High-Density Lipoprotein Changes in Alzheimer’s Disease Are APOE Genotype-Specific
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
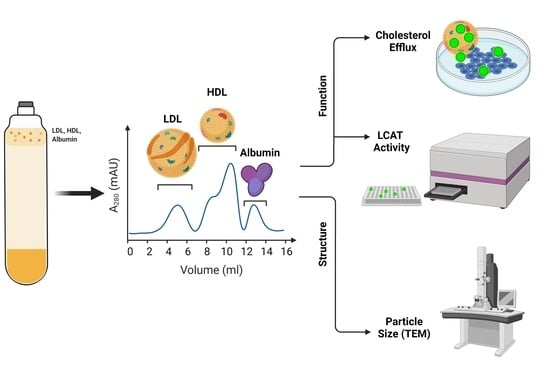
2.2. HDL Isolation and Analysis
2.2.1. Isolation Method
2.2.2. Cholesterol Efflux Capacity
2.2.3. LCAT Activity
2.2.4. HDL Particle Size
2.3. Cognitive Function Analysis
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
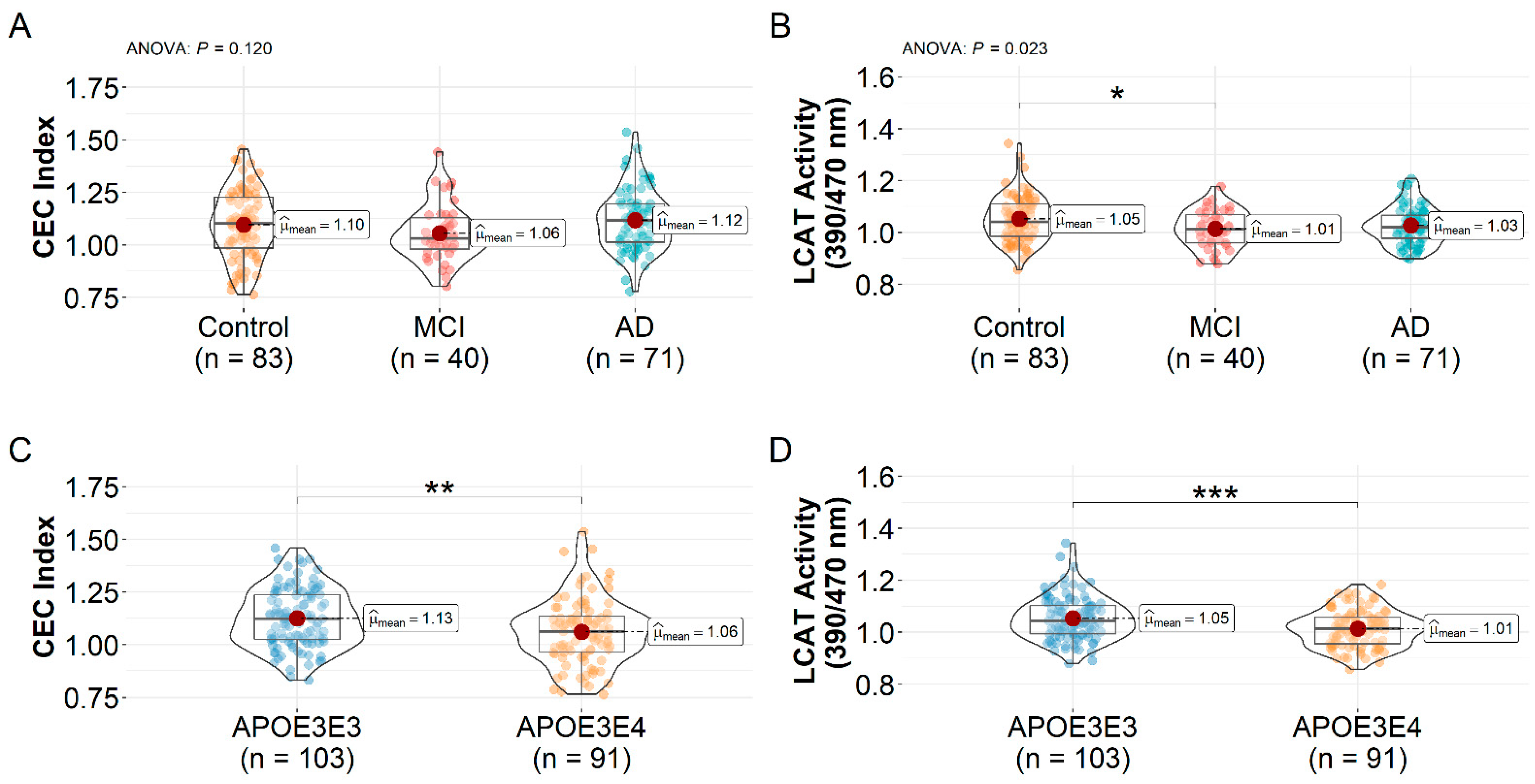
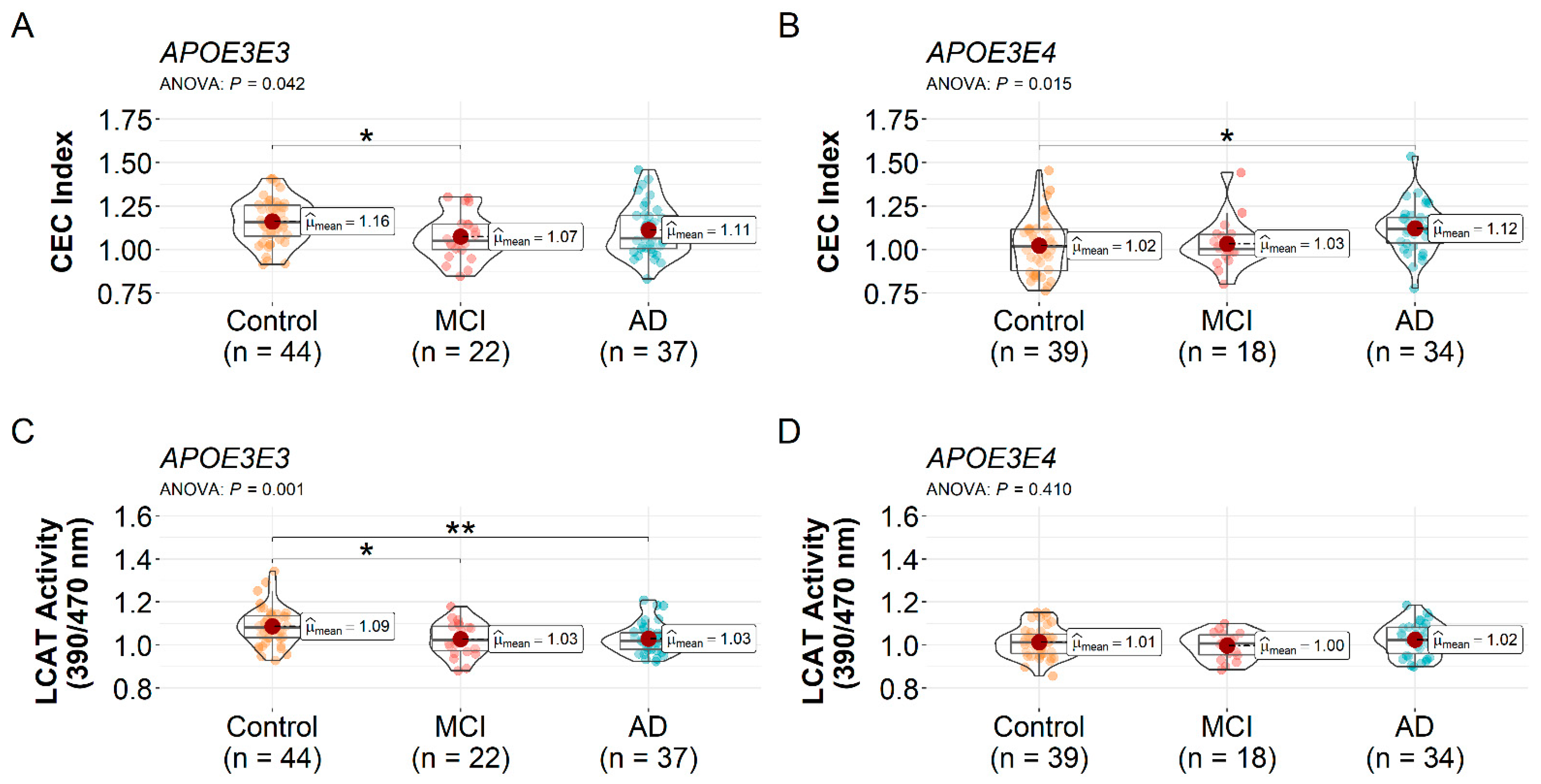
3.2. HDL CEC Index and LCAT Activity Differences
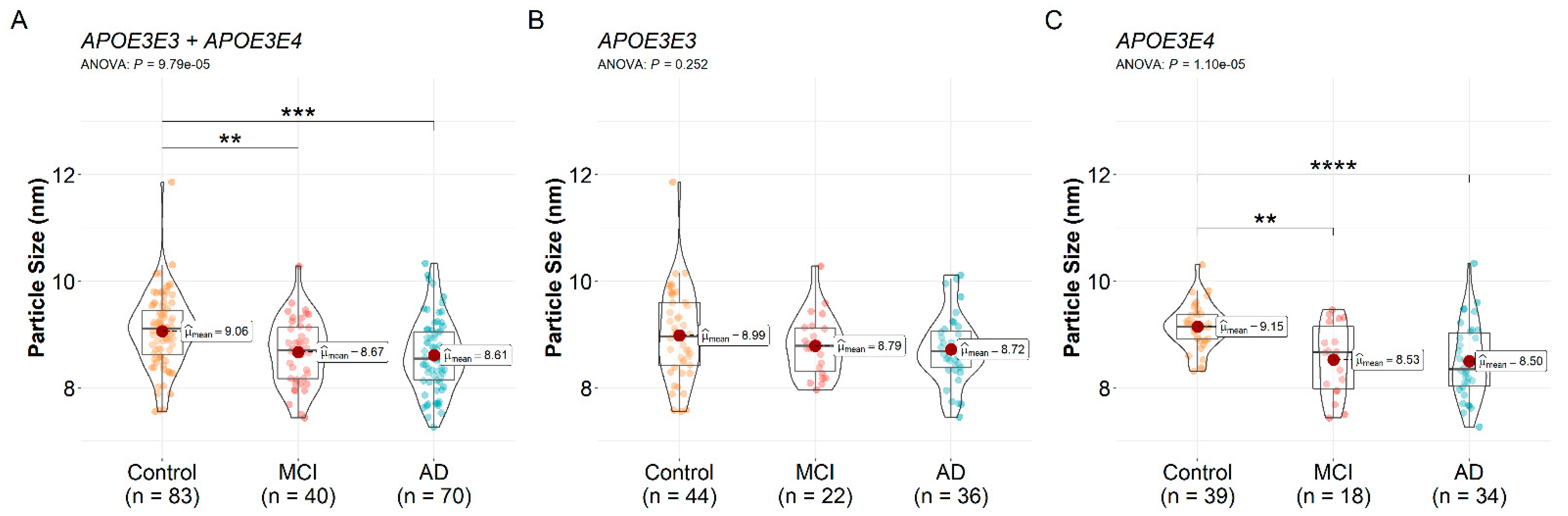
3.3. HDL Particle Size Differences
3.4. Correlation between HDL Function, Size, and Cognitive Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward Defining the Preclinical Stages of Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. J. Alzheimers Assoc. 2011, 7, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Zuin, M.; Cervellati, C.; Trentini, A.; Passaro, A.; Rosta, V.; Zimetti, F.; Zuliani, G. Association between Serum Concentrations of Apolipoprotein A-I (ApoA-I) and Alzheimer’s Disease: Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 984. [Google Scholar] [CrossRef]
- Merched, A.; Xia, Y.; Visvikis, S.; Serot, J.M.; Siest, G. Decreased High-Density Lipoprotein Cholesterol and Serum Apolipoprotein AI Concentrations Are Highly Correlated with the Severity of Alzheimer’s Disease☆. Neurobiol. Aging 2000, 21, 27–30. [Google Scholar] [CrossRef]
- Svensson, T.; Sawada, N.; Mimura, M.; Nozaki, S.; Shikimoto, R.; Tsugane, S. The Association between Midlife Serum High-Density Lipoprotein and Mild Cognitive Impairment and Dementia after 19 Years of Follow-Up. Transl. Psychiatry 2019, 9, 26. [Google Scholar] [CrossRef]
- Button, E.B.; Robert, J.; Caffrey, T.M.; Fan, J.; Zhao, W.; Wellington, C.L. HDL from an Alzheimer’s Disease Perspective. Curr. Opin. Lipidol. 2019, 30, 224–234. [Google Scholar] [CrossRef]
- Holzer, M.; Trieb, M.; Konya, V.; Wadsack, C.; Heinemann, A.; Marsche, G. Aging Affects High-Density Lipoprotein Composition and Function. Biochim. Biophys. Acta 2013, 1831, 1442–1448. [Google Scholar] [CrossRef] [Green Version]
- Soran, H.; Hama, S.; Yadav, R.; Durrington, P.N. HDL Functionality. Curr. Opin. Lipidol. 2012, 23, 353–366. [Google Scholar] [CrossRef]
