Arterial Calcifications in Patients with Liver Cirrhosis Are Linked to Hepatic Deficiency of Pyrophosphate Production Restored by Liver Transplantation
Abstract
:1. Introduction
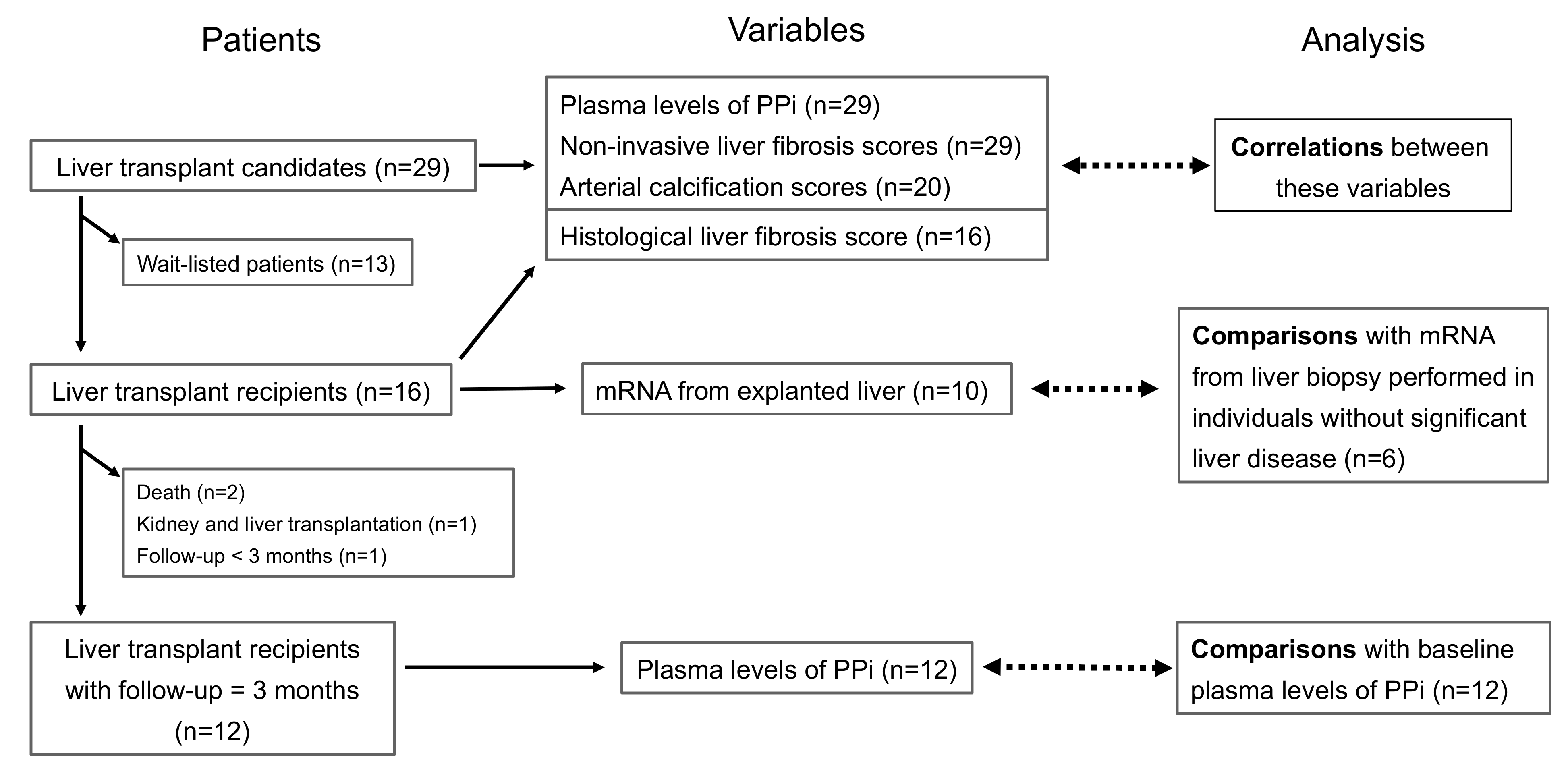
2. Materials and Methods
2.1. Patients
