; Nakase, H.; Uose, S.; Inai, M.; Matsushima, Y.; Katamura, K.; Ohmori, K.; Chiba, T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology 2000, 118, 573–581. [Google Scholar] [CrossRef]
- Pattabathula, K.; Waters, P.S.; Hwang, J.; Bettington, M.; Singh, M.; Bryant, R.D.; Cavallucci, D.J.; O’Rourke, N. Diagnostic and therapeutic considerations in biopsy-proven type 2 autoimmune pancreatitis: Comparative analysis with biopsy-proven type 1 autoimmune pancreatitis. ANZ J. Surg. 2020, 91, 907–914. [Google Scholar] [CrossRef]
- Raina, A.; Yadav, D.; Krasinskas, A.M.; McGrath, K.M.; Khalid, A.; Sanders, M.; Whitcomb, D.C.; Slivka, A. Evaluation and Management of Autoimmune Pancreatitis: Experience at a Large US Center. Am. J. Gastroenterol. 2009, 104, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Rogger, T.M.; De Marchi, G.; Brozzi, L.; Amodio, A.; Orsolini, G.; de Pretis, N.; Bellocchi, M.C.C.; Crinò, S.F.; Gabbrielli, A.; Ciccocioppo, R.; et al. Immunoglobulin G4-Related Disease Responder Index Correlates With the Risk of 1-Year Relapse in Type 1 Autoimmune Pancreatitis. Pancreas 2021, 50, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Chung, J.B.; Park, S.W.; Lee, J.K.; Lee, K.T.; Lee, W.J.; Moon, J.H.; Cho, K.B.; Kang, D.W.; Hwang, J.-H.; et al. Review of 67 patients with autoimmune pancreatitis in Korea: A multicenter nationwide study. Pancreas 2008, 37, 377–385. [Google Scholar] [CrossRef]
- Sadler, R.; Chapman, R.W.; Simpson, D.; Soonawalla, Z.F.; Waldegrave, E.L.; Burden, J.M.; Mishab, S.A.; Ferry, B.L. The diagnostic significance of serum IgG4 levels in patients with autoimmune pancreatitis: A UK study. Eur. J. Gastroenterol. Hepatol. 2011, 23, 139–145. [Google Scholar] [CrossRef]
- Sah, R.P.; Chari, S.T.; Pannala, R.; Sugumar, A.; Clain, J.E.; Levy, M.J.; Pearson, R.K.; Smyrk, T.C.; Petersen, B.T.; Topazian, M.D.; et al. Differences in Clinical Profile and Relapse Rate of Type 1 Versus Type 2 Autoimmune Pancreatitis. Gastroenterology 2010, 139, 140–148. [Google Scholar] [CrossRef]
- Sah, R.P.; Pannala, R.; Zhang, L.; Graham, R.; Sugumar, A.; Chari, S.T. Eosinophilia and Allergic Disorders in Autoimmune Pancreatitis. Am. J. Gastroenterol. 2010, 105, 2485–2491. [Google Scholar] [CrossRef]
- Sanchez Castanon, M.; Zuliani, V.; Amodio, A.; Campagnola, P.; Granato, A.; Gabbrielli, A.; Benini, L.; López Hoyos, M.; Frulloni, L. Role of Amylase-alpha2A Autoantibodies in the Diagnosis of Autoimmune Pancreatitis. Pancreas 2015, 44, 1078–1082. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Castañón, M.; Heras-Castaño, G.D.L.; Gomez, C.; López-Hoyos, M. Differentiation of autoimmune pancreatitis from pancreas cancer: Utility of anti-amylase and anti-carbonic anhydrase II autoantibodies. Autoimmun. Highlights 2011, 3, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Sandanayake, N.S.; Church, N.I.; Chapman, M.H.; Johnson, G.J.; Dhar, D.K.; Amin, Z.; Deheragoda, M.G.; Novelli, M.; Winstanley, A.; Rodriguez-Justo, M.; et al. Presentation and Management of Post-treatment Relapse in Autoimmune Pancreatitis/Immunoglobulin G4-Associated Cholangitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Tahara, J.; Takayama, Y.; Akao, J.; Ajihara, T.; Nagao, K.; Shiratori, K.; Tokushige, K. Assessment of the Rate of Decrease in Serum IgG4 Level of Autoimmune Pancreatitis Patients in Response to Initial Steroid Therapy as a Predictor of Subsequent Relapse. Pancreas 2016, 45, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, M.; Kodama, Y.; Sekiguchi, K.; Kuwada, T.; Tomono, T.; Kuriyama, K.; Yamazaki, H.; Morita, T.; Marui, S.; Sogabe, Y.; et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci. Transl. Med. 2018, 10, eaaq0997. [Google Scholar] [CrossRef] [Green Version]