- Lewis, T.L.; Cao, D.; Lu, H.; Mans, R.A.; Su, Y.R.; Jungbauer, L.; Linton, M.F.; Fazio, S.; LaDu, M.J.; Li, L. Overexpression of Human Apolipoprotein A-I Preserves Cognitive Function and Attenuates Neuroinflammation and Cerebral Amyloid Angiopathy in a Mouse Model of Alzheimer Disease. J. Biol. Chem. 2010, 285, 36958–36968. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; Berrougui, H.; Pawelec, G.; Fulop, T. Impairment of the ABCA1 and SR-BI-Mediated Cholesterol Efflux Pathways and HDL Anti-Inflammatory Activity in Alzheimer’s Disease. Mech. Ageing Dev. 2012, 133, 20–29. [Google Scholar] [CrossRef]
- Hinton, L.; Carter, K.; Reed, B.R.; Beckett, L.; Lara, E.; DeCarli, C.; Mungas, D. Recruitment of a Community-Based Cohort for Research on Diversity and Risk of Dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.W.; Harvey, D.J.; Beckett, L.A.; Green, R.; Farias, S.T.; Reed, B.R.; Olichney, J.M.; Mungas, D.M.; DeCarli, C. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol. 2015, 72, 1295–1303. [Google Scholar] [CrossRef]
- Zheng, J.J.; Agus, J.K.; Hong, B.V.; Tang, X.; Rhodes, C.H.; Houts, H.E.; Zhu, C.; Kang, J.W.; Wong, M.; Xie, Y.; et al. Isolation of HDL by Sequential Flotation Ultracentrifugation Followed by Size Exclusion Chromatography Reveals Size-Based Enrichment of HDL-Associated Proteins. Sci. Rep. 2021, 11, 16086. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.V.; Zhu, C.; Wong, M.; Sacchi, R.; Rhodes, C.H.; Kang, J.W.; Arnold, C.D.; Adu-Afarwuah, S.; Lartey, A.; Oaks, B.M.; et al. Lipid-Based Nutrient Supplementation Increases High-Density Lipoprotein (HDL) Cholesterol Efflux Capacity and Is Associated with Changes in the HDL Glycoproteome in Children. ACS Omega 2021, 6, 32022–32031. [Google Scholar] [CrossRef] [PubMed]
- Minami-Takano, A.; Iwata, H.; Miyosawa, K.; Shiozawa, T.; Hayashi, H.; Funamizu, T.; Ishii, K.; Nozaki, Y.; Tabuchi, H.; Sekita, G.; et al. The Association between Impairment of HDL Cholesterol Efflux Capacity and Atrial Remodeling in Atrial Fibrillation. Sci. Rep. 2021, 11, 3547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tong, H.; Garewal, M.; Ren, G. Optimized Negative-Staining Electron Microscopy for Lipoprotein Studies. Biochim. Biophys. Acta 2013, 1830, 2150–2159. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Mungas, D.; Reed, B.R.; Marshall, S.C.; González, H.M. Development of Psychometrically Matched English and Spanish Language Neuropsychological Tests for Older Persons. Neuropsychology 2000, 14, 209–223. [Google Scholar] [CrossRef]
