Association between Advanced Glycation End-Products and Sarcopenia in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. sRAGE, esRAGE, and cRAGE Quantification
2.3. AGE Quantification
2.4. Assessment of Sarcopenia
2.5. Anthropometric Measurements
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Advanced Glycation End-Products
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Romanelli, M.C. Sarcopenia in Chronic Kidney Disease: Focus on Advanced Glycation End Products as Mediators and Markers of Oxidative Stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Larsson, T.E. Chronic Kidney Disease: A Clinical Model of Premature Aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, K.; Chiodini, P.; Ceriello, A.; Giugliano, D. A nomogram to estimate the proportion of patients at hemoglobin A1c target. Acta Diabetol. 2014, 51, 305–311. [Google Scholar] [CrossRef]
- De Souza, V.A.; De Oliveira, D.; Mansur, H.N.; Fernandes, N.M.D.S.; Bastos, M.G. Sarcopenia in Chronic Kidney Disease. J. Bras. Nefrol. 2015, 37, 95–105. [Google Scholar] [CrossRef]
- Yabuuchi, J.; Ueda, S.; Yamagishi, S.-I.; Nohara, N.; Nagasawa, H.; Wakabayashi, K.; Matsui, T.; Yuichiro, H.; Kadoguchi, T.; Otsuka, T.; et al. Association of advanced glycation end products with sarcopenia and frailty in chronic kidney disease. Sci. Rep. 2020, 10, 17647. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary Advanced Glycation End Products and Aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Inoue, H.; Takeuchi, M. Oral administration of AST-120 (Kremezin) is a promising therapeutic strategy for advanced glycation end product (AGE)-related disorders. Med. Hypotheses 2007, 69, 666–668. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Linden, E.; Cai, W.; He, J.C.; Xue, C.; Li, Z.; Winston, J.; Vlassara, H.; Uribarri, J. Endothelial Dysfunction in Patients with Chronic Kidney Disease Results from Advanced Glycation End Products (AGE)-Mediated Inhibition of Endothelial Nitric Oxide Synthase through RAGE Activation. Clin. J. Am. Soc. Nephrol. 2008, 3, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Kellow, N.J.; Savige, G.S. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: A systematic review. Eur. J. Clin. Nutr. 2013, 67, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Sorci, G.; Riuzzi, F.; Giambanco, I.; Donato, R. RAGE in tissue homeostasis, repair and regeneration. Biochim. Biophys. Acta 2013, 1833, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Daffu, G.; Del Pozo, C.H.; O’Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical Roles for RAGE in the Pathogenesis of Oxidative Stress in Cardiovascular Diseases and Beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef] [Green Version]
