Abstract
Limb girdle muscular dystrophies (LGMD), caused by mutations in 29 different genes, are the fourth most prevalent group of genetic muscle diseases. Although the link between LGMD and its genetic origins has been determined, LGMD still represent an unmet medical need. Here, we describe a platform for modeling LGMD based on the use of human induced pluripotent stem cells (hiPSC). Thanks to the self-renewing and pluripotency properties of hiPSC, this platform provides a renewable and an alternative source of skeletal muscle cells (skMC) to primary, immortalized, or overexpressing cells. We report that skMC derived from hiPSC express the majority of the genes and proteins that cause LGMD. As a proof of concept, we demonstrate the importance of this cellular model for studying LGMDR9 by evaluating disease-specific phenotypes in skMC derived from hiPSC obtained from four patients.
1. Introduction
Limb girdle muscular dystrophies (LGMD) refer to a heterogeneous group of rare genetic neuromuscular diseases which lead to progressive weakness and wasting of proximal pelvic and shoulder girdle muscles [1,2,3]. With an estimated prevalence of 1.63 per 100,000 people LGMD represent the fourth most common group of muscular dystrophies [4]. From a genetic point of view, LGMD subtypes are caused by genetic alterations of various genes playing a critical role in muscle function, maintenance, and repair [5,6]. Since the first LGMD gene identification reported in 1995 [7], the number of LGMD subtypes has grown to a total of 29, according to the last revised nomenclature [8]. Classified according to their inheritance pattern, LGMD subtypes are autosomal dominant (LGMD D) or autosomal recessive (LGMD R) types affecting 27 different proteins of the nucleus, sarcoplasm, sarcomere, sarcolemma, and extracellular matrix (Table S1). While several gene replacement therapies [9,10,11,12,13,14] have been proposed over the years, no curative treatment is currently available for any types of LGMD.
Over the past decade, considerable efforts have been reported in deciphering the molecular mechanisms associated with LGMD. Understanding the multiple molecular pathways associated with LGMD requires the development of appropriate cellular and animal models that at least partially recapitulate the characteristics of these diseases. Animal models are currently preferred to in vitro models for studies on muscular dystrophies [15,16] and the assessment of therapeutic strategies [17]. However, although most of these animal models recapitulate the pathological phenotypes observed in humans, some of these models appear relatively normal or with a mild dystrophic phenotype [18] or even present impairments that are not reported in patients [19]. Alternatively, over the past decades, several in vitro human models have been developed to study phenotypes associated with LGMD. Among these, primary skeletal muscle cells (skMC) from muscle biopsies represent the most commonly reported biological resource, despite the difficulty of their generation and their limited proliferative capacity [20]. To overcome these issues, several in vitro models were developed through transdifferentiation of non-muscle cells into skeletal cells by forced expression of myogenic transcription factors [21]. More recently, immortalization of primary cells with hTERT and CDK4 was reported as successful for modeling LGMD [22,23], including LGMDD4/R1 [24], LGMDR2 [25,26,27], and LGMDR12 [28]. While these different models present several advantages in terms of proliferation and maturity, the development of transgene-free cellular models from noninvasive procedures was proposed through the use of pluripotent stem cells.
Derived from embryos [29,30,31,32] or generated through reprogramming [33,34], pluripotent stem cells have the unique capacity to unlimitedly self-renew and differentiate into any cell type in the organism [35]. Over the years, thanks to the development of efficient protocols of differentiation for a growing number of cell types, embryonic stem cells (ES) and human induced pluripotent stem cells (hiPSC) have been reported as efficient alternative models to primary or genetically modified cells. Since their discovery in humans, respectively in 1998 and 2007, the use of these cells has been explored for a broad range of applications, leading to the identification of new molecular mechanisms [36,37], new pathological phenotypes [38,39,40,41], and the development of new therapies [42,43,44,45] (reviewed in Karagiannis et al., 2019) [46]. Although successfully applied to a number of genetic diseases, pathological modeling using pluripotent stem cells remained challenging for application to muscular disorders over almost a decade because of the lack of protocols for generating homogeneous populations of myotubes. Since 2012, several studies have reported methods to overcome this limitation by differentiating hiPSC into myogenic cells using transgene-based methods [47,48,49] or small molecules recapitulating developmental myogenesis [50,51,52,53,54,55,56,57]. Among these protocols, Caron et al. described a three-step process that efficiently differentiated hiPSC into mature skMC in less than 26 days using combinations of small molecules and growth factors identified by high-throughput screening [51]. Recently, several studies have highlighted the use of human pluripotent stem cells—and, more particularly, hiPSC—for studying neuromuscular disorders [58,59,60,61,62], including LGMD [49,63,64,65,66]. In the present study, we report that hiPSC-derived skMC provide a reliable and robust in vitro tool for investigating LGMD subtypes. To do so, we used a robust protocol of differentiation to generate pure populations of skeletal myoblasts (skMb) and terminally differentiated myotubes (skMt) from hiPSC [51]. Taking advantage of this model, we monitored and characterized the expression and localization of all of the genes and proteins that cause LGMD subtypes. As a proof of concept, such a cellular model was used to model LGMDR9 by analyzing alpha-dystroglycan (α-DG) glycosylation in fukutin-related protein (FKRP)-deficient skMt derived from the hiPSC of four patients.
2. Materials and Methods
2.1. Cell Lines
Experiments were performed with human immortalized myoblasts from unaffected individuals or with hiPSC from control and LGMDR9-affected patients. The myoblast cell lines were established from human muscular biopsies and obtained from the MyoLine immortalization platform of the Institut de Myologie (Paris, France), with the agreement of the subjects through their signature on an informed consent form and anonymization before immortalization, in line with the EU GDPR regulation. The four cell lines used are the following: C25, AB678, AB1079, AB1167. The WT1 and WT2 hiPSC lines were reprogrammed from healthy IMR-90 lung fibroblasts obtained from the ATCC Cell Lines Biology Collection (Washington, DC, USA) and from GM1869 provided by the Coriell Cell Repository (Camden, NJ, USA) [67,68]. The WT3 hiPSC line is a commercial cell line provided by Phenocell (Grasse, France). Wild type eGFP-tagged hiPSC lines, AICS-37 and AICS-48, were provided by the Allen Institute and distributed by the Coriell Cell Repository (Camden, NJ, USA): troponin I (TNN) and titin (TTN) were endogenously tagged with mEGFP at the C-terminus site using CRISPR-Cas9 technology. The FKRP1 hiPSC line was reprogrammed by Phenocell (Grasse, France) from LGMDR9 fibroblasts (heterozygous FKRP c.899T > C c.962C > A) provided by Genethon’s Cell Bank (Evry, France). FKRP2 and FKRP3 hiPSC lines were also reprogrammed by Phenocell (Grasse, France) from LGMDR9 fibroblasts (heterozygous c.826C > A c.534G > T and c.826C > A c.872delA, respectively) provided by the Coriell Institute (Camden, NJ, USA). The FKRP4 hiPSC line (heterozygous c.826C > A c.135C > T) was provided by the LGMD2I Research Fund.