- Mungas, D.; Reed, B.R.; Crane, P.K.; Haan, M.N.; González, H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further Development and Psychometric Characteristics. Psychol. Assess. 2004, 16, 347–359. [Google Scholar] [CrossRef]
- Daly, E.; Zaitchik, D.; Copeland, M.; Schmahmann, J.; Gunther, J.; Albert, M. Predicting Conversion to Alzheimer Disease Using Standardized Clinical Information. Arch. Neurol. 2000, 57, 675–680. [Google Scholar] [CrossRef] [Green Version]
- DeCarli, C.; Maisog, J.; Murphy, D.G.; Teichberg, D.; Rapoport, S.I.; Horwitz, B. Method for Quantification of Brain, Ventricular, and Subarachnoid CSF Volumes from MR Images. J. Comput. Assist. Tomogr. 1992, 16, 274–284. [Google Scholar] [CrossRef] [PubMed]
- DeCarli, C.; Miller, B.L.; Swan, G.E.; Reed, T.; Wolf, P.A.; Garner, J.; Jack, L.; Carmelli, D. Predictors of Brain Morphology for the Men of the NHLBI Twin Study. Stroke 1999, 30, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Thalamuthu, A.; Mather, K.A.; Crawford, J.; Ulanova, M.; Wong, M.W.K.; Pickford, R.; Sachdev, P.S.; Braidy, N. Plasma Lipidome Is Dysregulated in Alzheimer’s Disease and Is Associated with Disease Risk Genes. Transl. Psychiatry 2021, 11, 344. [Google Scholar] [CrossRef]
- Rader, D.J.; Tall, A.R. Is It Time to Revise the HDL Cholesterol Hypothesis? Nat. Med. 2012, 18, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shea, S.; Stein, J.H.; Jorgensen, N.W.; McClelland, R.L.; Tascau, L.; Shrager, S.; Heinecke, J.W.; Yvan-Charvet, L.; Tall, A.R. Cholesterol Mass Efflux Capacity, Incident Cardiovascular Disease, and Progression of Carotid Plaque. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef]
- Yassine, H.N.; Feng, Q.; Chiang, J.; Petrosspour, L.M.; Fonteh, A.N.; Chui, H.C.; Harrington, M.G. ABCA1-Mediated Cholesterol Efflux Capacity to Cerebrospinal Fluid Is Reduced in Patients With Mild Cognitive Impairment and Alzheimer’s Disease. J. Am. Heart Assoc. 2016, 5, e002886. [Google Scholar] [CrossRef] [Green Version]