- Leonardis, D.; Basta, G.; Mallamaci, F.; Cutrupi, S.; Pizzini, P.; Tripepi, R.; De Caterina, R.; Zoccali, C. Circulating soluble receptor for advanced glycation end product (sRAGE) and left ventricular hypertrophy in patients with chronic kidney disease (CKD). Nutr. Metab. Cardiovasc. Dis. 2012, 22, 748–755. [Google Scholar] [CrossRef]
- Kratochvilová, M.; Zakiyanov, O.; Kalousová, M.; Kříha, V.; Zima, T.; Tesař, V. Associations of Serum Levels of Advanced Glycation end Products with Nutrition Markers and Anemia in Patients with Chronic Kidney Disease. Ren. Fail. 2011, 33, 131–137. [Google Scholar] [CrossRef]
- Koyama, H.; Shoji, T.; Yokoyama, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Emoto, M.; Shoji, T.; Tamei, H.; Matsuki, H.; et al. Plasma Level of Endogenous Secretory RAGE Is Associated With Components of the Metabolic Syndrome and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2005, 25, 2587–2593. [Google Scholar] [CrossRef]
- Assar, S.H.; Moloney, C.; Lima, M.; Magee, R.; Ames, J.M. Determination of N ɛ-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids 2009, 36, 317–326. [Google Scholar] [CrossRef]
- Dalal, M.; Ferrucci, L.; Sun, K.; Beck, J.; Fried, L.P.; Semba, R.D. Elevated Serum Advanced Glycation End Products and Poor Grip Strength in Older Community-Dwelling Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 132–137. [Google Scholar] [CrossRef]
- Semba, R.D.; Bandinelli, S.; Sun, K.; Guralnik, J.M.; Ferrucci, L. Relationship of an advanced glycation end product, plasma carboxymethyl-lysine, with slow walking speed in older adults: The InCHIANTI study. Eur. J. Appl. Physiol. 2009, 108, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-Y.; Yang, R.-S.; Sheu, M.-L.; Chan, D.-C.; Yang, T.-H.; Tsai, K.-S.; Chiang, C.-K.; Liu, S.-H. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J. Pathol. 2016, 238, 470–482. [Google Scholar] [CrossRef]
- Dozio, E.; Vettoretti, S.; Caldiroli, L.; Nerini-Molteni, S.; Tacchini, L.; Ambrogi, F.; Messa, P.; Romanelli, M.M.C. Advanced Glycation End Products (AGE) and Soluble Forms of AGE Receptor: Emerging Role as Mortality Risk Factors in CKD. Biomedicines 2020, 8, 638. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Makita, Z.; Shiroshita, K.; Ueda, T.; Fusegawa, T.; Kuwajima, S.; Takeuchi, M.; Koike, T. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism 1998, 47, 1348–1353. [Google Scholar] [CrossRef]
- Guerin-Dubourg, A.; Cournot, M.; Planesse, C.; Debussche, X.; Meilhac, O.; Rondeau, P.; Bourdon, E. Association between Fluorescent Advanced Glycation End-Products and Vascular Complications in Type 2 Diabetic Patients. BioMed Res. Int. 2017, 2017, 7989180. [Google Scholar] [CrossRef]