2.2. Trial Procedures
2.3. Calculation of Scores
2.4. Specific Dosages
2.5. Outcomes
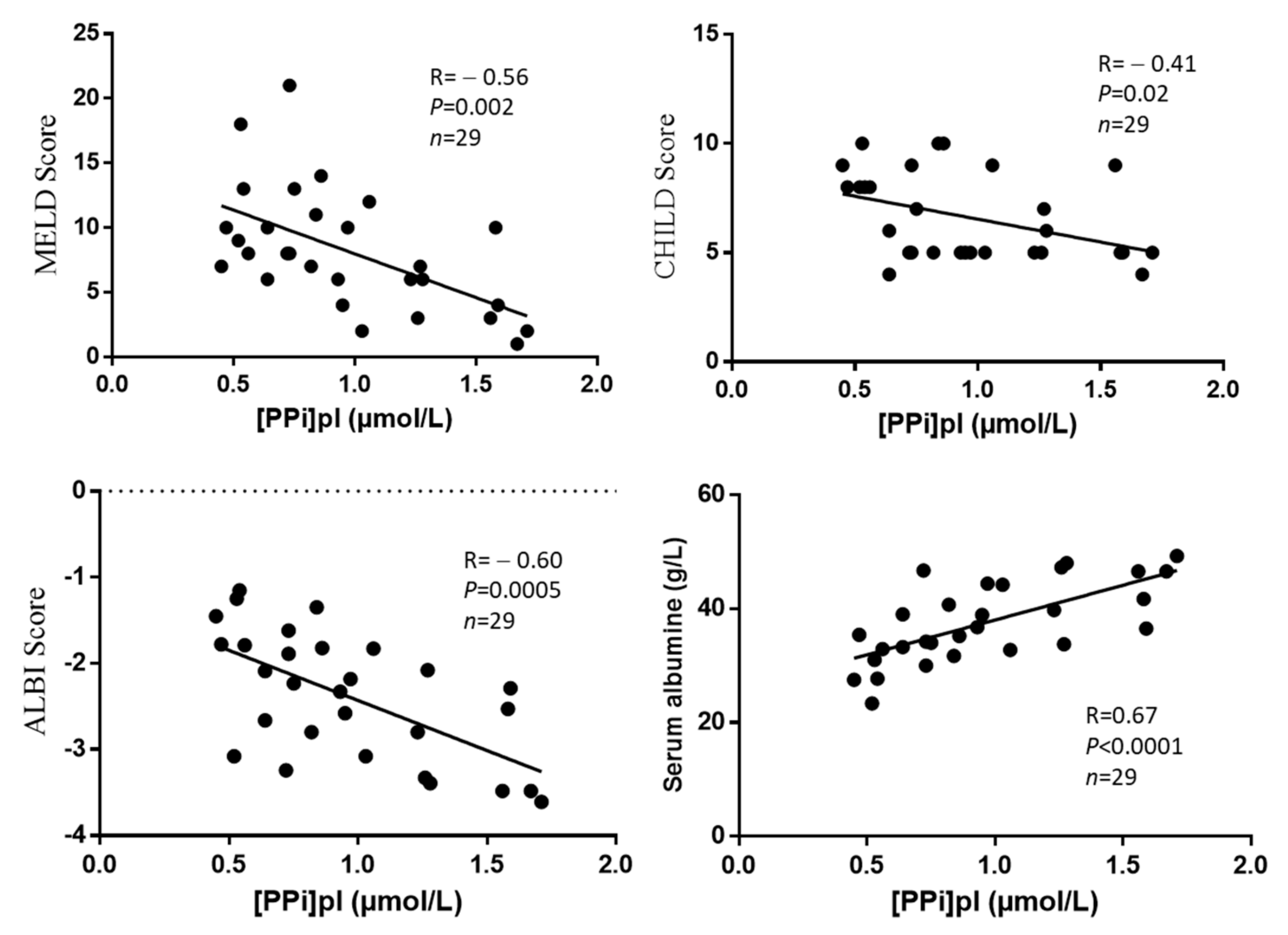
- Association between [PPi]pl and liver fibrosis indexes;
- Association between [PPi]pl and albumin or clinical scores of liver failure.