- Marchi, C.; Adorni, M.P.; Caffarra, P.; Ronda, N.; Spallazzi, M.; Barocco, F.; Galimberti, D.; Bernini, F.; Zimetti, F. ABCA1- and ABCG1-Mediated Cholesterol Efflux Capacity of Cerebrospinal Fluid Is Impaired in Alzheimer’s Disease. J. Lipid Res. 2019, 60, 1449–1456. [Google Scholar] [CrossRef]
- Elias-Sonnenschein, L.S.; Viechtbauer, W.; Ramakers, I.H.G.B.; Verhey, F.R.J.; Visser, P.J. Predictive Value of APOE-Ε4 Allele for Progression from MCI to AD-Type Dementia: A Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1149–1156. [Google Scholar] [CrossRef]
- Blacker, D.; Haines, J.L.; Rodes, L.; Terwedow, H.; Go, R.C.; Harrell, L.E.; Perry, R.T.; Bassett, S.S.; Chase, G.; Meyers, D.; et al. ApoE-4 and Age at Onset of Alzheimer’s Disease: The NIMH Genetics Initiative. Neurology 1997, 48, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Femlak, M.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Rysz, J. The Role and Function of HDL in Patients with Diabetes Mellitus and the Related Cardiovascular Risk. Lipids Health Dis. 2017, 16, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escolà-Gil, J.C.; Rotllan, N.; Julve, J.; Blanco-Vaca, F. Reverse Cholesterol Transport Dysfunction Is a Feature of Familial Hypercholesterolemia. Curr. Atheroscler. Rep. 2021, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Davidson, W.S.; Silva, R.A.G.D.; Chantepie, S.; Lagor, W.R.; Chapman, M.J.; Kontush, A. Proteomic Analysis of Defined HDL Subpopulations Reveals Particle-Specific Protein Clusters: Relevance to Antioxidative Function. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 870–876. [Google Scholar] [CrossRef]
- Kaysen, G.A. Metabolic Syndrome and Renal Failure: Similarities and Differences. Panminerva Med. 2006, 48, 151–164. [Google Scholar]
- Oulhaj, A.; Wilcock, G.K.; Smith, A.D.; de Jager, C.A. Predicting the Time of Conversion to MCI in the Elderly: Role of Verbal Expression and Learning. Neurology 2009, 73, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Denny, K.G.; Harvey, D.; Farias, S.T.; Mungas, D.; DeCarli, C.; Beckett, L. Progression from Normal Cognition to Mild Cognitive Impairment in a Diverse Clinic-Based and Community-Based Elderly Cohort. Alzheimers Dement. J. Alzheimers Assoc. 2017, 13, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.E.; Weissberger, G.; Kuklenyik, Z.; He, X.; Meuret, C.; Parekh, T.; Rees, J.C.; Parks, B.A.; Gardner, M.S.; King, S.M.; et al. The Small HDL Particle Hypothesis of Alzheimer’s Disease. Alzheimers Dement. 2022. [Google Scholar] [CrossRef]