2.2. Cell Culture and Differentiation
Immortalized myoblasts were cultivated in a growth medium: 1 volume of 199 medium (Invitrogen, Waltham, MA, USA) for 4 volumes of DMEM (Invitrogen, Waltham, MA, USA), supplemented with 20% FCS (Sigma, Saint Louis, MO, USA), 25 µg/mL fetuin (Life Technologies, Carlsbad, CA, USA), 5 ng/mL hEGF (Life Technologies, Carlsbad, CA, USA), 0.5 ng/mL bFGF (Life Technologies, Carlsbad, CA, USA), 0.2 µg/mL dexamethasone (Sigma, Saint Louis, MO, USA), and 5 µg/mL insulin (Sigma, Saint Louis, MO, USA). Cells were seeded on 0.1% gelatin and maintained in a humidified atmosphere of 5% CO2 at 37 °C. Differentiation was induced at confluence by replacing the growth medium with DMEM supplemented with 10 μg/mL insulin (Sigma, Saint Louis, MO, USA) for 7 days. Control and LGMDR9 hiPSC lines were maintained and expanded using a single-cell method on feeder-free coated dishes (Matrigel, Corning, NY, USA) with iPS-Brew XF medium (Macs, Miltenyi Biotech, Bergisch Gladbach, Germany). hiPSC were differentiated into skMC following a protocol developed by Geneabiocell ® (Caron et al., 2016). Briefly, hiPSC were seeded in collagen I-coated plates (Biocoat, DB Biosciences, Franklin Lakes, NJ, USA) and maintained for 10 days in skeletal muscle induction medium (SKM01, AMSBIO, Abingdon, United Kingdom) with a passage at day 7. Cells were then dissociated with 0.05% trypsin and seeded once again onto collagen I-coated plates for 7 days in skeletal myoblast medium (SKM02, AMSBIO, Abingdon, United Kingdom) until freezing. skMb were thawed on collagen I-coated glass slides in skeletal myoblast medium and incubated at confluence with skeletal muscle differentiation medium (SKM03, AMSBIO, Abingdon, United Kingdom) for 5 to 7 supplementary days. hiPSC, skMb, and skMt were analyzed at days 0, 17, and 24 of differentiation, respectively.
2.3. Flow Cytometry
A single-cell suspension of hiPSC was collected after chemical dissociation with accutase (Invitrogen, Waltham, MA, USA), centrifuged at 900 rpm for 5 min, and resuspended in 2% FBS in cold PBS. Cells were stained with fluorescent dye-conjugated antibodies for 30 min on ice and protected from light. Antibodies: APC-conjugated TRA1-80 (Millipore, Burlington, MA, USA) and PE-conjugated SSEA4 (Millipore, Burlington, MA, USA). Cells were washed in cold PBS before being sorted by a MACSquant analyzer (MiltenyiBiotec, Bergisch Gladbach, Germany). Data were analyzed with FlowJo Software (BD Biosciences, Franklin Lakes, NJ, USA ).
2.4. Multiplex Fluorescence In Situ Hybridization (m-FISH) Karyotype Analysis
Cells were blocked in metaphase with colchicine (Eurobio, Paris, France) for 90 min, warmed with a hypotonic solution, and fixed with a Carnoy fixative. A M-FISH 24Xcite probe (MetaSystems, Heidelberg, Germany) and ProLong Gold Antifade Mountant with DAPI (ThermoFisher Scientific, Waltham, MA, USA) were used for m-FISH staining. Seventy metaphases were acquired with Metafer MetaSystems software coupled to an AxioImager Z2 (Zeiss, Oberkochen, Germany) microscope equipped with a camera cool cube and 10X and 63X objectives. Images were analyzed with Isis software (MetaSystems).
2.5. Immunofluorescence Analysis
Cells at days 0, 17, or 24 of skMC differentiation were fixed with 4% paraformaldehyde for 10 min at room temperature. For two antibodies (FKTN and TCAP), cells were pre-fixed and then fixed in cold methanol for 5 min at room temperature. After 3 washes in phosphate-buffered saline (PBS), cells were permeabilized, or not, with 0.5% Triton X-100 for 5 min and blocked in PBS solution supplemented with 1% bovine serum albumin (BSA, Sigma, Saint Louis, MO, USA) for 1 h at room temperature. Cells were stained for specific markers overnight at 4 °C using the antibodies listed in Table S3. After 3 washes in PBS, staining was revealed by appropriate Alexa Fluor secondary antibodies (ThermoFisher Scientific, Waltham, MA, USA; 1:1000) in the dark for 1 h at room temperature, and nuclei were visualized with Hoechst 33,342 (Invitrogen; 1:2000). Cell imaging was carried out with a Zen Black software-associated LSM-800 confocal microscope (Zeiss, Oberkochen, Germany) with a 20X or 63X objective.
2.6. Time-Lapse Imaging of skMt Differentiation
Time-lapses of skMt were recorded as previously described, from day 0 to day 5 of differentiation. Imaging of endogenous GFP-tagged titin and troponin signaling was carried out with an Incucyte® S3 Live-Cell Analysis system (Sartorius, Gottingen, Germany) or a LSM-800 confocal microscope (Zeiss, Oberkochen, Germany) with a 20X objective, using Zen Black software. Differentiation efficiency was measured as the percentage of GFP-tagged cells at each time point using ImageJ software and normalized to the total number of skMt. An algorithm was used to determine the percentage of cells when at least 50% of the area of an identified nucleus was covered by GFP staining. Differentiation efficiency was calculated from 5 random fields comprising at least 300 cells. The results are expressed as mean values +/− S.E.M. *** p < 0.005 (Student’s t-test).
2.7. Quantitative PCR
Total RNAs were isolated using the RNeasy Mini extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A DNase I digestion was performed to degrade DNA in the sample. RNA levels and quality were checked using the NanoDrop technology. A total of 500 ng of RNA was used for reverse transcription using the SuperScript III reverse transcription kit (Invitrogen, Waltham, MA, USA). Quantitative polymerase chain reaction (qPCR) analysis was performed using a QuantStudio 12 K Flex real-time PCR system (Applied biosystem, Waltham, MA, USA) and Luminaris Probe qPCR Master Mix (Thermo Scientific, Waltham, MA, USA), following the manufacturers’ instructions. Gene expression analysis was performed using the TaqMan gene expression Master Mix (Roche, Basel, Switzerland), following the manufacturer’s protocol. Quantification of gene expression was based on the DeltaCt method and normalized to 18S expression (Assay HS_099999). The primers and probe sequences that were designed are listed in Table S2. The sequences of the remaining genes are commercially available (Applied biosystem, Waltham, MA, USA): DYSF (Hs01002513), FKRP (Hs00748199), TTN (Hs00399225), ANO5 (Hs01381106), and DAG1 (Hs00189308).
2.8. Western Immunoblotting
Whole-cell lysate of control and LGMDR9-skMt were collected after 7 days of differentiation. Proteins were extracted with RIPA lysis buffer (Thermo Scientific, Waltham, MA, USA) supplemented with 1X Protease Inhibitor-Complete ULTRA tablets mini (Roche, Basel, Switzerland) and 1X benzonase nuclease HC (Millipore, Burlington, MA, USA) for 1 h at 4 °C. Protein concentration was measured using the Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA), and the absorbance at 562 nm was evaluated using a CLARIOstar® microplate reader (BMG Labtech, Ortenberg, Germany). For α-DG protein detection, a total of 130 μg of protein was loaded and run on Mini-PROTEAN 4–12% bis-tris protein gels (BioRad, Hercules, CA, USA) and then transferred to nitrocellulose membranes with a Trans-Blot Turbo Transfer system (Biorad, Hercules, CA, USA), following the manufacturer’s instructions. Membranes were then blocked in Odyssey blocking buffer (Li-Cor, Lincoln, NE, USA) for 1 h at room temperature. Incubation with primary antibodies diluted in Odyssey blocking buffer was carried out at 4 °C for between 2 h and overnight for the mouse anti-α-DG-IIH6 1:50 (DSHB, Iowa city, IA, USA), the mouse anti-α-DG-IIH6 1:1000 (Millipore, Burlington, MA, USA), and the mouse anti-β-DG 1:200 (DSHB, Iowa city, IA, USA). Washing was carried out 3 times for 10 min at room temperature with TBS + 0.1% Tween20, and the membranes were incubated with a donkey anti-mouse antibody IRDye-800CW 1:5000 (Li-Cor, Lincoln, NE, USA) in blocking buffer. Washing was carried out, and proteins were detected by fluorescence (Odyssey, Li-Cor, Lincoln, NE, USA), following the manufacturer’s instructions.
2.9. Statistical Analysis
Data are presented as means ± SD unless otherwise specified. Statistical analysis was performed using the Student’s t-test, and p-values ≤ 0.05 (*), 0.01 (**), and 0.005 (***) were considered significant.