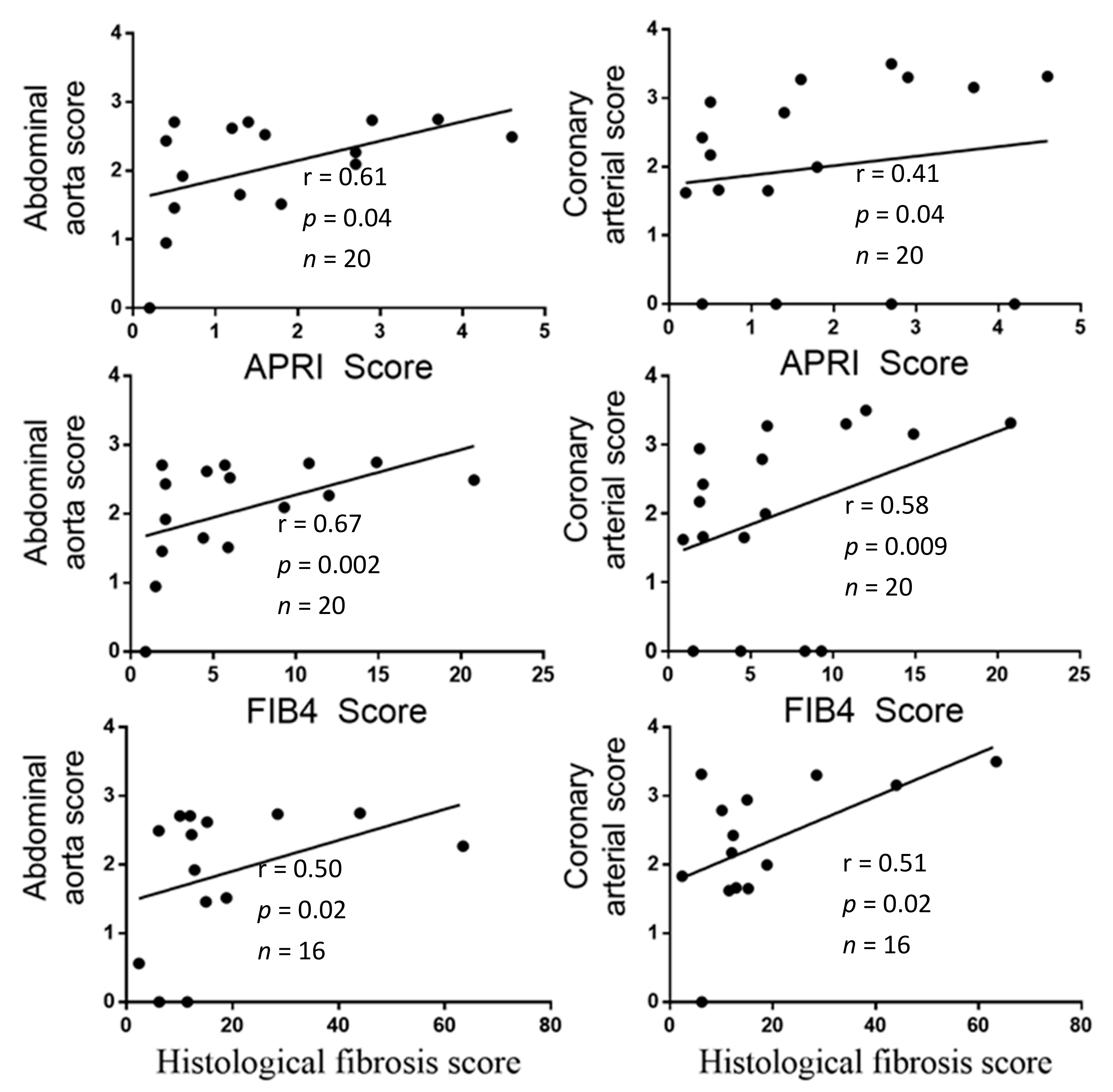
- Association between [PPi]pl and AC indexes;
- Association between liver fibrosis indexes and AC indexes;
- Association between albumin or clinical scores of liver failure and AC indexes.
- the correlation between plasma ALP activity and [PPi]pl at baseline;
- the comparison of mRNA levels of ABCC6, ENPP1, ALPL, and NT5E between the liver biopsies from the explanted liver from patients undergoing LT and liver biopsies from a control group of individuals without liver disease;
- the comparison between plasma ALP activity in LT candidates at baseline and plasma ALP activity in LT recipients three months after LT.