- Vettoretti, S.; Caldiroli, L.; Armelloni, S.; Ferrari, C.; Cesari, M.; Messa, P. Sarcopenia is Associated with Malnutrition but Not with Systemic Inflammation in Older Persons with Advanced CKD. Nutrients 2019, 11, 1378. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [Green Version]
- Molinari, P.; Caldiroli, L.; Dozio, E.; Rigolini, R.; Giubbilini, P.; Romanelli, M.M.C.; Messa, P.; Vettoretti, S. AGEs and sRAGE Variations at Different Timepoints in Patients with Chronic Kidney Disease. Antioxidants 2021, 10, 1994. [Google Scholar] [CrossRef]
- Jandeleit-Dahm, K.A.; Lassila, M.; Allen, T.J. Advanced Glycation End Products in Diabetes-Associated Atherosclerosis and Renal Disease: Interventional Studies. Ann. N. Y. Acad. Sci. USA 2005, 1043, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Jaber, B.L.; Balakrishnan, V.S. Chronic kidney disease and acquired mitochondrial myopathy. Curr. Opin. Nephrol. Hypertens. 2018, 27, 113–120. [Google Scholar] [CrossRef]
- Yazdi, P.G.; Moradi, H.; Yang, J.-Y.; Wang, P.H.; Vaziri, N.D. Skeletal muscle mitochondrial depletion and dysfunction in chronic kidney disease. Int. J. Clin. Exp. Med. 2013, 6, 532–539. [Google Scholar]
- Su, Z.; Klein, J.D.; Du, J.; Franch, H.A.; Zhang, L.; Hassounah, F.; Hudson, M.B.; Wang, X.H. Chronic kidney disease induces autophagy leading to dysfunction of mitochondria in skeletal muscle. Am. J. Physiol. Physiol. 2017, 312, F1128–F1140. [Google Scholar] [CrossRef]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef]
- Payne, G.W. Effect of Inflammation on the Aging Microcirculation: Impact on Skeletal Muscle Blood Flow Control. Microcirculation 2006, 13, 343–352. [Google Scholar] [CrossRef]
- Mills, K.T.; Zhang, W.; Bundy, J.D.; Chen, C.-S.; Kelly, T.N. A systematic analysis of world-wide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2016, 88, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Waqas, K.; Chen, J.; Trajanoska, K.; Ikram, M.A.; Uitterlinden, A.G.; Rivadeneira, F.; Zillikens, M.C. Skin Autofluorescence, a Noninvasive Biomarker for Advanced Glycation End-products, Is Associated with Sarcopenia. J. Clin. Endocrinol. Metab. 2022, 107, e793–e803. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Lindholm, B.; Kaysen, G.A.; Bergström, J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol. Dial. Transplant. 2000, 15, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.; Lee, S.-R.; Park, B.-S.; Kim, J.-S. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin. Exp. Res. 2012, 24, 412–422. [Google Scholar] [CrossRef]