3. Results
3.1. Generation and Characterization of Skeletal Muscle Cells from Human Induced Pluripotent Stem Cells
The workflow to generate skMb and multinucleated skMt from hiPSC is represented in Figure 1A. First, the pluripotency capacities of three healthy hiPSC lines (WT1,2,3) were characterized by analyzing the expression of Oct4 and Nanog through immunostaining (Figure 1B) and quantifying SSEA4/TRA1-80 by flow cytometry (Figure S1). Myogenic differentiation was then induced using a three-step protocol of differentiation [51]. Briefly, SKM01 medium was used for 10 days to initiate the differentiation of hiPSC into myogenic precursors before their maturation into myoblasts in SKM02 medium and their terminal differentiation into myotubes in SKM03 medium. To demonstrate the efficacy and robustness of this protocol of differentiation, MyoD and desmin expressions were assessed by immunostaining in skMb, showing homogeneity of labeling (Figure 1C). Terminal differentiation of skMt was then characterized by measuring the expression of myogenin and titin by immunostaining (Figure 1D). To quantify gene expression changes throughout the differentiation protocol, the same markers were monitored by qPCR, revealing, on the one hand, a significant time-dependent decrease in Oct4 and Nanog in skMb and skMt compared to hiPSC (Figure 1E) and, on the other hand, a significant induction of MyoD, desmin, MyoG, and titin in skMb and skMt (Figure 1F,G).
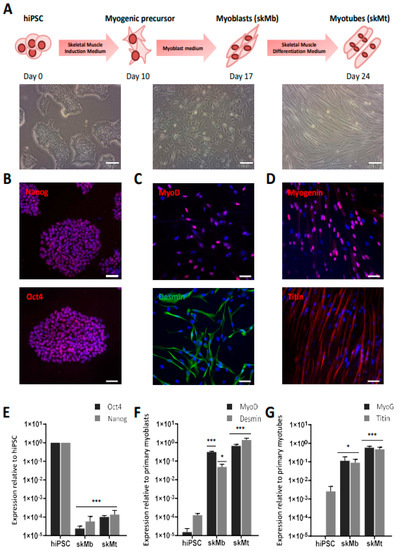
Figure 1.
Differentiation of hiPSC into skMC. (A) Experimental workflow of skMC differentiation from hiPSC using the Genea Biocells protocol. Representative pictures of hiPSC, skeletal myoblasts (skMb), and myotubes (skMt) with bright field acquisition. Scale bar = 50 µm. (B) Characterization of the expression of pluripotency markers Oct4 and Nanog by immunostaining in hiPSC (C) Characterization of the expression of MyoD and desmin by immunostaining in skMb (D) Characterization of the expression of MyoG and titin by immunostaining in skMt. Nuclei are labeled by Hoechst staining (blue). Scale bar = 50 µm. (E) Measurement of Oct4 and Nanog expression by qPCR in hiPSC, skMb, and skMt. (F) Measurement of MyoD and desmin expression by qPCR in hiPSC, skMb, and skMt. (G) Measurement of MyoG and titin expression by qPCR in hiPSC, skMb, and skMt. Gene expression analyses are normalized to hiPSC, primary myoblasts, or primary myotubes. Data are shown as the mean of skMC differentiations of three control cell lines +/− SD. * 0.01 < p ≤ 0.05 or *** p ≤ 0.005 (Student’s t-test). Abbreviations: hiPSC, human induced pluripotent stem cells; skMb, skeletal myoblasts; skMt, skeletal myotubes; MyoG, myogenin.
To further characterize the terminal differentiation of skMt derived from hiPSC and visualize sarcomeric structure formation in real time, we used two gene-edited hiPSC lines expressing titin (TTN) or troponin I (TNN) proteins fused to GFP (AICS-48 and AIC-37). After having validated their pluripotency capacities by measuring the expression of SSEA4/TRA1-80 using flow cytometry (Figure S2A), TTN-GFP and TNN-GFP hiPSC were differentiated using the same protocol. Analysis by qPCR confirmed the efficient myogenic differentiation of these cells by showing a significant decrease in Oct4 and Nanog expression in skMb and skMt compared to undifferentiated hiPSC (Figure S2B) and a significant increase in MyoD, desmin, MyoG, and titin in skMb and skMt (Figure S2B). Terminal differentiation of TTN-GFP and TNN-GFP skMt was then followed in real time using a time-lapse analysis of GFP expression from day 0 to day 7 (Movies S1 and S2). Confocal microscopy analysis of GFP expression and localization in skMt derived from these two hiPSC cell lines revealed the appearance of GFP in both cell lines from day 3 onwards (Figure 2A). Quantification of GFP-positive cells showed that half of the cells expressed GFP from day 3 of maturation and up to 80% at day 5 (Figure 2B,C). Organization of the sarcomeric structures in skMt was further analyzed by characterizing the Z-disk, I-band, and M-line using specific antibodies, as shown in Figure 2D. Confocal analysis of immunostaining and the GFP signal was performed in TTN-GFP skMt (Figure 2E) and TNN-GFP skMt (Figure S2C), revealing a regular, transverse, and repetitive organization of sarcomeres. Finally, quantification of TTN-GFP skMt presenting a striated pattern revealed a time-dependent maturation from 40% at day 2 to 80% at day 5 (Figure 2F).
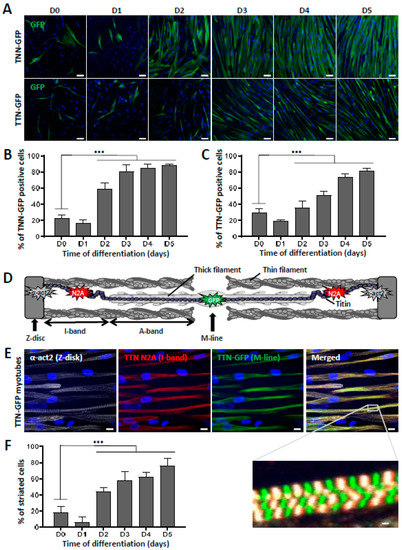
Figure 2.
Striation pattern appears during terminal myogenic differentiation. (A–C): Troponin I(TNN)- and titin(TTN)-eGFP dynamics during myotube maturation. (A) Confocal images of TNN- and TTN-eGFP-tagged cell lines were obtained from day 0 to day 5 after induction of myotube maturation. Nuclei were labeled by Hoechst staining. Scale bar = 50 µm. Quantification of the percentage of cells terminally differentiated, presented as the percentage of (B) TNN- or (C) TTN-positive cells out of the total number of myotubes. Each chart represents the mean +/− SD (n = 5) for a representative experiment; *** p ≤ 0.005 (Student’s t-test). (D–F): Analysis of sarcomeric organization during myotube maturation. (D) Schematic representation of sarcomeric architecture in skeletal muscle: Z-disk composed of α-actinin, I-band made up of actin thin filaments, A-band comprised of myosin thick filaments, and the M-line where filaments are anchored. Titin spans the entire half-sarcomere: from the Z-disk with the N-terminal extremity to the M-line, by way of the I-band, specifically recognized by the titin N2A antibody. (E) Immunofluorescent staining and confocal acquisition of sarcomere structures with TTN-eGFP-tagged cells at day 5 of myotube maturation. Nuclei are labeled by Hoechst staining. Scale bar = 10 µm. White box denotes an enlargement of co-staining to visualize the cross-striation pattern of myotubes. Scale bar = 1 µm. (F) Quantification of the number of striated cells relative to the total number of skMt during time course differentiation of the TTN-eGFP-tagged cell line. Each chart represents the mean +/− SD (n = 10) for a representative experiment; *** p ≤ 0.005 (Student’s t-test). Abbreviations: TNN, troponin I; TTN, titin; α-act2, alpha-actinin-2.
3.2. Characterization of LGMD Genes and Proteins in Myotubes Derived from Human Induced Pluripotent Stem Cells
Mutations in 27 genes are reported to cause 29 distinct LGMD (Table S1). The expression of these 27 genes was measured by qPCR in skMt derived from three healthy hiPSC lines (WT1,2,3) and myotubes derived from four healthy immortalized myoblast cell lines (C25, AB678, AB1079, AB1167) (Figure 3). Given that collagen type 6 is composed of three genetic components, from alpha 1 to alpha 3 chains, three genes were monitored for this LGMD subtype, resulting in a total of 29 genes measured by gene expression in these two sources of myotubes. Analysis of this qPCR revealed similar levels of expression for the majority of these genes, both in skMt and immortalized myotubes: DNAJB6, TNPO3, HNRNPDL, CAPN3, COL6A3, DYSF, SGCB, SGCG, SGCD, TCAP, TRIM32, POMT1, FKTN, POMT2, DAG1, TRAPPC11, GMPPB, and POGLUT1. Among these 29 genes, two genes revealed significant differences (10x fold change); on the one hand, ANO5, which was upregulated in skMt and, on the other hand, LAMA2, which was significantly upregulated in immortalized myotubes.

