Oxidative Stress and Arginine/Nitric Oxide Pathway in Red Blood Cells Derived from Patients with Prediabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Collection
2.3. Oral Glucose Tolerance Test
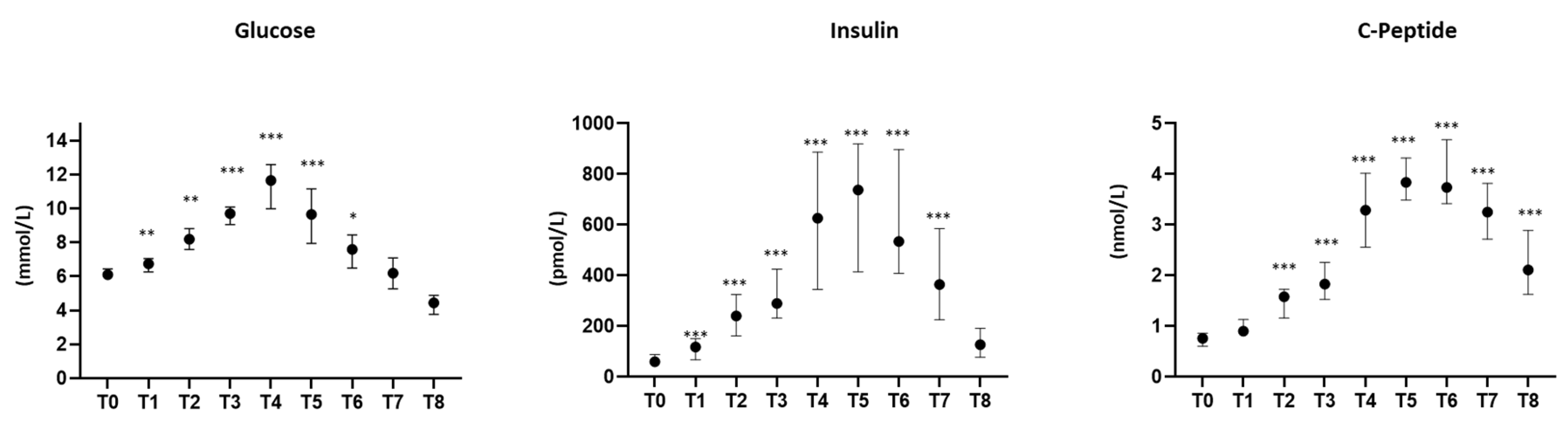
2.4. Insulin Resistance and β-Cell Function Evaluation
2.5. Biochemical Analyses
2.6. Arg/NO Metabolic Pathway
2.7. Oxidative Stress
2.8. Nitric Oxide Synthase Analysis in Red Blood Cells
2.9. Phosphatidylserine Exposure
2.10. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
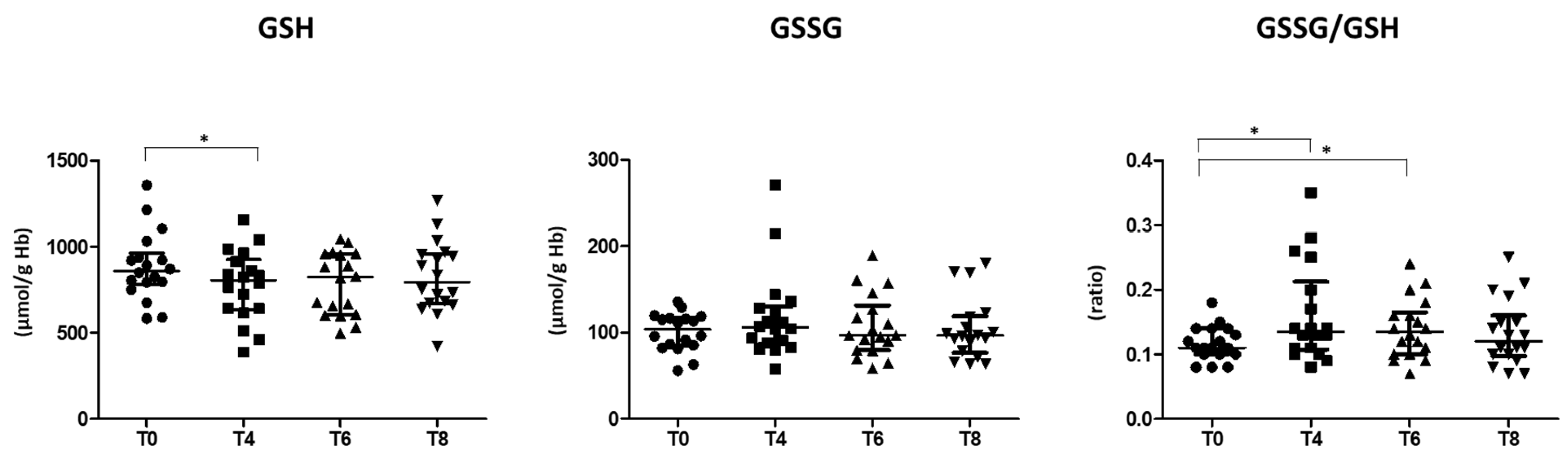
3.2. Oxidative Stress Status
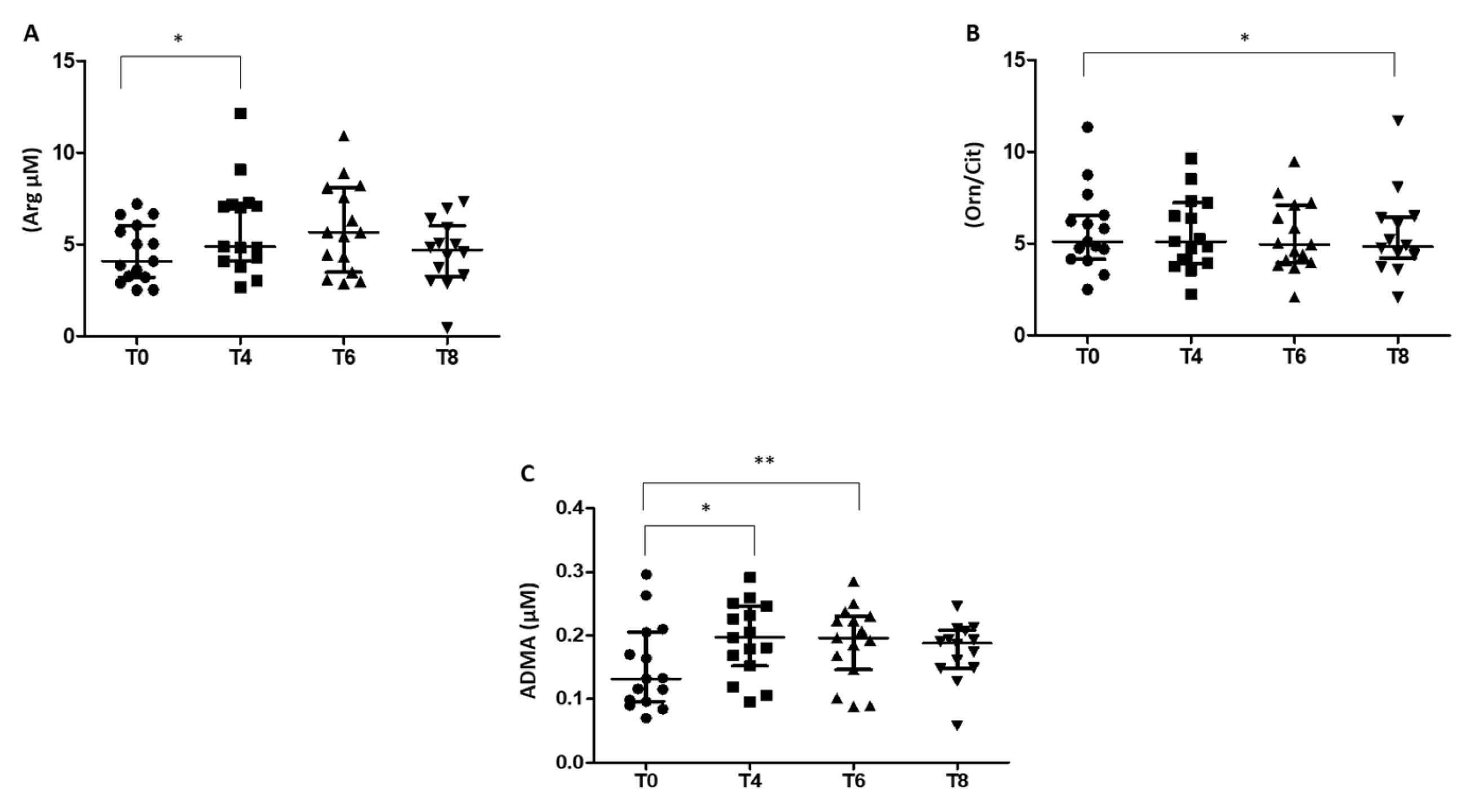
3.3. Arg/NO Pathway in RBCs of Prediabetic Patients
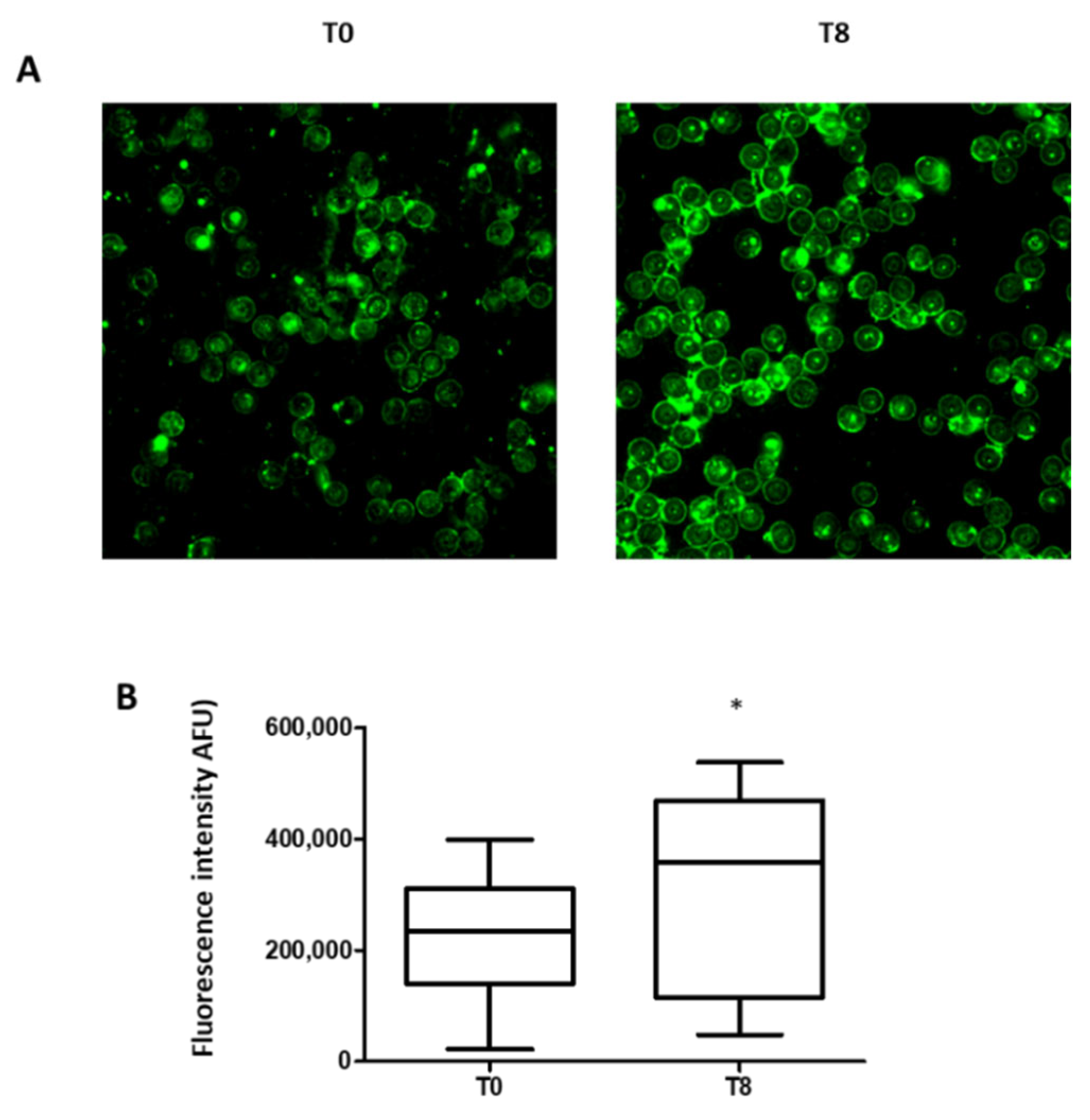
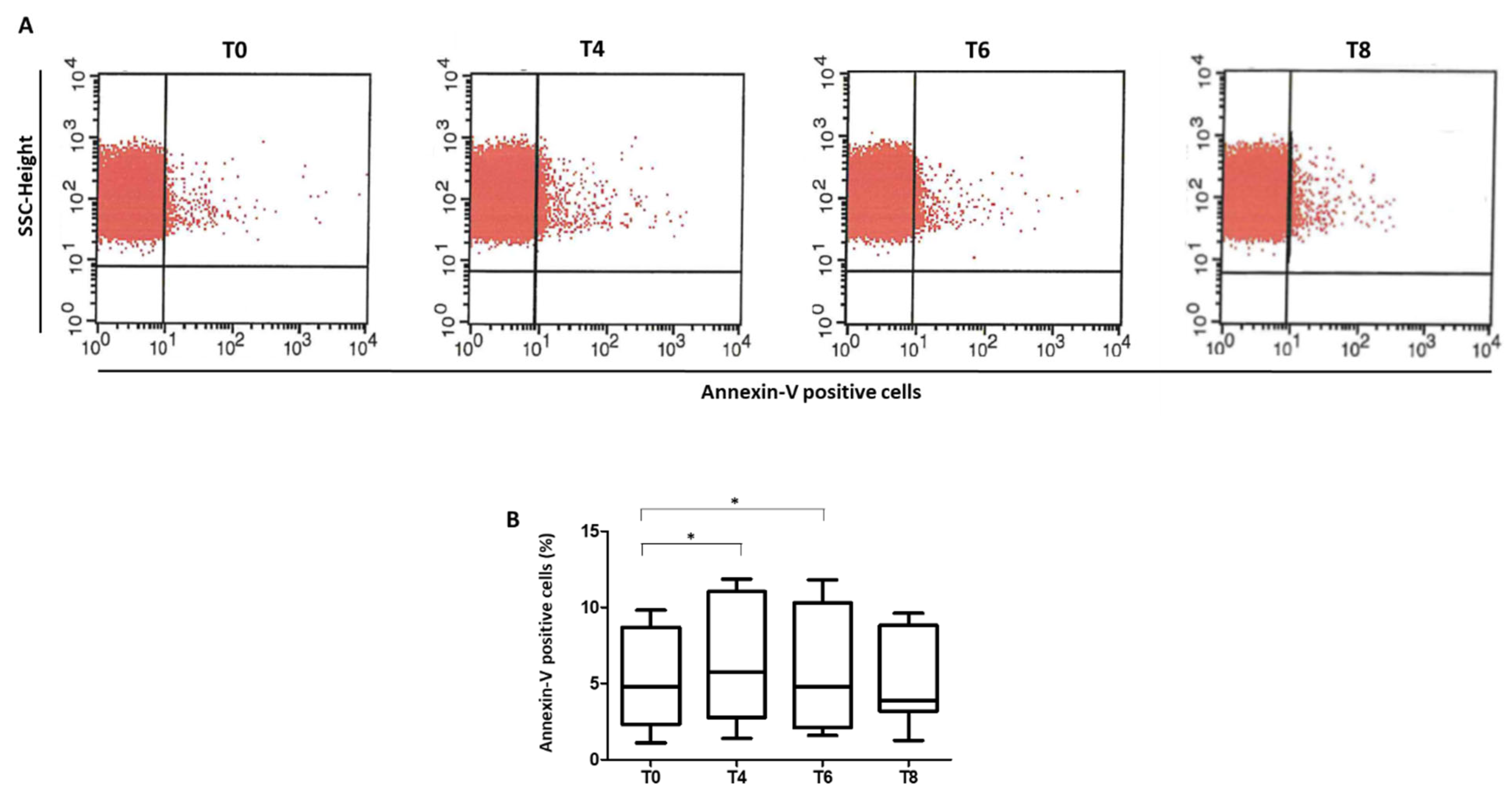
3.4. Phosphatidylserine Exposure in RBCs
3.5. Red Cell Distribution Width
4. Discussion
4.1. Oxidative Stress Status
4.2. Arginine/Nitric Oxide Pathway
4.3. Eryptotic Process
4.4. Red Cell Distribution Width