- Van Valkenburgh, J.; Meuret, C.; Martinez, A.E.; Kodancha, V.; Solomon, V.; Chen, K.; Yassine, H.N. Understanding the Exchange of Systemic HDL Particles Into the Brain and Vascular Cells Has Diagnostic and Therapeutic Implications for Neurodegenerative Diseases. Front. Physiol. 2021, 12, 700847. [Google Scholar] [CrossRef]
- Yamamoto, S.; Narita, I.; Kotani, K. The Macrophage and Its Related Cholesterol Efflux as a HDL Function Index in Atherosclerosis. Clin. Chim. Acta 2016, 457, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Cavigiolio, G.; Brot, N.; Oda, M.N.; Heinecke, J.W. Methionine Oxidation Impairs Reverse Cholesterol Transport by Apolipoprotein A-I. Proc. Natl. Acad. Sci. USA 2008, 105, 12224–12229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedrick, C.C.; Thorpe, S.R.; Fu, M.X.; Harper, C.M.; Yoo, J.; Kim, S.M.; Wong, H.; Peters, A.L. Glycation Impairs High-Density Lipoprotein Function. Diabetologia 2000, 43, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashyap, S.R.; Osme, A.; Ilchenko, S.; Golizeh, M.; Lee, K.; Wang, S.; Bena, J.; Previs, S.F.; Smith, J.D.; Kasumov, T. Glycation Reduces the Stability of ApoAI and Increases HDL Dysfunction in Diet-Controlled Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 388–396. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Control | MCI | AD | F(df, df Error) or χ2(df) | p-Value |
|---|---|---|---|---|---|
| n combined | 83 | 40 | 71 | n/a | n/a |
| APOE3E3 | 44 | 22 | 37 | n/a | n/a |
| APOE3E4 | 39 | 18 | 34 | n/a | n/a |
| Sex proportion, (male/female), combined | 35:48 | 23:17 | 27:44 | χ2(2) = 4.10 | 0.129 |
| APOE3E3 | 18:26 | 12:10 | 15:22 | χ2(2) = 1.34 | 0.511 |
| APOE3E4 | 17:22 | 11:7 | 12:22 | χ2(2) = 3.18 | 0.203 |
| Age, years, mean ± SD, (n), combined | 75.5 ± 7.0 (83) | 78.0 ± 7.0 (40) | 78.9 ± 7.2 (71) | F(2, 191) = 4.82 | 0.009 ab |
| APOE3E3 | 75.9 ± 7.0 (44) | 78.6 ± 7.7 (22) | 79.7 ± 8.4 (37) | F(2, 100) = 2.55 | 0.080 |
| APOE3E4 | 75.0 ± 7.1 (39) | 77.3 ± 6.2 (18) | 78.1 ± 5.7 (34) | F(2, 88) = 2.28 | 0.108 |
| BMI, kg/m2, mean ± SD, (n), combined | 28.1 ± 4.6 (81) | 27.1 ± 4.2 (39) | 26.6 ± 4.6 (64) | F(2, 181) = 2.00 | 0.138 |