Figure 3.
Gene expression analysis of LGMD genes in skMt. Gene expression analysis of LGMD genes in skMt derived from three healthy hiPSC lines (WT1,2,3) at day 7 of myotube maturation. Fold changes (on log 10 scale) for ∆∆CT, relative to myotubes derived from four healthy primary myoblast cell lines (C25, AB678, AB1079, AB1167), are plotted as a group of data points and organized by gene according to the last revised LGMD classification. The pink horizontal line in each group of data points represents the mean for the group; * 0.01 < p ≤ 0.05 or ** 0.005 < p ≤ 0.01 or *** p < 0.005. Abbreviations: ANO5, anoctamin 5; CAPN3, calpain 3; COL6, collagen 6; DAG1, dystroglycan 1; DNAJB6, DnaJ heat shock protein family member B6; DYSF, dysferlin; FKRP, fukutin-related protein; FKTN, fukutin; GMPPB, GDP-mannose pyrophosphorylase B; HNRNPDL, heterogeneous nuclear ribonucleoprotein D-like; ISPD, isoprenoid synthase domain-containing protein; LAMA2, laminin subunit alpha 2; PLEC, plectin; POGLUT1, protein O-glucosyltransferase 1; POMGNT, protein O-linked mannose N-acetylglucosaminyltransferase; POMT, protein O-mannosyltransferase; SGC, sarcoglycan; TCAP, titin-cap; TNPO3, transportin 3; TRAPPC11, trafficking protein particle complex subunit 11; TRIM32, tripartite motif-containing 32; TTN, titin.
We then evaluated the expression of the corresponding proteins by immunostaining in skMt derived from one representative control hiPSC line (Figure 4). Confocal analysis of the expression of the dystrophin-associated glycoprotein complex (DGC) components revealed positive staining of dystroglycan 1 (DAG1) and beta, delta, and gamma sarcoglycans (SGCB, SGCD, and SGCG), while the alpha subunit (SGCA) was not detected in skMt. Analysis of sarcomeric components showed positive expression and localization within sarcomeres of calpain-3 (CAPN3), telethonin (TCAP), and titin (TTN), while a more diffuse, cross-striated pattern of plectin (PLEC) was also detected in skMt. We then evaluated the expression of proteins involved in DG glycosylation. Immunostaining of POMT2, POGLUT1, and FKRP showed a punctiform perinuclear localization of proteins, while localization was cytoplasmic for POMGnt1 and 2, POMT1, FKTN, GMPPB, and ISPD. Regarding the proteins involved in trafficking, analysis of dysferlin expression by immunostaining revealed the presence of the protein at the plasma membrane and also in the cytoplasm of skMt, whereas TNPO3 and TRAPPC11 labeling was cytoplasmic. Staining of DNAJB6 resulted in a signal in the nucleus and the cytoplasm, whereas that of anoctamin 5 (ANO5/TMEM16E) and tripartite motif-containing protein 32 (TRIM32) revealed a punctiform signal in the cytoplasm. With regard to the nuclear HNRNPDL protein, staining was observed in the nucleus of skMt, as expected. Finally, analysis of the extracellular matrix proteins performed in non-permeabilized conditions confirmed the presence of laminin-2 (LAMA2) in the sarcolemma. Immunostaining of collagen 6 alpha subunits 1–3 (COL6A1, COL6A2, COL6A3) revealed a punctiform signal across skMt. Altogether, qPCR and immunostaining data show that hiPSC-derived skMt express the majority of LGMD genes at a comparable level to immortalized myotubes and that the related encoded proteins are present in the expected cellular compartment.
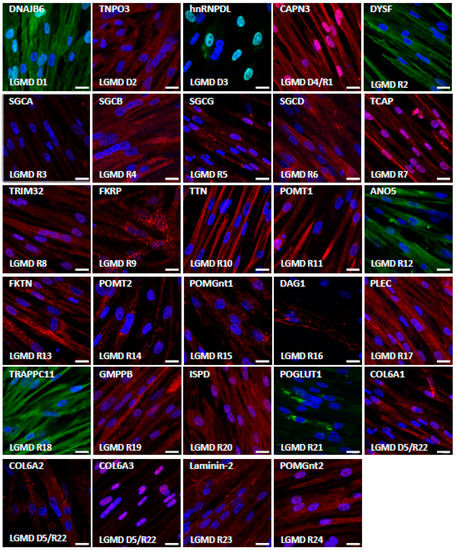
Figure 4.
Characterization of LGMD proteins in skMt. Representative images of LGMD protein immunostaining in skMt derived from one control hiPSC line at day 5 of maturation. Nuclei are labeled by Hoechst staining. Scale bar = 20µm. Abbreviations: ANO5, anoctamin 5; CAPN3, calpain 3; COL6, collagen 6; DAG1, dystroglycan 1; DNAJB6, DnaJ heat shock protein family member B6; DYSF, dysferlin; FKRP, fukutin-related protein; FKTN, fukutin; GMPPB, GDP-mannose pyrophosphorylase B; hnRNPDL, heterogeneous nuclear ribonucleoprotein D-like; ISPD, isoprenoid synthase domain-containing protein; LAMA2, laminin subunit alpha 2; PLEC, plectin; POGLUT1, protein O-glucosyltransferase 1; POMGnt, protein O-linked mannose N-acetylglucosaminyltransferase; POMT, protein O-mannosyltransferase; SGC, sarcoglycan; TCAP, titin-cap; TNPO3, transportin 3; TRAPPC11, trafficking protein particle complex subunit 11; TRIM32, tripartite motif-containing 32; TTN, titin.
3.3. Derivation and Characterization of Myotubes from FKRP-Deficient Human Induced Pluripotent Stem Cells
As a proof of concept, we investigated the pathological features of LGMDR9 using hiPSC. To do so, a hiPSC cell line from one patient was provided by the LGMD2I Research Fund, and three additional hiPSC lines carrying mutations in the FKRP gene were generated by reprogramming patient cells. Pluripotency of the four LGMDR9 hiPSC lines was confirmed by measuring the expression of Oct4 and Nanog using qPCR (Figure S3A) and immunostaining (Figure 5A). The pluripotency status of these hiPSC lines was also validated by measuring the expression of SSEA4 and TRA1-80 using flow cytometry (Figure S3B). Finally, chromosomal stability was analyzed using the multiplex fluorescence in situ hybridization (m-FISH) technique, revealing the absence of significant abnormalities (Figure S3C). Following this quality control assessment, the four FKRP-deficient hiPSC lines were further differentiated into skMb and skMt and compared to one representative healthy hiPSC line. Molecular characterization of skMb derived from FKRP-deficient hiPSC revealed no difference from the healthy skMb in terms of MyoD expression (Figure S4A). FKRP-deficient skMb were secondarily differentiated into skMt, showing positive sarcomeric staining for myosin heavy chain (MHC), titin, and α-actinin (Figure 5B and Figure S4B), which suggested that the absence of FKRP does not affect either the initial or the terminal stages of differentiation. Analysis of α-DG glycosylation was then performed by immunostaining in FKRP-deficient skMt and compared to the representative healthy relatives. Confocal analyses of IIH6 staining revealed the absence of a glycosylated α-DG signal in the FKRP1 line (c.899T > C c.962C > A mutation) but not in the other cell lines (FKRP2,3,4) when compared to the healthy control (Figure 5B). This result was subsequently confirmed by Western blot, showing that IIH6 staining was decreased in the same cell line (Figure 5C).