2.6. Statistical Analysis
2.6.1. Sample Size Calculation
2.6.2. Statistics
3. Results
3.1. Description of the Study
3.2. Description of the Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Mosler, T.P.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorzi, A.; Brunetti, G.; Cardaioli, F.; D’Arcangelo, F.; Fabris, T.; Gambato, M.; Iliceto, S.; Martini, A.; Mattesi, G.; Peluso, C.; et al. Coronary artery calcium on standard chest computed tomography predicts cardiovascular events after liver transplantation. Int. J. Cardiol. 2021, 339, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Dogan, S.; Celikbilek, M.; Yilmaz, Y.K.; Sarikaya, S.; Zararsiz, G.; Serin, H.I.; Borekci, E.; Akyol, L.; Pirti, I.; Davarci, S.E. Association between liver fibrosis and coronary heart disease risk in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Ishiba, H.; Sumida, Y.; Kataoka, S.; Kuroda, M.; Akabame, S.; Tomiyasu, K.; Tanaka, M.; Arai, M.; Taketani, H.; Seko, Y.; et al. Association of coronary artery calcification with liver fibrosis in Japanese patients with non-alcoholic fatty liver disease. Hepatol. Res. 2016, 46, 1107–1117. [Google Scholar] [CrossRef]
- You, S.C.; Kim, K.J.; Kim, S.U.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Hepatic fibrosis assessed using transient elastography independently associated with coronary artery calcification. J. Gastroenterol. Hepatol. 2015, 30, 1536–1542. [Google Scholar] [CrossRef]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Poupon, R. Liver alkaline phosphatase: A missing link between choleresis and biliary inflammation. Hepatology 2015, 61, 2080–2090. [Google Scholar] [CrossRef]
- Corpechot, C.; Chazouilleres, O.; Rousseau, A.; Le Gruyer, A.; Habersetzer, F.; Mathurin, P.; Goria, O.; Potier, P.; Minello, A.; Silvain, C.; et al. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N. Engl. J. Med. 2018, 378, 2171–2181. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Apekey, T.A.; Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 2014, 236, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Monseu, M.; Dubois, S.; Boursier, J.; Aube, C.; Gagnadoux, F.; Leftheriotis, G.; Ducluzeau, P.H. Osteoprotegerin levels are associated with liver fat and liver markers in dysmetabolic adults. Diabetes Metab. 2016, 42, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Varadi, A.; Plomp, A.; Bergen, A.A.; Oude Elferink, R.P.; Borst, P.; et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedinszki, D.; Szeri, F.; Kozak, E.; Pomozi, V.; Tokesi, N.; Mezei, T.R.; Merczel, K.; Letavernier, E.; Tang, E.; Le Saux, O.; et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol. Med. 2017, 9, 1463–1470. [Google Scholar] [CrossRef]
- Laurain, A.; Rubera, I.; Duranton, C.; Rutsch, F.; Nitschke, Y.; Ray, E.; Vido, S.; Sicard, A.; Leftheriotis, G.; Favre, G. Alkaline Phosphatases Account for Low Plasma Levels of Inorganic Pyrophosphate in Chronic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 586831. [Google Scholar] [CrossRef]
- Zhao, J.; Kingman, J.; Sundberg, J.P.; Uitto, J.; Li, Q. Plasma PPi Deficiency is the Major, But Not the Exclusive, Cause of Ectopic Mineralization in an Abcc6−/− Mouse Model of PXE. J. Investig. Dermatol. 2017, 137, 2336–2343. [Google Scholar] [CrossRef] [Green Version]
- Favre, G.; Laurain, A.; Aranyi, T.; Szeri, F.; Fulop, K.; Le Saux, O.; Duranton, C.; Kauffenstein, G.; Martin, L.; Leftheriotis, G. The ABCC6 Transporter: A New Player in Biomineralization. Int. J. Mol. Sci. 2017, 18, 1941. [Google Scholar] [CrossRef]