- Song, T.J.; Kim, M.-H.; Moon, S.-H.; Eum, J.B.; Park, D.H.; Lee, S.S.; Seo, D.W.; Lee, S.K. The Combined Measurement of Total Serum IgG and IgG4 May Increase Diagnostic Sensitivity for Autoimmune Pancreatitis Without Sacrificing Specificity, Compared With IgG4 Alone. Am. J. Gastroenterol. 2010, 105, 1655–1660. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; Waragai, Y.; Asama, H.; Takasumi, M.; et al. Efficacy of Steroid Pulse Therapy for Autoimmune Pancreatitis Type 1: A Retrospective Study. PLoS ONE 2015, 10, e0138604. [Google Scholar] [CrossRef]
- Suzuki, D.; Shimizu, K.; Tokushige, K. Relative Rise of Serum IgG4 Levels After Steroid Therapy for Autoimmune Pancreatitis Predicts the Likelihood of Relapse. Pancreas 2018, 47, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Kamisawa, T.; Takuma, K.; Anjiki, H.; Egawa, N.; Kurata, M.; Honda, G.; Tsuruta, K.; Setoguchi, K.; Obayashi, T.; et al. Serum IgG4 concentrations and IgG4-related sclerosing disease. Clin. Chim. Acta 2009, 408, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Kamisawa, T.; Takuma, K.; Hara, S.; Kuruma, S.; Inaba, Y. Differences between diffuse and focal autoimmune pancreatitis. World J. Gastroenterol. 2012, 18, 2099–2104. [Google Scholar] [CrossRef]
- Taguchi, M.; Kihara, Y.; Nagashio, Y.; Yamamoto, M.; Otsuki, M.; Harada, M. Decreased production of immunoglobulin M and A in autoimmune pancreatitis. J. Gastroenterol. 2009, 44, 1133–1139. [Google Scholar] [CrossRef]
- Takizawa, S.; Endo, T.; Wanjia, X.; Tanaka, S.; Takahashi, M.; Kobayashi, T. HSP 10 is a new autoantigen in both autoimmune pancreatitis and fulminant type 1 diabetes. Biochem. Biophys. Res. Commun. 2009, 386, 192–196. [Google Scholar] [CrossRef]
- Takuma, K.; Kamisawa, T.; Tabata, T.; Inaba, Y.; Egawa, N.; Igarashi, Y. Short-term and long-term outcomes of autoimmune pancreatitis. Eur. J. Gastroenterol. Hepatol. 2011, 23, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Talar-Wojnarowska, R.; Gąsiorowska, A.; Olakowski, M.; Dranka-Bojarowska, D.; Lampe, P.; Śmigielski, J.; Kujawiak, M.; Grzegorczyk, J.; Malecka-Panas, E. Utility of serum IgG, IgG4 and carbonic anhydrase II antibodies in distinguishing autoimmune pancreatitis from pancreatic cancer and chronic pancreatitis. Adv. Med. Sci. 2014, 59, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, T.; Nouso, K.; Kato, H.; Miyahara, K.; Dohi, C.; Morimoto, Y.; Kinugasa, H.; Akimoto, Y.; Matsumoto, K.; Yamamoto, N.; et al. Alteration of serum N-glycan profile in patients with autoimmune pancreatitis. Pancreatology 2016, 16, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Heerde, M.J.; Buijs, J.; Hansen, B.E.; de Waart, M.; van Eijck, C.H.; Kazemier, G.; Pek, C.J.; Poley, J.W.; Bruno, M.J.; Kuipers, E.J.; et al. Serum level of Ca 19-9 increases ability of IgG4 test to distinguish patients with autoimmune pancreatitis from those with pancreatic carcinoma. Dig. Dis. Sci. 2014, 59, 1322–1329. [Google Scholar] [CrossRef]