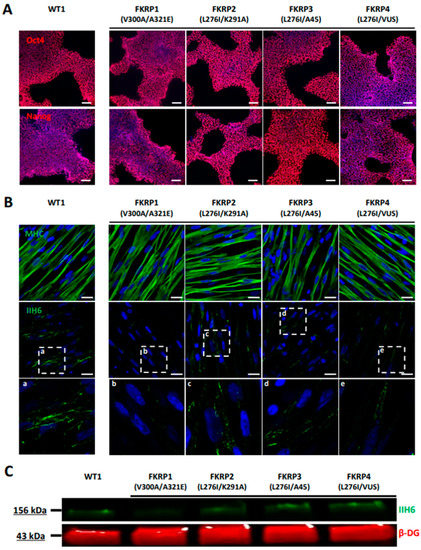
Figure 5.
Differentiation and characterization of LGMDR9-affected hiPSC into SkMC. (A) Immunostaining analysis of Oct4 and Nanog in one control and four LGMDR9 hiPSC lines at day 0 of differentiation. (B) Immunostaining images of MHC (upper panel) and α-DG glycosylation with IIH6 antibody (middle panel) in one control and four LGMDR9-derived skMt at day 24 of differentiation. White boxes, annotated from “a” to “e”, delineate enlargement of IIH6 staining to visualize higher magnification of α-DG glycosylation in the bottom panel. Nuclei are labeled by Hoechst staining. Scale bar = 20 µm. (C) Immunoblot analysis of α-DG glycosylation in skMt at day 24 of differentiation. β-DG was used as the loading control. Abbreviations: MHC, myosin heavy chain; β-DG, beta-dystroglycan.
4. Discussion
The main result of this study is the demonstration that myotubes derived from hiPSC can be used as a common biological resource to study a variety of LGMD. Following terminal differentiation, we reveal here that skMt are sufficiently mature to express the majority of LGMD-causing genes as well as their related encoded proteins, comparably to immortalized myotubes obtained from muscle biopsies. As a proof-of-concept study, we further demonstrated that skMt derived from FKRP-deficient hiPSC express the hallmark of LGMDR9 according to their mutations. Altogether, our results demonstrate that hiPSC represent a valuable unlimited resource for modeling LGMD and assessing in vitro efficacy of future LGMD therapies.
While similar in terms of clinical features, LGMD represent a highly heterogeneous group of diseases. Despite the high number of clinical or molecular studies of LGMD subtypes, our current knowledge of the molecular mechanisms underlying these diseases poses many challenges. In this context, the development of a common cellular platform to compare pathological pathways in multiple LGMD would provide a major step forward in their comprehension. So far, hiPSC have been used to explore individual LGMD [64,66], but no studies have documented the possibility of using these cells for all types of LGMD. A first study using hiPSC to model one LGMD was reported in 2013 [49]. In this proof-of-concept study, the authors showed that LGMDR2 hiPSC-derived myotubes obtained by stable genomic MyoD1 induction exhibited a defective membrane repair capacity following laser lesion that was rescued by full-length dysferlin gene transfer. More recently, El-Battrawy et al. [63] explored ion channel dysfunctions in cardiomyocytes derived from LGMDR9 hiPSC and successfully identified a significant reduction of systolic and diastolic intracellular calcium concentrations in those cells compared to healthy cells. In our study, we explored the possibility of going further by using hiPSC derivatives to model all LGMD genetic subtypes. To do so, we first evaluated the level of expression of all of the genes that cause LGMD and related proteins in myotubes differentiated from healthy hiPSC. qPCR and immunostaining data revealed that the majority of these genes and proteins were expressed in hiPSC-derived skMt at similar levels to primary myotubes. Beyond the overview of the LGMD that can be studied using hiPSC, this result suggests the use of hiPSC-derived skMt as a platform to improve our understanding of common pathways or phenotypes of several LGMD in a unique cellular model. Although useful to decipher pathological mechanisms or the development of new therapies, two-dimensional (2D) culture models of skeletal muscle cannot fully recapitulate the organization and the function of living muscle tissues, as shown with the recent development of functional 3D muscle tissues such as “myo bundles” [69].
As a proof of concept, we then generated and studied hiPSC carrying several mutations of the FKRP gene. Genetic variants in the FKRP gene cause a broad spectrum of phenotypes in LGMDR9 patients [70], ranging from mild muscle weakness and atrophy to more severe symptoms, with loss of ambulation, dilated cardiomyopathy, and respiratory [71] and mental impairment [72]. From a molecular perspective, FKRP is involved in α-DG glycosylation, as shown by Brockington et al. [73]. While the regulation of α-DG glycosylation appears to be a valuable therapeutic target for the treatment of LGMDR9 [74], other studies indicate, on the one hand, that several FKRP mutations do not affect α-DG glycosylation [75] and, on the other hand, that there is no correlation between the severity of LGMDR9 and α-DG glycosylation [76]. Taken together, these studies suggest that the detailed molecular mechanisms underpinning muscular symptoms and pathological pathways in LGMDR9 are still to be elucidated. In our study, we used one hiPSC line provided by the LGMD2I Research Fund and generated three extra hiPSC lines with different mutations. After differentiation into skMt, our results confirmed that the absence of FKRP does not lead to a systematic decrease in α-DG glycosylation in LGMDR9. Only one cell line (FKRP1 with the V300A/A321E mutation) was shown to affect this phenotype, in line with previous studies [75].
5. Conclusions
In the current study, we demonstrated that skMt derived from hiPSC can be used to model a large panel of LGMD. We showed that almost all genes and proteins involved in LGMD were expressed. We also demonstrated the biological relevance of this model derived from hiPSC through its ability to recapitulate the pathological phenotype identified for LGMDR9. Overall, this study opens up new perspectives on the use of this cellular model to study molecular mechanisms associated with LGMD but also for the development of high-content phenotypic assays and associated drug screenings.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biomedicines10061428/s1, Figure S1. Pluripotency characterization of control hiPSC lines Figure S2. Molecular characterization of TNN- and TTN-eGFP hiPSC and skMC Figure S3. Characterization of control and LGMDR9-affected hiPSC Figure S4. Characterization of FKRP-affected hiPSC differentiation into SkMC. Movie S1. Troponin I-eGFP skMt maturation. Movie S2. Titin-eGFP skMt maturation Table S1. Classification and nomenclature of LGMD. Table S2. Primers used for the measurement of the 27 genes causing LGMD by qPCR Table S3. Antibodies used for immunostaining of the 27 proteins causing LGMD.