| APOE3E3 | 28.6 ± 4.5 (43) | 27.8 ± 5.1 (21) | 26.2 ± 4.7 (32) | F(2, 93) = 2.49 | 0.089 |
| APOE3E4 | 27.5 ± 4.6 (38) | 26.3 ± 2.7 (18) | 27.0 ± 4.9 (32) | F(2, 85) = 0.42 | 0.658 |
| Ethnicity proportion, (African American/Asian/Hispanic/White), combined | 18:2:19:44 | 4:1:4:31 | 7:1:11:52 | χ2(6) = 10.97 | 0.089 |
| Diabetes at sample collection, %, (n), combined | 34% (76) | 7.9% (38) | 4.9% (61) | χ2(2) = 22.94 | <0.001abc |
| APOE3E3 | 39% (41) | 9.5% (21) | 0% (32) | χ2(2) = 19.30 | <0.001 abc |
| APOE3E4 | 29% (35) | 5.9% (17) | 10% (29) | χ2(2) = 5.64 | 0.060 |
| History of diabetes, %, (n), combined | 39% (83) | 10% (39) | 5.6% (71) | χ2(2) = 28.50 | <0.001 abc |
| APOE3E3 | 39% (44) | 14% (21) | 0% (37) | χ2(2) = 19.51 | <0.001 abc |
| APOE3E4 | 38% (39) | 5.6% (18) | 12% (34) | χ2(2) = 8.52 | 0.014 abc |
| History of hypercholesterolemia, %, (n), combined | 74% (81) | 60% (40) | 54% (70) | χ2(2) = 6.70 | 0.035 ab |
| APOE3E3 | 62% (42) | 64% (22) | 47% (36) | χ2(2) = 2.21 | 0.331 |
| APOE3E4 | 87% (39) | 56% (18) | 62% (34) | χ2(2) = 8.52 | 0.014 abc |
| History of hypertension, %, (n), combined | 72% (83) | 65% (40) | 76% (71) | χ2(2) = 1.56 | 0.459 |
| APOE3E3 | 75% (44) | 77% (22) | 76% (37) | χ2(2) = 0.04 | 0.980 |
| APOE3E4 | 69% (39) | 50% (18) | 76% (34) | χ2(2) = 3.84 | 0.147 |
| Genotype | Group | ANOVA | Post hoc Comparison p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MCI | AD | F | df | df Error | p-Value | Control vs. MCI | Control vs. AD | MCI vs. AD | |
| CEC Index | ||||||||||
| APOE3E3 + APOE3E4 | 1.10 ± 0.16 | 1.06 ± 0.14 | 1.12 ± 0.14 | 2.14 | 2 | 191 | 0.120 | - | - | - |
| APOE3E3 | 1.16 ± 0.13 | 1.07 ± 0.13 | 1.11 ± 0.15 | 3.26 | 2 | 100 | 0.042 | 0.042 | 0.246 | 0.551 |
| APOE3E4 | 1.02 ± 0.16 | 1.03 ± 0.14 | 1.12 ± 0.14 | 4.38 | 2 | 88 | 0.015 | 0.964 | 0.016 | 0.114 |
| LCAT Activity (390/470 nm) | ||||||||||
| APOE3E3 + APOE3E4 | 1.05 ± 0.09 | 1.01 ± 0.07 | 1.03 ± 0.07 | 3.87 | 2 | 191 | 0.023 | 0.030 | 0.118 | 0.661 |
| APOE3E3 | 1.09 ± 0.09 | 1.03 ± 0.08 | 1.03 ± 0.07 | 7.00 | 2 | 100 | 0.001 | 0.012 | 0.004 | 0.995 |
| APOE3E4 | 1.01 ± 0.07 | 1.00 ± 0.06 | 1.02 ± 0.07 | 0.91 | 2 | 88 | 0.410 | - | - | - |
| HDL Particle Diameter (nm) | ||||||||||