- Bergen, A.A.; Plomp, A.S.; Schuurman, E.J.; Terry, S.; Breuning, M.; Dauwerse, H.; Swart, J.; Kool, M.; van Soest, S.; Baas, F.; et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 228–231. [Google Scholar] [CrossRef]
- Le Saux, O.; Urban, Z.; Tschuch, C.; Csiszar, K.; Bacchelli, B.; Quaglino, D.; Pasquali-Ronchetti, I.; Pope, F.M.; Richards, A.; Terry, S.; et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Ringpfeil, F.; Lebwohl, M.G.; Christiano, A.M.; Uitto, J. Pseudoxanthoma elasticum: Mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6001–6006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.S.; Kucukosmanoglu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.; Bergen, A.A.; Gorgels, T.G.; Borst, P.; van de Wetering, K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, K.; Hayashi, K.; Dang, K.; Hayashi, M.; Boyd, C.D. Analysis of ABCC6 (MRP6) in normal human tissues. Histochem. Cell Biol. 2005, 123, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.I.; van Driel, I.R.; Goding, J.W. Identification and characterization of a soluble form of the plasma cell membrane glycoprotein PC-1 (5′-nucleotide phosphodiesterase). Eur. J. Biochem. 1993, 217, 421–428. [Google Scholar] [CrossRef]
- Pomozi, V.; Brampton, C.; van de Wetering, K.; Zoll, J.; Calio, B.; Pham, K.; Owens, J.B.; Marh, J.; Moisyadi, S.; Varadi, A.; et al. Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am. J. Pathol. 2017, 187, 1258–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Back, M.; Aranyi, T.; Cancela, M.L.; Carracedo, M.; Conceicao, N.; Leftheriotis, G.; Macrae, V.; Martin, L.; Nitschke, Y.; Pasch, A.; et al. Endogenous Calcification Inhibitors in the Prevention of Vascular Calcification: A Consensus Statement from the COST Action EuroSoftCalcNet. Front. Cardiovasc. Med. 2018, 5, 196. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Patouraux, S.; Rousseau, D.; Bonnafous, S.; Lebeaupin, C.; Luci, C.; Canivet, C.M.; Schneck, A.S.; Bertola, A.; Saint-Paul, M.C.; Iannelli, A.; et al. CD44 is a key player in non-alcoholic steatohepatitis. J. Hepatol. 2017, 67, 328–338. [Google Scholar] [CrossRef]
- Bekri, S.; Gual, P.; Anty, R.; Luciani, N.; Dahman, M.; Ramesh, B.; Iannelli, A.; Staccini-Myx, A.; Casanova, D.; Ben Amor, I.; et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 2006, 131, 788–796. [Google Scholar] [CrossRef]
- Anty, R.; Bekri, S.; Luciani, N.; Saint-Paul, M.C.; Dahman, M.; Iannelli, A.; Amor, I.B.; Staccini-Myx, A.; Huet, P.M.; Gugenheim, J.; et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am. J. Gastroenterol. 2006, 101, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, F.; Valla, D. Assessment of prognosis of cirrhosis. Semin. Liver Dis. 2008, 28, 110–122. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Legrand, A.; Pujol, C.; Durand, C.M.; Mesnil, A.; Rubera, I.; Duranton, C.; Zuily, S.; Sousa, A.B.; Renaud, M.; Boucher, J.L.; et al. Pseudoxanthoma elasticum overlaps hereditary spastic paraplegia type 56. J. Intern. Med. 2021, 289, 709–725. [Google Scholar] [CrossRef]
- Li, Q.; Kingman, J.; van de Wetering, K.; Tannouri, S.; Sundberg, J.P.; Uitto, J. Abcc6 Knockout Rat Model Highlights the Role of Liver in PPi Homeostasis in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2017, 137, 1025–1032. [Google Scholar] [CrossRef]