Author Contributions
X.N. and I.R. were responsible for the experimental design and project management. C.B. and M.G. performed the characterization of TTN and TNN hiPSC differentiations. C.B., M.G., M.B., E.P., L.H. and E.G. developed the hiPSC differentiation protocol and carried out molecular analysis of LGMD genes and proteins. M.B. generated, characterized, and differentiated FKRP-deficient cells. J.D. generated the immortalized cells. C.B., X.N. and I.R. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
I-Stem and Genethon are part of the Biotherapies Institute for Rare Diseases, supported by the Association Française contre les Myopathies (AFM-Téléthon). This research was funded by grants from INSERM, the domaine d’intéret majeur (DIM) Biothérapies, université Paris-Saclay, Genopole, and the European Commission. The authors thank Marc Peschanski (I-Stem) for helpful discussions, the “technological research team” of I-Stem and Marine Faivre (Genethon) for technical support; and the Platform for Immortalization of Human Cells from the Centre of Research in Myology, Institute of Myology, Paris for providing immortalized muscular cells. In memory of our friend and colleague Jackie Gide.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guglieri, M.; Straub, V.; Bushby, K.; Lochmüller, H. Limb–Girdle Muscular Dystrophies. Curr. Opin. Neurol. 2008, 21, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Savarese, M. Genetic Basis of Limb-Girdle Muscular Dystrophies: The 2014 Update. Acta Myol. 2014, 33, 1–12. [Google Scholar] [PubMed]
- Walton, J.N.; Nattrass, F.J. On the Classification, Natural History and Treatment of the Myopathies. Brain 1954, 77, 169–231. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.K.; Korngut, L.; Fiest, K.M.; Dykeman, J.; Day, L.J.; Pringsheim, T.; Jette, N. A Systematic Review and Meta-Analysis on the Epidemiology of the Muscular Dystrophies. Can. J. Neurol. Sci. 2016, 43, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Liewluck, T.; Milone, M. Untangling the Complexity of Limb-girdle Muscular Dystrophies. Muscle Nerve 2018, 58, 167–177. [Google Scholar] [CrossRef]
- Wicklund, M.P.; Kissel, J.T. The Limb-Girdle Muscular Dystrophies. Neurol. Clin. 2014, 32, 729–749. [Google Scholar] [CrossRef]
- Richard, I.; Broux, O.; Fougerousse, F.; Chiannilkulchai, N.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C.; Hillaire, D.; et al. Mutations in the Proteolytic Enzyme Calpain 3 Cause Limb-Girdle Muscular Dystrophy Type 2A. Cell 1995, 81, 27–40. [Google Scholar] [CrossRef]
- Straub, V.; Murphy, A.; Udd, B.; Corrado, A.; Aymé, S.; Bönneman, C.; de Visser, M.; Hamosh, A.; Jacobs, L.; Khizanishvili, N.; et al. 229th ENMC International Workshop: Limb Girdle Muscular Dystrophies—Nomenclature and Reformed Classification Naarden, The Netherlands, 17–19 March 2017. Neuromuscul. Disord. 2018, 28, 702–710. [Google Scholar] [CrossRef]
- Holt, K.H.; Lim, L.E.; Straub, V.; Venzke, D.P.; Duclos, F.; Anderson, R.D.; Davidson, B.L.; Campbell, K.P. Functional Rescue of the Sarcoglycan Complex in the BIO 14.6 Hamster Using δ-Sarcoglycan Gene Transfer. Mol. Cell 1998, 1, 841–848. [Google Scholar] [CrossRef]
- Li, J.; Dressman, D.; Tsao, Y.P.; Sakamoto, A.; Hoffman, E.P.; Xiao, X. RAAV Vector-Mediated Sarcogylcan Gene Transfer in a Hamster Model for Limb Girdle Muscular Dystrophy. Gene 1999, 6, 74–82. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.; Tsao, Y.-P.; Dressman, D.; Hoffman, E.P.; Watchko, J.F. Full Functional Rescue of a Complete Muscle (TA) in Dystrophic Hamsters by Adeno-Associated Virus Vector-Directed Gene Therapy. J. Virol. 2000, 74, 1436–1442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allamand, V.; Donahue, K.M.; Straub, V.; Davisson, R.L.; Davidson, B.L.; Campbell, K.P. Early Adenovirus-Mediated Gene Transfer Effectively Prevents Muscular Dystrophy in Alpha-Sarcoglycan-Deficient Mice. Gene 2000, 7, 1385–1391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartoli, M.; Roudaut, C.; Martin, S.; Fougerousse, F.; Suel, L.; Poupiot, J.; Gicquel, E.; Noulet, F.; Danos, O.; Richard, I. Safety and Efficacy of AAV-Mediated Calpain 3 Gene Transfer in a Mouse Model of Limb-Girdle Muscular Dystrophy Type 2A. Mol. Ther. 2006, 13, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, P.C.; Griffin, D.A.; Pozsgai, E.R.; Johnson, R.W.; Grose, W.E.; Heller, K.N.; Shontz, K.M.; Montgomery, C.L.; Liu, J.; Clark, K.R.; et al. AAV.Dysferlin Overlap Vectors Restore Function in Dysferlinopathy Animal Models. Ann. Clin. Transl. Neurol. 2015, 2, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Allamand, V. Animal Models for Muscular Dystrophy: Valuable Tools for the Development of Therapies. Hum. Mol. Genet. 2000, 9, 2459–2467. [Google Scholar] [CrossRef]
- Ng, R.; Banks, G.B.; Hall, J.K.; Muir, L.A.; Ramos, J.N.; Wicki, J.; Odom, G.L.; Konieczny, P.; Seto, J.; Chamberlain, J.R.; et al. Animal Models of Muscular Dystrophy. Prog. Mol. Biol. Transl. Sci. 2012, 105, 83–111. [Google Scholar] [CrossRef]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal Models of Duchenne Muscular Dystrophy: From Basic Mechanisms to Gene Therapy. Dis. Models Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef]
- Ackroyd, M.R.; Skordis, L.; Kaluarachchi, M.; Godwin, J.; Prior, S.; Fidanboylu, M.; Piercy, R.J.; Muntoni, F.; Brown, S.C. Reduced Expression of Fukutin Related Protein in Mice Results in a Model for Fukutin Related Protein Associated Muscular Dystrophies. Brain 2008, 132, 439–451. [Google Scholar] [CrossRef]
- Vafiadaki, E.; Reis, A.; Keers, S.; Harrison, R.; Anderson, L.V.B.; Raffelsberger, T.; Ivanova, S.; Hoger, H.; Bittner, R.E.; Bushby, K.; et al. Cloning of the Mouse Dysferlin Gene and Genomic Characterization of the SJL-Dysf Mutation. Neuroreport 2001, 12, 625–629. [Google Scholar] [CrossRef]
- Aas, V.; Bakke, S.S.; Feng, Y.Z.; Kase, E.T.; Jensen, J.; Bajpeyi, S.; Thoresen, G.H.; Rustan, A.C. Are Cultured Human Myotubes Far from Home? Cell Tissue Res. 2013, 354, 671–682. [Google Scholar] [CrossRef]
- Bar-Nur, O.; Gerli, M.F.M.; Di Stefano, B.; Almada, A.E.; Galvin, A.; Coffey, A.; Huebner, A.J.; Feige, P.; Verheul, C.; Cheung, P.; et al. Direct Reprogramming of Mouse Fibroblasts into Functional Skeletal Muscle Progenitors. Stem Cell Rep. 2018, 10, 1505–1521. [Google Scholar] [CrossRef] [PubMed]
- Mamchaoui, K.; Trollet, C.; Bigot, A.; Negroni, E.; Chaouch, S.; Wolff, A.; Kandalla, P.K.; Marie, S.; Di Santo, J.; St Guily, J.L.; et al. Immortalized Pathological Human Myoblasts: Towards a Universal Tool for the Study of Neuromuscular Disorders. Skelet. Muscle 2011, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Thorley, M.; Duguez, S.; Mazza, E.M.C.; Valsoni, S.; Bigot, A.; Mamchaoui, K.; Harmon, B.; Voit, T.; Mouly, V.; Duddy, W. Skeletal Muscle Characteristics Are Preserved in HTERT/Cdk4 Human Myogenic Cell Lines. Skelet. Muscle 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Lasa-Elgarresta, J.; Mosqueira-Martín, L.; González-Imaz, K.; Marco-Moreno, P.; Gerenu, G.; Mamchaoui, K.; Mouly, V.; López de Munain, A.; Vallejo-Illarramendi, A. Targeting the Ubiquitin-Proteasome System in Limb-Girdle Muscular Dystrophy With CAPN3 Mutations. Front. Cell Dev. Biol. 2022, 10, 822563. [Google Scholar] [CrossRef] [PubMed]