| APOE3E3 + APOE3E4 | 9.06 ± 0.69 | 8.67 ± 0.63 | 8.61 ± 0.68 | 9.70 | 2 | 190 | <0.001 | 0.008 | <0.001 | 0.901 |
| APOE3E3 | 8.99 ± 0.85 | 8.79 ± 0.58 | 8.72 ± 0.64 | 1.40 | 2 | 99 | 0.252 | - | - | - |
| APOE3E4 | 9.15 ± 0.42 | 8.53 ± 0.67 | 8.50 ± 0.70 | 13.04 | 2 | 88 | <0.001 | 0.001 | <0.001 | 0.985 |
| Variable | Group | Kruskal–Wallis Test | |||||
|---|---|---|---|---|---|---|---|
| Control n = 83 | MCI n = 40 | AD n = 71 | χ2 | df | p-Value | ||
| Cognitive | Verbal memory score, | 0.24 | −0.97 | −1.41 | 87.72 | 2 | <0.001 |
| median (25th, 75th), | (−0.39, 0.67) | (−1.37, −0.73) | (−1.90, −1.06) | ||||
| (n) | (69) | (35) | (40) | ||||
| Executive function score, | −0.03 | −0.37 | −0.96 | 54.29 | 2 | <0.001 | |
| median (25th, 75th), | (−0.27, 0.44) | (−0.60, −0.07) | (−1.52, −0.55) | ||||
| (n) | (68) | (35) | (44) | ||||
| Semantic memory score, | 0.50 | 0.23 | −0.50 | 32.95 | 2 | <0.001 | |
| median (25th, 75th), | (−0.08, 0.91) | (−0.12, 0.66) | (−1.01, 0.17) | ||||
| (n) | (68) | (35) | (43) | ||||
| Spatial score, | 0.42 | 0.14 | −0.86 | 32.09 | 2 | <0.001 | |
| median (25th, 75th), | (−0.18, 0.83) | (−0.21, 0.48) | (−1.36, −0.05) | ||||
| (n) | (67) | (35) | (37) | ||||
| Functional | CDR sum of boxes, | 0.00 | 3.00 | 5.00 | 94.79 | 2 | <0.001 |
| median (25th, 75th), | (0.00, 0.50) | (1.50, 3.50) | (3.38, 7.00) | ||||
| (n) | (59) | (33) | (40) | ||||
| Imaging a | WMH volume, | 0.00 | 0.01 | 0.01 | 18.27 | 2 | <0.001 |
| median (25th, 75th), | (0.00, 0.01) | (0.01, 0.02) | (0.00, 0.01) | ||||
| (n) | (65) | (33) | (40) | ||||
| Cognitive | Functional | Imaging a | ||||
|---|---|---|---|---|---|---|
| Characteristic | Verbal Memory Score | Executive Function Score | Semantic Memory Score | Spatial Score | CDR Sum of Boxes | WMH Volume |
| n | 144 | 147 | 146 | 139 | 132 | 138 |
| CEC Index | ||||||
| r | 0.02 | 0.01 | −0.10 | −0.01 | 0.04 | 0.12 |
| 95% CI | (−0.15, 0.19) | (−0.15, 0.18) | (−0.26, 0.07) | (−0.27, 0.07) | (−0.14, 0.21) | (−0.05, 0.29) |
| p-value | 0.826 | 0.877 | 0.234 | 0.245 | 0.693 | 0.149 |
| Adjusted b r | 0.02 | 0.03 | −0.07 | −0.08 | 0.05 | 0.12 |
| Adjusted b 95% CI | (−0.14, 0.19) | (−0.15, 0.19) | (−0.23, 0.09) | (−0.24, 0.08) | (−0.12, 0.23) | (−0.04, 0.29) |
| Adjusted b p-value | 0.824 | 0.720 | 0.397 | 0.334 | 0.571 | 0.160 |
| LCAT Activity (390/470 nm) | ||||||