- Miglionico, R.; Armentano, M.F.; Carmosino, M.; Salvia, A.M.; Cuviello, F.; Bisaccia, F.; Ostuni, A. Dysregulation of gene expression in ABCC6 knockdown HepG2 cells. Cell. Mol. Biol. Lett. 2014, 19, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Tevar, A.M.; Garcia-Fernandez, M.; Murcia-Casas, B.; Rioja-Villodres, J.; Carrillo, J.L.; Camacho, M.; Van Gils, M.; Sanchez-Chaparro, M.A.; Vanakker, O.; Valdivielso, P. Plasma inorganic pyrophosphate and alkaline phosphatase in patients with pseudoxanthoma elasticum. Ann. Transl. Med. 2019, 7, 798. [Google Scholar] [CrossRef]
- Savinov, A.Y.; Salehi, M.; Yadav, M.C.; Radichev, I.; Millan, J.L.; Savinova, O.V. Transgenic Overexpression of Tissue-Nonspecific Alkaline Phosphatase (TNAP) in Vascular Endothelium Results in Generalized Arterial Calcification. J. Am. Heart Assoc. 2015, 4, e002499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brichacek, A.L.; Benkovic, S.A.; Chakraborty, S.; Nwafor, D.C.; Wang, W.; Jun, S.; Dakhlallah, D.; Geldenhuys, W.J.; Pinkerton, A.B.; Millan, J.L.; et al. Systemic inhibition of tissue-nonspecific alkaline phosphatase alters the brain-immune axis in experimental sepsis. Sci. Rep. 2019, 9, 18788. [Google Scholar] [CrossRef] [Green Version]
- Sharples, E.J.; Pereira, D.; Summers, S.; Cunningham, J.; Rubens, M.; Goldsmith, D.; Yaqoob, M.M. Coronary artery calcification measured with electron-beam computerized tomography correlates poorly with coronary artery angiography in dialysis patients. Am. J. Kidney Dis. 2004, 43, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, L.I.; Polak, J.F.; Cupples, L.A.; Hannan, M.T.; Kiel, D.P.; Wilson, P.W. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 1997, 132, 245–250. [Google Scholar] [CrossRef]
- London, G.M.; Guerin, A.P.; Marchais, S.J.; Metivier, F.; Pannier, B.; Adda, H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transplant. 2003, 18, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Marchais, S.J.; Guerin, A.P.; Metivier, F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr. Opin. Nephrol. Hypertens. 2005, 14, 525–531. [Google Scholar] [CrossRef]
- Evrard, S.; Delanaye, P.; Kamel, S.; Cristol, J.P.; Cavalier, E.; On behalf of the SFBC/SN Joined Working Group on Vascular Calcifications. Vascular calcification: From pathophysiology to biomarkers. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 438, 401–414. [Google Scholar] [CrossRef]
- Braun, J.; Oldendorf, M.; Moshage, W.; Heidler, R.; Zeitler, E.; Luft, F.C. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am. J. Kidney Dis. 1996, 27, 394–401. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B.; Marchais, S.J.; Guerin, A.P. Calcification of the aortic valve in the dialyzed patient. J. Am. Soc. Nephrol. 2000, 11, 778–783. [Google Scholar] [CrossRef]
- Lomashvili, K.A.; Khawandi, W.; O’Neill, W.C. Reduced plasma pyrophosphate levels in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 2495–2500. [Google Scholar] [CrossRef]
- O’Neill, W.C.; Sigrist, M.K.; McIntyre, C.W. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 187–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanova, M.; Younossi, Z.M. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin. Gastroenterol. Hepatol. 2012, 10, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Hernaez, R.; Bonekamp, S.; Kamel, I.R.; Brancati, F.L.; Guallar, E.; Clark, J.M. Non-alcoholic fatty liver disease and mortality among US adults: Prospective cohort study. BMJ 2011, 343, d6891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Ryu, S.; Sung, K.C.; Cho, Y.K.; Sung, E.; Kim, H.N.; Jung, H.S.; Yun, K.E.; Ahn, J.; Shin, H.; et al. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: Evidence from the Kangbuk Samsung Health Study. Gut 2019, 68, 1667–1675. [Google Scholar] [CrossRef]