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183–1197. [CrossRef] [PubMed]
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [CrossRef] [PubMed]
- Vaccaro, O.; Ruffa, G.; Imperatore, G.; Iovino, V.; Rivellese, A.A.; Riccardi, G. Risk of diabetes in the new diagnostic category of impaired fasting glucose: A prospective analysis. Diabetes Care 1999, 22, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Muller, D.C.; Nathan, D.M.; Blake, D.R.; Andres, R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003, 52, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, O.; Riccardi, G. Changing the definition of impaired fasting glucose: Impact on the classification of individuals and risk definition. Diabetes Care 2005, 28, 1786–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forouhi, N.G.; Balkau, B.; Borch-Johnsen, K.; Dekker, J.; Glumer, C.; Qiao, Q.; Spijkerman, A.; Stolk, R.; Tabac, A.; Wareham, N.J.; et al. The threshold for diagnosing impaired fasting glucose: A position statement by the European Diabetes Epidemiology Group. Diabetologia 2006, 49, 822–827. [Google Scholar] [CrossRef] [Green Version]
- Bonora, E.; Kiechl, S.; Oberhollenzer, F.; Egger, G.; Bonadonna, R.C.; Muggeo, M.; Willeit, J. Impaired glucose tolerance, Type II diabetes mellitus and carotid atherosclerosis: Prospective results from the Bruneck Study. Diabetologia 2000, 43, 156–164. [Google Scholar] [CrossRef]
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Zenari, L.; Saggiani, F.; Poli, M.; Perbellini, S.; et al. The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med. 2004, 21, 52–58. [Google Scholar] [CrossRef]
- Sung, J.; Song, Y.M.; Ebrahim, S.; Lawlor, D.A. Fasting blood glucose and the risk of stroke and myocardial infarction. Circulation 2009, 119, 812–819. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garber, A.J.; Handelsman, Y.; Einhorn, D.; Bergman, D.A.; Bloomgarden, Z.T.; Fonseca, V.; Garvey, W.T.; Gavin, J.R., 