| r | 0.17 | 0.13 | −0.13 | −0.10 | −0.20 | −0.05 |
| 95% CI | (0.01, 0.33) | (−0.03, 0.29) | (−0.29, 0.04) | (−0.26, 0.08) | (−0.37, −0.03) | (−0.22, 0.12) |
| p-value | 0.037 | 0.105 | 0.132 | 0.265 | 0.019 | 0.562 |
| Adjusted b r | 0.18 | 0.15 | −0.10 | −0.08 | −0.20 | −0.06 |
| Adjusted b 95% CI | (0.01, 0.33) | (−0.02, 0.29) | (−0.27, 0.07) | (−0.26, 0.07) | (−0.35, −0.02) | (−0.21, 0.10) |
| Adjusted b p-value | 0.033 | 0.069 | 0.231 | 0.358 | 0.025 | 0.512 |
| Particle Size (nm) | ||||||
| r | 0.31 | 0.21 | 0.07 | 0.08 | −0.31 | −0.17 |
| 95% CI | (0.15, 0.45) | (0.04, 0.36) | (−0.10, 0.24) | (−0.09, 0.25) | (−0.46, −0.14) | (−0.33, 0.00) |
| p-value | <0.001 | 0.013 | 0.396 | 0.330 | <0.001 | 0.049 |
| Adjusted b r | 0.31 | 0.21 | 0.08 | 0.09 | −0.31 | −0.17 |
| Adjusted b 95% CI | (0.15, 0.45) | (0.05, 0.34) | (−0.08, 0.23) | (−0.08, 0.25) | (−0.45, −0.14) | (−0.32, 0.02) |
| Adjusted b p-value | <0.001 | 0.007 | 0.331 | 0.313 | <0.001 | 0.053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, B.V.; Zheng, J.; Agus, J.K.; Tang, X.; Lebrilla, C.B.; Jin, L.-W.; Maezawa, I.; Erickson, K.; Harvey, D.J.; DeCarli, C.S.; et al. High-Density Lipoprotein Changes in Alzheimer’s Disease Are APOE Genotype-Specific. Biomedicines 2022, 10, 1495. https://doi.org/10.3390/biomedicines10071495
Hong BV, Zheng J, Agus JK, Tang X, Lebrilla CB, Jin L-W, Maezawa I, Erickson K, Harvey DJ, DeCarli CS, et al. High-Density Lipoprotein Changes in Alzheimer’s Disease Are APOE Genotype-Specific. Biomedicines. 2022; 10(7):1495. https://doi.org/10.3390/biomedicines10071495
Chicago/Turabian StyleHong, Brian V., Jingyuan Zheng, Joanne K. Agus, Xinyu Tang, Carlito B. Lebrilla, Lee-Way Jin, Izumi Maezawa, Kelsey Erickson, Danielle J. Harvey, Charles S. DeCarli, and et al. 2022. "High-Density Lipoprotein Changes in Alzheimer’s Disease Are APOE Genotype-Specific" Biomedicines 10, no. 7: 1495. https://doi.org/10.3390/biomedicines10071495
APA StyleHong, B. V., Zheng, J., Agus, J. K., Tang, X., Lebrilla, C. B., Jin, L.-W., Maezawa, I., Erickson, K., Harvey, D. J., DeCarli, C. S., Mungas, D. M., Olichney, J. M., Farias, S. T., & Zivkovic, A. M. (2022). High-Density Lipoprotein Changes in Alzheimer’s Disease Are APOE Genotype-Specific. Biomedicines, 10(7), 1495. https://doi.org/10.3390/biomedicines10071495