- Cea, L.A.; Bevilacqua, J.A.; Arriagada, C.; Cárdenas, A.M.; Bigot, A.; Mouly, V.; Sáez, J.C.; Caviedes, P. The Absence of Dysferlin Induces the Expression of Functional Connexin-Based Hemichannels in Human Myotubes. BMC Cell Biol. 2016, 17, 15. [Google Scholar] [CrossRef]
- Carmeille, R.; Bouvet, F.; Tan, S.; Croissant, C.; Gounou, C.; Mamchaoui, K.; Mouly, V.; Brisson, A.R.; Bouter, A. Membrane Repair of Human Skeletal Muscle Cells Requires Annexin-A5. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2267–2279. [Google Scholar] [CrossRef]
- Philippi, S.; Bigot, A.; Marg, A.; Mouly, V.; Spuler, S.; Zacharias, U. Dysferlin-Deficient Immortalized Human Myoblasts and Myotubes as a Useful Tool to Study Dysferlinopathy. PLoS Curr. 2012, 4, RRN1298. [Google Scholar] [CrossRef]
- Chandra, G.; Defour, A.; Mamchoui, K.; Pandey, K.; Mishra, S.; Mouly, V.; Sreetama, S.; Mahad Ahmad, M.; Mahjneh, I.; Morizono, H.; et al. Dysregulated Calcium Homeostasis Prevents Plasma Membrane Repair in Anoctamin 5/TMEM16E-Deficient Patient Muscle Cells. Cell Death Discov. 2019, 5, 118. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Martin, G.R. Isolation of a Pluripotent Cell Line from Early Mouse Embryos Cultured in Medium Conditioned by Teratocarcinoma Stem Cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef]
- Shamblott, M.J.; Axelman, J.; Wang, S.; Bugg, E.M.; Littlefield, J.W.; Donovan, P.J.; Blumenthal, P.D.; Huggins, G.R.; Gearhart, J.D. Derivation of Pluripotent Stem Cells from Cultured Human Primordial Germ Cells. Dev. Biol. 1998, 95, 13726–13731. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Sci. New Ser. 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Wobus, A.M.; Boheler, K.R. Embryonic Stem Cells: Prospects for Developmental Biology and Cell Therapy. Physiol. Rev. 2005, 85, 635–678. [Google Scholar] [CrossRef]
- Laustriat, D.; Gide, J.; Barrault, L.; Chautard, E.; Benoit, C.; Auboeuf, D.; Boland, A.; Battail, C.; Artiguenave, F.; Deleuze, J.-F.; et al. In Vitro and In Vivo Modulation of Alternative Splicing by the Biguanide Metformin. Mol. Ther. Nucleic Acids 2015, 4, e262. [Google Scholar] [CrossRef]
- Nissan, X.; Blondel, S.; Navarro, C.; Maury, Y.; Denis, C.; Girard, M.; Martinat, C.; De Sandre-Giovannoli, A.; Levy, N.; Peschanski, M. Unique Preservation of Neural Cells in Hutchinson- Gilford Progeria Syndrome Is Due to the Expression of the Neural-Specific MiR-9 MicroRNA. Cell Rep. 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Allouche, J.; Bellon, N.; Saidani, M.; Stanchina-Chatrousse, L.; Masson, Y.; Patwardhan, A.; Gilles-Marsens, F.; Delevoye, C.; Domingues, S.; Nissan, X.; et al. In Vitro Modeling of Hyperpigmentation Associated to Neurofibromatosis Type 1 Using Melanocytes Derived from Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 9034–9039. [Google Scholar] [CrossRef]
- Estronca, L.; Francisco, V.; Pitrez, P.; Honório, I.; Carvalho, L.; Vazão, H.; Blersch, J.; Rai, A.; Nissan, X.; Simon, U.; et al. Induced Pluripotent Stem Cell-Derived Vascular Networks to Screen Nano–Bio Interactions. Nanoscale Horiz. 2021, 6, 245–259. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Saidani, M.; Allouche, J.; Egesipe, A.L.; Hoch, L.; Bruge, C.; Sigaudy, S.; De Sandre-Giovannoli, A.; Levy, N.; Baldeschi, C.; et al. Pathological Modelling of Pigmentation Disorders Associated with Hutchinson-Gilford Progeria Syndrome (HGPS) Revealed an Impaired Melanogenesis Pathway in IPS-Derived Melanocytes. Sci. Rep. 2018, 8, 9112. [Google Scholar] [CrossRef]
- Moreau, A.; Reisqs, J.; Delanoe-Ayari, H.; Pierre, M.; Janin, A.; Deliniere, A.; Bessière, F.; Meli, A.C.; Charrabi, A.; Lafont, E.; et al. Deciphering DSC2 Arrhythmogenic Cardiomyopathy Electrical Instability: From Ion Channels to ECG and Tailored Drug Therapy. Clin. Transl. Med. 2021, 11, e319. [Google Scholar] [CrossRef] [PubMed]
- Egesipe, A.-L.; Blondel, S.; Lo Cicero, A.; Jaskowiak, A.-L.; Navarro, C.; Sandre-Giovannoli, A.D.; Levy, N.; Peschanski, M.; Nissan, X. Metformin Decreases Progerin Expression and Alleviates Pathological Defects of Hutchinson–Gilford Progeria Syndrome Cells. npj Aging Mech. Dis. 2016, 2, 16026. [Google Scholar] [CrossRef] [PubMed]
- Harhouri, K.; Navarro, C.; Depetris, D.; Mattei, M.; Nissan, X.; Cau, P.; De Sandre-Giovannoli, A.; Lévy, N. MG 132-induced Progerin Clearance Is Mediated by Autophagy Activation and Splicing Regulation. EMBO Mol. Med. 2017, 9, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Jaskowiak, A.-L.; Egesipe, A.-L.; Tournois, J.; Brinon, B.; Pitrez, P.R.; Ferreira, L.; de Sandre-Giovannoli, A.; Levy, N.; Nissan, X. A High Throughput Phenotypic Screening Reveals Compounds That Counteract Premature Osteogenic Differentiation of HGPS IPS-Derived Mesenchymal Stem Cells. Sci. Rep. 2016, 6, 34798. [Google Scholar] [CrossRef] [PubMed]
- Pitrez, P.R.; Estronca, L.; Monteiro, L.M.; Colell, G.; Vazão, H.; Santinha, D.; Harhouri, K.; Thornton, D.; Navarro, C.; Egesipe, A.-L.; et al. Vulnerability of Progeroid Smooth Muscle Cells to Biomechanical Forces Is Mediated by MMP13. Nat. Commun. 2020, 11, 4110. [Google Scholar] [CrossRef]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Darabi, R.; Arpke, R.W.; Irion, S.; Dimos, J.T.; Grskovic, M.; Kyba, M.; Perlingeiro, R.C.R. Human ES- and IPS-Derived Myogenic Progenitors Restore DYSTROPHIN and Improve Contractility upon Transplantation in Dystrophic Mice. Cell Stem Cell 2012, 10, 610–619. [Google Scholar] [CrossRef]
- Maffioletti, S.M.; Gerli, M.F.M.; Ragazzi, M.; Dastidar, S.; Benedetti, S.; Loperfido, M.; VandenDriessche, T.; Chuah, M.K.; Tedesco, F.S. Efficient Derivation and Inducible Differentiation of Expandable Skeletal Myogenic Cells from Human ES and Patient-Specific IPS Cells. Nat. Protoc. 2015, 10, 941–958. [Google Scholar] [CrossRef]
- Tanaka, A.; Woltjen, K.; Miyake, K.; Hotta, A.; Ikeya, M.; Yamamoto, T.; Nishino, T.; Shoji, E.; Sehara-Fujisawa, A.; Manabe, Y.; et al. Efficient and Reproducible Myogenic Differentiation from Human IPS Cells: Prospects for Modeling Miyoshi Myopathy In Vitro. PLoS ONE 2013, 8, e61540. [Google Scholar] [CrossRef]
- Borchin, B.; Chen, J.; Barberi, T. Derivation and FACS-Mediated Purification of PAX3+/PAX7+ Skeletal Muscle Precursors from Human Pluripotent Stem Cells. Stem Cell Rep. 2013, 1, 620–631. [Google Scholar] [CrossRef]