- Chen, C.H.; Nien, C.K.; Yang, C.C.; Yeh, Y.H. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig. Dis. Sci. 2010, 55, 1752–1760. [Google Scholar] [CrossRef]
- Li, Q.; Sundberg, J.P.; Levine, M.A.; Terry, S.F.; Uitto, J. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle 2015, 14, 1082–1089. [Google Scholar] [CrossRef] [Green Version]


| Gene | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|
| ABCC6 | AAGGAACCACCATCAGGAGGAG | ACCAGCGACACAGAGAAGAGG |
| ENPP1 | CCGTGGACAGAAATGACAGTTTC | ATGGACAGGACTAAGAGGAATTCTAAA |
| ALPL | TACAAGCACTCCCACTTCATCTG | GCTCGAAGAGACCCAATAGGTAGT |
| NT5E | GGGCGGAAGGTTCCTGTAG | GAGGAGCCATCCAGATAGACA |
| RPLP0 | CAGATCCGCATGTCCCTTCG | AACACAAAGCCCACATTCCC |
| Patients | Follow up of the Patients | Sex | Age (years) | BMI (kg/m²) | Pugh–Child | Fib-4 | Liver Diseases | Hepatocellular Carcinoma |
|---|---|---|---|---|---|---|---|---|
| 1 | LTR dead before M3 | F | 51 | 24 | 5 | 1.10 | relapsing hepatocellular carcinoma | yes |
| 2 | LTR available at M3 | M | 41 | 21 | 7 | 8.36 | cryptogenetic | no |
| 3 | LTR available at M3 | F | 61 | 21 | 5 | 1.70 | papillomatosis | no |
| 4 | LTC at M3 | F | 49 | 21 | 8 | 9.41 | viral hepatitis C | yes |
| 5 | LTC at M3 | M | 53 | 26 | 7 | 4.46 | alcohol | yes |
| 6 | LTC at M3 | M | 66 | 26 | 9 | 1.55 | hemochromatosis | no |
| 7 | LTC at M3 | M | 58 | 27 | 6 | 2.10 | viral hepatitis C and alcohol | yes |
| 8 | LTR available at M3 | F | 56 | 28 | 9 | 4.68 | alcohol | no |
| 9 | LTC at M3 | M | 61 | 26 | 5 | 0.87 | viral hepatitis C | yes |
| 10 | LTR available at M3 | M | 45 | 33 | 8 | ND | alcohol | no |
| 11 | LTR available at M3 | M | 62 | 33 | 8 | 5.93 | NASH | yes |
| 12 | LTR available at M3 | M | 66 | 21 | 5 | 1.95 | viral hepatitis B | yes |
| 13 | LTC at M3 | F | 57 | 21 | 5 | 2.13 | viral hepatitis C | yes |
| 14 | LTR available at M3 | M | 60 | 38 | 5 | 5.74 | viral hepatitis C and alcohol | yes |
| 15 | LTC at M3 | M | 56 | 29 | 5 | 1.91 | viral hepatitis C | yes |
| 16 | LTC at M3 | M | 59 | 30 | 9 | 15.0 | alcohol | no |
| 17 | LTC at M3 | M | 63 | 38 | 5 | 6.02 | alcohol and NASH | yes |
| 18 | LTR available at M3 | M | 63 | 30 | 10 | 10.8 | alcohol and NASH | no |
| 19 | LTR available at M3 | M | 72 | 25 | 6 | 20.9 | cryptogenetic | no |
| 20 | LTC at M3 | F | 57 | 28 | 5 | 12.1 | viral hepatitis C and alcohol | yes |
| 21 | LTR available at M3 | M | 61 | 24 | 9 | alcohol | yes | |
| 22 | LTR available at M3 | M | 53 | 28 | 5 | 3.82 | viral hepatitis C | yes |
| 23 | LTR dead before M3 | M | 51 | 27 | 5 | 1.41 | viral hepatitis C and B | yes |
| 24 | LTC at M3 | M | 64 | 27 | 5 | 4.80 | viral hepatitis C | yes |
| 25 | LTC at M3 | F | 57 | 31 | 10 | 10.5 | NASH | no |
| 26 | LTC at M3 | M | 58 | 21 | 5 | 1.79 | viral hepatitis C and alcohol | yes |
| 27 | Liver and kidney transplant recipient | M | 58 | 21 | 5 | 11.5 | viral hepatitis B, delta and alcohol | yes |
| 28 | LTR not available at M3 | M | 67 | 32 | 8 | 5.82 | alcohol | yes |
| 29 | LTR available at M3 | M | 63 | 24 | 1 | 7.24 | alcohol | no |