- Budui, S.L.; Rossi, A.P.; Zamboni, M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015, 12, 22–26. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor- With Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [Green Version]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory Markers and Loss of Muscle Mass (Sarcopenia) and Strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26, 389–399. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Choi, H.; Baik, S.H.; et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean Sarcopenic Obesity Study. Clin. Endocrinol. 2013, 78, 525–532. [Google Scholar] [CrossRef]
- Fukasawa, H.; Ishigaki, S.; Kinoshita-Katahashi, N.; Yasuda, H.; Kumagai, H.; Furuya, R. Plasma levels of the pro-inflammatory protein S100A12 (EN-RAGE) are associated with muscle and fat mass in hemodialysis patients: A cross-sectional study. Nutr. J. 2014, 13, 48. [Google Scholar] [CrossRef] [Green Version]
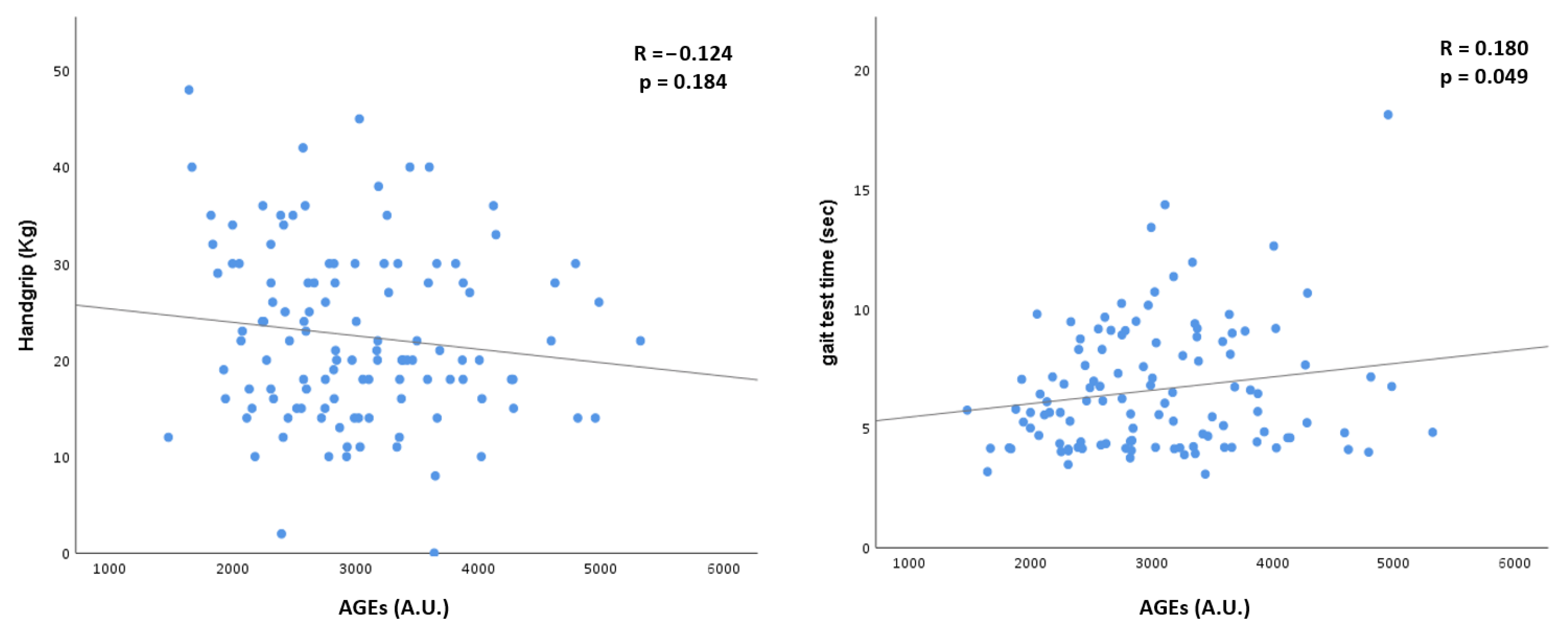
| Variables | Overall Cohort (n = 117) | N-Src (n = 91) | Src (n = 26) | p |
|---|---|---|---|---|
| General characteristics | ||||
| Age, (years) | 80 ± 11 | 76 ± 12 | 83 ± 6 | 0.001 |
| Males, n (%) | 82 (70) | 62 (68) | 20 (77) | 0.28 |
| Diabetes, n (%) | 65 (56) | 52 (65) | 13 (50) | 0.65 |
| Hypertension, n (%) | 104 (89) | 81 (89) | 23 (88.5) | 0.91 |
| BMI, (kg/m2) | 28 ± 5 | 28.6 ± 4.8 | 24.8 ± 3.9 | <0.0001 |
| eGFR, (mL/min/1.73 m2) | 25 ± 11 | 26 ± 11 | 22 ± 8 | 0.08 |
| Creatinine clearance, (mL/min/1.73 m2) | 28 ± 16 | 30 ± 17 | 23 ± 13 | 0.039 |
| Metabolic characteristics | ||||
| Uric Acid, (mg/dL) | 6 ± 1.5 | 6.1 ± 1.4 | 6 ± 1.8 | 0.42 |
| HbA1c, (mmol/dL) | 47 ± 11 | 47 ± 10 | 48 ± 15 | 0.83 |
| Total Cholesterol, (mg/dL) | 168 ± 37 | 167 ± 36 | 173 ± 39 | 0.84 |
| Albumin, (g/dL) | 4 ± 0.4 | 4.0 ± 0.3 | 4.1 ± 0.5 | 0.43 |
| Prealbumin, (mg/dL) | 28 ± 5 | 29 ± 5 | 27 ± 6 | 0.12 |
| CRP, (mg/dL) | 0.4 ± 0.7 | 0.3 ± 0.4 | 0.8 ± 1.3 | 0.003 |
| Variables | N-Src (n = 91) | Src (n = 26) | p | p (eGFR Weighted) |
|---|---|---|---|---|
| AGEs (arbitrary unit) | 2912 ± 722 | 3405 ± 951 | 0.005 | 0.02 |
| sRAGE (pg/mL) | 2338 ± 1280 | 2411 ± 1268 | 0.86 | 0.63 |
| esRAGE (pg/mL) | 656 ± 503 | 713 ± 448 | 0.60 | 0.96 |
| cRAGE (pg/mL) | 1693 ± 937 | 1698 ± 902 | 0.93 | 0.51 |
| AGEs/sRAGE (arbitrary unit) | 1.59 ± 0.89 | 1.8 ± 1.2 | 0.23 | 0.19 |
| Variables | Yes | Not | p | p |
|---|---|---|---|---|
| (eGFR Weighted) | ||||
| Reduced MAMC, n (%) | 37 (31) | 80 (69) | <0.0001 | <0.0001 |
| AGEs (arbitrary unit) | 3322 ± 919 | 2883 ± 700 | 0.005 | 0.049 |