- Van Toorenenbergen, A.W.; van Heerde, M.J.; van Buuren, H.R. Potential value of serum total IgE for differentiation between autoimmune pancreatitis and pancreatic cancer. Scand. J. Immunol. 2010, 72, 444–448. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Maier, P.; Maetzel, H.; Valente, R.; Pozzi-Mucelli, R.; Moro, C.F.; Haas, S.L.; Said, K.; Verbeke, C.S.; Maisonneuve, P.; et al. Immunoglobulin G subtypes-1 and 2 differentiate immunoglobulin G4-associated sclerosing cholangitis from primary sclerosing cholangitis. United Eur. Gastroenterol. J. 2020, 8, 584–593. [Google Scholar] [CrossRef]
- Xin, L.; He, Y.-X.; Zhu, X.-F.; Zhang, Q.-H.; Hu, L.-H.; Zou, D.-W.; Jin, Z.-D.; Chang, X.-J.; Zheng, J.-M.; Zuo, C.-J.; et al. Diagnosis and treatment of autoimmune pancreatitis: Experience with 100 patients. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 642–648. [Google Scholar] [CrossRef]
- Yan, T.; Ke, Y.; Chen, Y.; Xu, C.; Yu, C.; Li, Y. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19-9, globulin, eosinophils and hemoglobin. PLoS ONE 2017, 12, e0174735. [Google Scholar] [CrossRef]
- Yanagisawa, N.; Haruta, I.; Shimizu, K.; Furukawa, T.; Higuchi, T.; Shibata, N.; Shiratorib, K.; Yagia, J. Identification of commensal flora-associated antigen as a pathogenetic factor of autoimmune pancreatitis. Pancreatology 2014, 14, 100–106. [Google Scholar] [CrossRef]
- Trampert, D.C.; Hubers, L.M.; van de Graaf, S.F.J.; Beuers, U. On the role of IgG4 in inflammatory conditions: Lessons for IgG4-related disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1401–1409. [Google Scholar] [CrossRef]
- Van der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martinez-Martinez, P.; Vermeulen, E.; den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubers, L.M.; Vos, H.; Schuurman, A.R.; Erken, R.; Elferink, R.P.O.; Burgering, B.; van de Graaf, S.; Beuers, U. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut 2017, 67, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Diaz, L.A.; Fairley, J.; Giudice, G.J.; Liu, Z. The Anti-Desmoglein 1 Autoantibodies in Pemphigus Vulgaris Sera are Pathogenic. J. Investig. Dermatol. 1999, 112, 739–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiokawa, M.; Kodama, Y.; Kuriyama, K.; Yoshimura, K.; Tomono, T.; Morita, T.; Kakiuchi, N.; Matsumori, T.; Mima, A.; Nishikawa, Y.; et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut 2016, 65, 1322–1332. [Google Scholar] [CrossRef]
- Kawa, S.; Ito, T.; Watanabe, T.; Maruyama, M.; Hamano, H.; Maruyama, M.; Muraki, T.; Arakura, N. The Utility of Serum IgG4 Concentrations as a Biomarker. Int. J. Rheumatol. 2012, 2012, 198314. [Google Scholar] [CrossRef]
- Ohara, H.; Nakazawa, T.; Sano, H.; Ando, T.; Okamoto, T.; Takada, H.; Hayashi, K.; Kitajima, Y.; Nakao, H.; Joh, T. Systemic Extrapancreatic Lesions Associated With Autoimmune Pancreatitis. Pancreas 2005, 31, 232–237. [Google Scholar] [CrossRef]
- Xiang, P.; Zhang, X.; Wang, C.; Lang, Y.; Xu, L.; Huang, L.; Shen, J.; Feng, S.-T. Pancreatic tumor in type 1 autoimmune pancreatitis: A diagnostic challenge. BMC Cancer 2019, 19, 814. [Google Scholar] [CrossRef]
- Schneider, A.; Hirth, M.; Münch, M.; Weiss, C.; Löhr, J.M.; Ebert, M.P.; Pfützer, R.H. Risk of Cancer in Patients with Autoimmune Pancreatitis: A Single-Center Experience from Germany. Digestion 2017, 95, 172–180. [Google Scholar] [CrossRef]