| Variables | LT Candidates (n = 29) | LT Recipients before LT (n = 12) | LT Recipients after LT (n = 12) | p |
|---|---|---|---|---|
| Interstitial fibrosis score (n = 16) | 12.2 [7.2–17.9] | 10.3 [5.2–16.1] | - | |
| FIB-4 | 4.7 [1.9–9.3] | 5.8 [3.3–8.8] | - | |
| APRI | 1.3 [0.5–2.7] | 1.6 [1.0–3.2] | - | |
| MELD score | 7 [3–12] | 8 [5–13] | - | |
| ALBI score | −2.3 [−3.1–1.8] | −2.1 [−2.8–1.7] | - | |
| Pugh–Child score | 6 [5–9] | 8 [5–9] | - | |
| [PPi]pl (µmol/L) | 0.86 [0.64–1.27] | 0.68 [0.52–0.80] | 1.40 [1.07–1.86] | 0.0005 |
| Fe PPi (%) | 7.6 [4.0–15.0] | 9.2 [5.7–18.8] | 9.3 [5.7–17.2] | 0.31 |
| eGFR (mL/mn/1.73 m2) | 94 [82–101] | 92 [81–98] | 73 [62–92] | 0.002 |
| Serum albumin (g/L) | 37 [33–44] | 35 [30–40] | 40 [37–44] | 0.009 |
| C-reactiv protein (mg/L) | 2 [1–8] | 7 [2–12] | 3 [1–8] | 0.20 |
| Plasma ALP activity (UI/L) | 138 [76–245] | 191 [87–322] | 81 [65–156] | 0.05 |
| Prothrombin level (%) | 76 [62–94] | 75 [60–89] | 100 [87–100] | 0.01 |
| Total bilirubin (µmol/L) | 14 [10–30] | 20 [10–44] | 9 [8–13] | 0.059 |
| AST (UI/L) | 46 [32–72] | 54 [37–91] | 23 [18–27] | 0.008 |
| ALT (UI/L) | 34 [28–39] | 37 [29–54] | 26 [20–28] | 0.04 |
| GGT (UI/L) | 55 [37–133] | 98 [30–162] | 24 [13–67] | 0.11 |
| Platelet count (×109/L) | 105 [71–216] | 85 [72–142] | 151 [124–233] | 0.008 |
| Variables | LT Recipients (n = 10) | Patients without Fibrosis (n = 6) | p |
|---|---|---|---|
| Pugh–Child score | 8 [5–9] | - | |
| APRI | 2.45 [0.83–3.63] | 0.23 [0.20–0.30] | 0.0004 |
| FIB-4 | 5.78 [3.24–13.24] | 0.43 [0.37–0.52] | 0.0004 |
| Plasma ALP activity (UI/L) | 244 [110–364] | 65 [58–83] | 0.001 |
| Total bilirubin (µmol/L) | 35 [15–145] | 6 [4–6] | 0.003 |
| Serum albumin (g/L) | 29 [26–33] | 46 [39–48] | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurain, A.; Rubera, I.; Razzouk-Cadet, M.; Bonnafous, S.; Albuquerque, M.; Paradis, V.; Patouraux, S.; Duranton, C.; Lesaux, O.; Lefthériotis, G.; et al. Arterial Calcifications in Patients with Liver Cirrhosis Are Linked to Hepatic Deficiency of Pyrophosphate Production Restored by Liver Transplantation. Biomedicines 2022, 10, 1496. https://doi.org/10.3390/biomedicines10071496
Laurain A, Rubera I, Razzouk-Cadet M, Bonnafous S, Albuquerque M, Paradis V, Patouraux S, Duranton C, Lesaux O, Lefthériotis G, et al. Arterial Calcifications in Patients with Liver Cirrhosis Are Linked to Hepatic Deficiency of Pyrophosphate Production Restored by Liver Transplantation. Biomedicines. 2022; 10(7):1496. https://doi.org/10.3390/biomedicines10071496
Chicago/Turabian StyleLaurain, Audrey, Isabelle Rubera, Micheline Razzouk-Cadet, Stéphanie Bonnafous, Miguel Albuquerque, Valérie Paradis, Stéphanie Patouraux, Christophe Duranton, Olivier Lesaux, Georges Lefthériotis, and et al. 2022. "Arterial Calcifications in Patients with Liver Cirrhosis Are Linked to Hepatic Deficiency of Pyrophosphate Production Restored by Liver Transplantation" Biomedicines 10, no. 7: 1496. https://doi.org/10.3390/biomedicines10071496
APA StyleLaurain, A., Rubera, I., Razzouk-Cadet, M., Bonnafous, S., Albuquerque, M., Paradis, V., Patouraux, S., Duranton, C., Lesaux, O., Lefthériotis, G., Tran, A., Anty, R., Gual, P., Iannelli, A., & Favre, G. (2022). Arterial Calcifications in Patients with Liver Cirrhosis Are Linked to Hepatic Deficiency of Pyrophosphate Production Restored by Liver Transplantation. Biomedicines, 10(7), 1496. https://doi.org/10.3390/biomedicines10071496