| sRAGE (pg/mL) | 2426 ± 1292 | 2350 ± 1287 | 0.77 | 0.58 |
| esRAGE (pg/mL) | 536 (398–707) | 552 (368–787) | 0.66 | 0.75 |
| cRAGE (pg/mL) | 1727 ± 921 | 1707 ± 959 | 0.91 | 0.53 |
| AGEs/sRAGE (arbitrary unit) | 1.8 ± 1.1 | 1.6 ± 0.9 | 0.25 | 0.19 |
| Reduced Gait Speed Test, n (%) | 76 (64) | 41 (36) | <0.0001 | 0.001 |
| AGEs (arbitrary unit) | 2977 ± 750 | 3101 ± 882 | 0.42 | 0.26 |
| sRAGE (pg/mL) | 2373 ± 1310 | 2376 ± 1249 | 0.99 | 0.90 |
| esRAGE (pg/mL) | 630 (368–776) | 526 (390–720) | 0.75 | 0.66 |
| cRAGE (pg/mL) | 1728 ± 972 | 1687 ± 902 | 0.82 | 0.89 |
| AGEs/sRAGE (arbitrary unit) | 1.6 ± 0.9 | 1.6 ± 1 | 0.94 | 0.93 |
| Reduced handgrip strength, n (%) | 68 (58) | 49 (42) | <0.0001 | <0.0001 |
| AGEs (arbitrary unit) | 3054 ± 809 | 2976 ± 787 | 0.60 | 0.78 |
| sRAGE (pg/mL) | 2343 ± 1330 | 2416 ± 1230 | 0.76 | 0.39 |
| esRAGE (pg/mL) | 543 (367–718) | 515 (384–787) | 0.50 | 0.84 |
| cRAGE (pg/mL) | 1662 ± 937 | 1782 ± 958 | 0.50 | 0.23 |
| AGEs/sRAGE (arbitrary unit) | 1.7 ± 1 | 1.5 ± 0.9 | 0.34 | 0.30 |
| Variables | Odds Ratio | Odds Ratio (CI) | p |
|---|---|---|---|
| AGEs (arbitrary unit) | 1.5 | (0.91–2.19) | 0.19 |
| eGFR, (mL/min/1.73 m2) | 0.99 | (0.93–1.06) | 0.84 |
| Age, (years) | 1.11 | (1.03–1.2) | 0.008 |
| CRP (mg/dL) | 2.2 | (1.08–4.5) | 0.029 |
| BMI (kg/m2) | 0.77 | (0.66–0.9) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, P.; Caldiroli, L.; Dozio, E.; Rigolini, R.; Giubbilini, P.; Corsi Romanelli, M.M.; Castellano, G.; Vettoretti, S. Association between Advanced Glycation End-Products and Sarcopenia in Patients with Chronic Kidney Disease. Biomedicines 2022, 10, 1489. https://doi.org/10.3390/biomedicines10071489
Molinari P, Caldiroli L, Dozio E, Rigolini R, Giubbilini P, Corsi Romanelli MM, Castellano G, Vettoretti S. Association between Advanced Glycation End-Products and Sarcopenia in Patients with Chronic Kidney Disease. Biomedicines. 2022; 10(7):1489. https://doi.org/10.3390/biomedicines10071489
Chicago/Turabian StyleMolinari, Paolo, Lara Caldiroli, Elena Dozio, Roberta Rigolini, Paola Giubbilini, Massimiliano M. Corsi Romanelli, Giuseppe Castellano, and Simone Vettoretti. 2022. "Association between Advanced Glycation End-Products and Sarcopenia in Patients with Chronic Kidney Disease" Biomedicines 10, no. 7: 1489. https://doi.org/10.3390/biomedicines10071489
APA StyleMolinari, P., Caldiroli, L., Dozio, E., Rigolini, R., Giubbilini, P., Corsi Romanelli, M. M., Castellano, G., & Vettoretti, S. (2022). Association between Advanced Glycation End-Products and Sarcopenia in Patients with Chronic Kidney Disease. Biomedicines, 10(7), 1489. https://doi.org/10.3390/biomedicines10071489