- Macinga, P.; Bajer, L.; Del Chiaro, M.; Chari, S.T.; Dite, P.; Frulloni, L.; Ikeura, T.; Kamisawa, T.; Kubota, K.; Naitoh, I.; et al. Pancreatic cancer in patients with autoimmune pancreatitis: A scoping review. Pancreatology 2021, 21, 928–937. [Google Scholar] [CrossRef]
- Moon, S.-H.; Kim, M.-H.; Park, D.H.; Hwang, C.Y.; Park, S.J.; Lee, S.S.; Seo, D.W. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut 2008, 57, 1704–1712. [Google Scholar] [CrossRef]
- Kim, S.; Park, B.K.; Seo, J.H.; Choi, J.; Choi, J.W.; Lee, C.K.; Chung, J.B.; Park, Y.; Kim, D.W. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci. Rep. 2020, 10, 8820. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Chari, S.T.; Frulloni, L.; Lerch, M.M.; Kamisawa, T.; Kawa, S.; Kim, M.-H.; Lévy, P.; Masamune, A.; Webster, G.; et al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology 2016, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Okazaki, K.; Kawa, S.; Ito, T.; Inui, K.; Irie, H.; Nishino, T.; Notohara, K.; Nishimori, I.; Tanaka, S.; et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J. Gastroenterol. 2014, 49, 961–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, K.; Iida, H.; Fujisawa, T.; Yoneda, M.; Inamori, M.; Abe, Y.; Kirikoshi, H.; Saito, S.; Ohshiro, H.; Kakuta, Y.; et al. Clinical factors predictive of spontaneous remission or relapse in cases of autoimmune pancreatitis. Gastrointest. Endosc. 2007, 66, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Shimosegawa, T.; Okazaki, K.; Nishino, T.; Watanabe, H.; Kanno, A.; Okumura, F.; Nishikawa, T.; Kobayashi, K.; Ichiya, T.; et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009, 58, 1504–1507. [Google Scholar] [CrossRef]
- Ito, T.; Nishimori, I.; Inoue, N.; Kawabe, K.; Gibo, J.; Arita, Y.; Okazaki, K.; Takayanagi, R.; Otsuki, M. Treatment for autoimmune pancreatitis: Consensus on the treatment for patients with autoimmune pancreatitis in Japan. J. Gastroenterol. 2007, 42, 50–58. [Google Scholar] [CrossRef]
- Tager, A.M.; LaCamera, P.; Shea, B.S.; Campanella, G.S.; Selman, M.; Zhao, Z.; Polosukhin, V.; Wain, J.; Karimi-Shah, B.A.; Kim, N.D.; et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 2007, 14, 45–54. [Google Scholar] [CrossRef]
- Pischke, S.; Hartl, J.; Pas, S.D.; Lohse, A.W.; Jacobs, B.C.; Van der Eijk, A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017, 66, 1082–1095. [Google Scholar] [CrossRef] [Green Version]
- De Buy Wenniger, L.J.; Culver, E.L.; Beuers, U. Exposure to occupational antigens might predispose to IgG4-related disease. Hepatology 2014, 60, 1453. [Google Scholar] [CrossRef] [Green Version]
- Guarneri, F.; Guarneri, C.; Benvenga, S. Helicobacter pylori and autoimmune pancreatitis: Role of carbonic anhydrase via molecular mimicry? J. Cell. Mol. Med. 2005, 9, 741–744. [Google Scholar] [CrossRef]
- Culver, E.L.; Smit, W.L.; Evans, C.; Sadler, R.; Cargill, T.; Makuch, M.; Wang, L.-M.; Ferry, B.; Klenerman, P.; Barnes, E. No evidence to support a role for Helicobacter pylori infection and plasminogen binding protein in autoimmune pancreatitis and IgG4-related disease in a UK cohort. Pancreatology 2017, 17, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Jesnowski, R.; Isaksson, B.; Möhrckea, C.; Bertsch, C.; Bulajic, M.; Schneider-Brachert, W.; Klöppel, G.; Lowenfels, A.B.; Maisonneuve, P.; Löhr, M. Helicobacter pylori in Autoimmune Pancreatitis and Pancreatic Carcinoma. Pancreatology 2010, 10, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Perugino, C.A.; AlSalem, S.B.; Mattoo, H.; Della-Torre, E.; Mahajan, V.; Ganesh, G.; Allard-Chamard, H.; Wallace, Z.; Montesi, S.B.; Kreuzer, J.; et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J. Allergy Clin. Immunol. 