- Caron, L.; Kher, D.; Lee, K.L.; McKernan, R.; Dumevska, B.; Hidalgo, A.; Li, J.; Yang, H.; Main, H.; Ferri, G.; et al. A Human Pluripotent Stem Cell Model of Facioscapulohumeral Muscular Dystrophy-Affected Skeletal Muscles. Stem Cells Transl. Med. 2016, 5, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Oginuma, M.; Al Tanoury, Z.; Gobert, B.; Sumara, O.; Hick, A.; Bousson, F.; Zidouni, Y.; Mursch, C.; Moncuquet, P.; et al. Differentiation of Pluripotent Stem Cells to Muscle Fiber to Model Duchenne Muscular Dystrophy. Nat. Biotechnol. 2015, 33, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Al Tanoury, Z.; Hestin, M.; Gobert, B.; Aivio, S.; Hick, A.; Cherrier, T.; Nesmith, A.P.; Parker, K.K.; Pourquié, O. Generation of Human Muscle Fibers and Satellite-like Cells from Human Pluripotent Stem Cells in Vitro. Nat. Protoc. 2016, 11, 1833–1850. [Google Scholar] [CrossRef]
- Shelton, M.; Metz, J.; Liu, J.; Carpenedo, R.L.; Demers, S.-P.; Stanford, W.L.; Skerjanc, I.S. Derivation and Expansion of PAX7-Positive Muscle Progenitors from Human and Mouse Embryonic Stem Cells. Stem Cell Rep. 2014, 3, 516–529. [Google Scholar] [CrossRef]
- Shelton, M.; Kocharyan, A.; Liu, J.; Skerjanc, I.S.; Stanford, W.L. Robust Generation and Expansion of Skeletal Muscle Progenitors and Myocytes from Human Pluripotent Stem Cells. Methods 2016, 101, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Swartz, E.W.; Baek, J.; Pribadi, M.; Wojta, K.J.; Almeida, S.; Karydas, A.; Gao, F.-B.; Miller, B.L.; Coppola, G. A Novel Protocol for Directed Differentiation of C9orf72-Associated Human Induced Pluripotent Stem Cells Into Contractile Skeletal Myotubes. Stem Cells Transl. Med. 2016, 5, 1461–1472. [Google Scholar] [CrossRef]
- Xu, C.; Tabebordbar, M.; Iovino, S.; Ciarlo, C.; Liu, J.; Castiglioni, A.; Price, E.; Liu, M.; Barton, E.R.; Kahn, C.R.; et al. A Zebrafish Embryo Culture System Defines Factors That Promote Vertebrate Myogenesis across Species. Cell 2013, 155, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Sci. New Ser. 2008, 321, 1218–1221. [Google Scholar] [CrossRef]
- Ebert, A.D.; Yu, J.; Rose, F.F.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced Pluripotent Stem Cells from a Spinal Muscular Atrophy Patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef]
- Lin, B.; Li, Y.; Han, L.; Kaplan, A.D.; Ao, Y.; Kalra, S.; Bett, G.C.L.; Rasmusson, R.L.; Denning, C.; Yang, L. Modeling and Study of the Mechanism of Dilated Cardiomyopathy Using Induced Pluripotent Stem Cells Derived from Individuals with Duchenne Muscular Dystrophy. Dis. Models Mech. 2015, 8, 457–466. [Google Scholar] [CrossRef]
- Park, I.-H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-Specific Induced Pluripotent Stem Cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Shoji, E.; Sakurai, H.; Nishino, T.; Nakahata, T.; Heike, T.; Awaya, T.; Fujii, N.; Manabe, Y.; Matsuo, M.; Sehara-Fujisawa, A. Early Pathogenesis of Duchenne Muscular Dystrophy Modelled in Patient-Derived Human Induced Pluripotent Stem Cells. Sci. Rep. 2015, 5, 12831. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Zhao, Z.; Lan, H.; Li, X.; Yücel, G.; Lang, S.; Sattler, K.; Schünemann, J.-D.; Zimmermann, W.-H.; Cyganek, L.; et al. Ion Channel Dysfunctions in Dilated Cardiomyopathy in Limb-Girdle Muscular Dystrophy. Circ. Genom. Precis. Med. 2018, 11, e001893. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aierdi, A.J.; Dehesa-Etxebeste, M.; Goicoechea, M.; Aiastui, A.; Richaud-Patin, Y.; Jiménez-Delgado, S.; Raya, A.; Naldaiz-Gastesi, N.; López de Munain, A. Patient-Specific IPSC-Derived Cellular Models of LGMDR1. Stem Cell Res. 2021, 53, 102333. [Google Scholar] [CrossRef]
- Tedesco, F.S.; Gerli, M.F.M.; Perani, L.; Benedetti, S.; Ungaro, F.; Cassano, M.; Antonini, S.; Tagliafico, E.; Artusi, V.; Longa, E.; et al. Transplantation of Genetically Corrected Human IPSC-Derived Progenitors in Mice with Limb-Girdle Muscular Dystrophy. Sci. Transl. Med. 2012, 4, 140ra89. [Google Scholar] [CrossRef]
- Wu, J.; Hunt, S.D.; Matthias, N.; Servián-Morilla, E.; Lo, J.; Jafar-Nejad, H.; Paradas, C.; Darabi, R. Generation of an Induced Pluripotent Stem Cell Line (CSCRMi001-A) from a Patient with a New Type of Limb-Girdle Muscular Dystrophy (LGMD) Due to a Missense Mutation in POGLUT1 (Rumi). Stem Cell Res. 2017, 24, 102–105. [Google Scholar] [CrossRef]
- Boissart, C.; Poulet, A.; Georges, P.; Darville, H.; Julita, E.; Delorme, R.; Bourgeron, T.; Peschanski, M.; Benchoua, A. Differentiation from Human Pluripotent Stem Cells of Cortical Neurons of the Superficial Layers Amenable to Psychiatric Disease Modeling and High-Throughput Drug Screening. Transl. Psychiatry 2013, 3, e294. [Google Scholar] [CrossRef]
- Darville, H.; Poulet, A.; Rodet-Amsellem, F.; Chatrousse, L.; Pernelle, J.; Boissart, C.; Héron, D.; Nava, C.; Perrier, A.; Jarrige, M.; et al. Human Pluripotent Stem Cell-Derived Cortical Neurons for High Throughput Medication Screening in Autism: A Proof of Concept Study in SHANK3 Haploinsufficiency Syndrome. EBioMedicine 2016, 9, 293–305. [Google Scholar] [CrossRef]
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering Human Pluripotent Stem Cells into a Functional Skeletal Muscle Tissue. Nat. Commun. 2018, 9, 126. [Google Scholar] [CrossRef]
- Mercuri, E.; Brockington, M.; Straub, V.; Quijano-Roy, S.; Yuva, Y.; Herrmann, R.; Brown, S.C.; Torelli, S.; Dubowitz, V.; Blake, D.J.; et al. Phenotypic Spectrum Associated with Mutations in the Fukutin-Related Protein Gene. Ann. Neurol. 2003, 53, 537–542. [Google Scholar] [CrossRef]
- Poppe, M.; Bourke, J.; Eagle, M.; Frosk, P.; Wrogemann, K.; Greenberg, C.; Muntoni, F.; Voit, T.; Straub, V.; Hilton-Jones, D.; et al. Cardiac and Respiratory Failure in Limb-Girdle Muscular Dystrophy 2I. Ann. Neurol. 2004, 56, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Valero de Bernabe, D. Mutations in the FKRP Gene Can Cause Muscle-Eye-Brain Disease and Walker-Warburg Syndrome. J. Med. Genet. 2004, 41, e61. [Google Scholar] [CrossRef] [PubMed]
- Brockington, M.; Blake, D.J.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Ponting, C.P.; Estournet, B.; Romero, N.B.; Mercuri, E.; et al. Mutations in the Fukutin-Related Protein Gene (FKRP) Cause a Form of Congenital Muscular Dystrophy with Secondary Laminin A2 Deficiency and Abnormal Glycosylation of a-Dystroglycan. Am. J. Hum. Genet. 2001, 69, 1198–1209. [Google Scholar] [CrossRef]
- Cataldi, M.P.; Lu, P.; Blaeser, A.; Lu, Q.L. Ribitol Restores Functionally Glycosylated α-Dystroglycan and Improves Muscle Function in Dystrophic FKRP-Mutant Mice. Nat. Commun. 2018, 9, 3448. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.F.; Gicquel, E.; Marsolier, J.; Richard, I. Functional and Cellular Localization Diversity Associated with Fukutin-related Protein Patient Genetic Variants. Hum. Mutat. 2019, 40, 1874–1885. [Google Scholar] [CrossRef]
- Alhamidi, M.; Brox, V.; Stensland, E.; Liset, M.; Lindal, S.; Nilssen, Ø. Limb Girdle Muscular Dystrophy Type 2I: No Correlation between Clinical Severity, Histopathology and Glycosylated α-Dystroglycan Levels in Patients Homozygous for Common FKRP Mutation. Neuromuscul. Disord. 2017, 27, 619–626. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).