2018, 143, 736–745.e6. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Li, W.; Yi, L.; Yu, S.; Fan, G.; Lu, T.; Yang, X.; Wang, G.; Zhang, N.; Ding, J.; et al. Hybrid kappa\lambda antibody is a new serological marker to diagnose autoimmune pancreatitis and differentiate it from pancreatic cancer. Sci. Rep. 2016, 6, 27415. [Google Scholar] [CrossRef] [Green Version]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Culver, E.L.; Van De Bovenkamp, F.S.; Derksen, N.I.L.; Koers, J.; Cargill, T.; Barnes, E.; de Neef, L.; Koeleman, C.A.M.; Aalberse, R.C.; Wuhrer, M.; et al. Unique patterns of glycosylation in immunoglobulin subclass G4-related disease and primary sclerosing cholangitis. J. Gastroenterol. Hepatol. 2018, 34, 1878–1886. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, C.-M.; Guo, T.; Qian, J.-M. Eosinophilia Associated with Chronic Pancreatitis. Pancreas 2009, 38, 149–153. [Google Scholar] [CrossRef]
- Fox, R.I. The distinct pathogenesis of IgG4 Mickulicz’s disease and Sjogren’s syndrome in Japan; the role of IL-21 and IL-6. Ann. Rheum. Dis. 2012, 71, 1919–1920. [Google Scholar] [CrossRef] [Green Version]
- Perugino, C.A.; Stone, J.H. IgG4-related disease: An update on pathophysiology and implications for clinical care. Nat. Rev. Rheumatol. 2020, 16, 702–714. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamashita, K.; Arai, Y.; Minaga, K.; Kamata, K.; Nagai, T.; Komeda, Y.; Takenaka, M.; Hagiwara, S.; Ida, H.; et al. Chronic Fibro-Inflammatory Responses in Autoimmune Pancreatitis Depend on IFN-α and IL-33 Produced by Plasmacytoid Dendritic Cells. J. Immunol. 2017, 198, 3886–3896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, K.; Yamamoto, M.; Takahashi, H.; Himi, T. Recent advances in knowledge regarding the head and neck manifestations of IgG4-related disease. Auris Nasus Larynx 2016, 44, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugic, A.; Verdejo Gil, C.; Mellenthin, C.; Vujasinovic, M.; Löhr, J.-M.; Mühldorfer, S. The Clinical Utility of Soluble Serum Biomarkers in Autoimmune Pancreatitis: A Systematic Review. Biomedicines 2022, 10, 1511. https://doi.org/10.3390/biomedicines10071511
Dugic A, Verdejo Gil C, Mellenthin C, Vujasinovic M, Löhr J-M, Mühldorfer S. The Clinical Utility of Soluble Serum Biomarkers in Autoimmune Pancreatitis: A Systematic Review. Biomedicines. 2022; 10(7):1511. https://doi.org/10.3390/biomedicines10071511
Chicago/Turabian StyleDugic, Ana, Cristina Verdejo Gil, Claudia Mellenthin, Miroslav Vujasinovic, J.-Matthias Löhr, and Steffen Mühldorfer. 2022. "The Clinical Utility of Soluble Serum Biomarkers in Autoimmune Pancreatitis: A Systematic Review" Biomedicines 10, no. 7: 1511. https://doi.org/10.3390/biomedicines10071511
APA StyleDugic, A., Verdejo Gil, C., Mellenthin, C., Vujasinovic, M., Löhr, J.-M., & Mühldorfer, S. (2022). The Clinical Utility of Soluble Serum Biomarkers in Autoimmune Pancreatitis: A Systematic Review. Biomedicines, 10(7), 1511. https://doi.org/10.3390/biomedicines10071511