3rd; Grunberger, G.; Horton, E.S.; et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: When do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008, 14, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Brannick, B.; Wynn, A.; Dagogo-Jack, S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp. Biol Med. 2016, 241, 1323–1331. [Google Scholar] [CrossRef] [Green Version]
- Rosen, P.N.; Kaplan, R.M.; David, K. Measuring outcomes of cataract surgery using the Quality of Well-Being Scale and VF-14 Visual Function Index. J. Cataract Refract. Surg. 2005, 31, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Hatemi, H.; Ozer, E.M.; Gonen, S.; Akcay, T. The erythrocyte glutathione levels during oral glucose tolerance test. J. Endocrinol. Investig. 1997, 20, 471–475. [Google Scholar] [CrossRef]
- Ceriello, A.; Bortolotti, N.; Crescentini, A.; Motz, E.; Lizzio, S.; Russo, A.; Ezsol, Z.; Tonutti, L.; Taboga, C. Antioxidant defences are reduced during the oral glucose tolerance test in normal and non-insulin-dependent diabetic subjects. Eur. J. Clin. Investig. 1998, 28, 329–333. [Google Scholar] [CrossRef]
- Lang, F.; Abed, M.; Lang, E.; Foller, M. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal. 2014, 21, 138–153. [Google Scholar] [CrossRef]
- Klarl, B.A.; Lang, P.A.; Kempe, D.S.; Niemoeller, O.M.; Akel, A.; Sobiesiak, M.; Eisele, K.; Podolski, M.; Huber, S.M.; Wieder, T.; et al. Protein kinase C mediates erythrocyte “programmed cell death” following glucose depletion. Am. J. Physiol. Cell Physiol. 2006, 290, C244–C253. [Google Scholar] [CrossRef] [Green Version]
- Lang, F.; Gulbins, E.; Lang, P.A.; Zappulla, D.; Foller, M. Ceramide in suicidal death of erythrocytes. Cell Physiol. Biochem. 2010, 26, 21–28. [Google Scholar] [CrossRef]
- Lang, K.S.; Duranton, C.; Poehlmann, H.; Myssina, S.; Bauer, C.; Lang, F.; Wieder, T.; Huber, S.M. Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003, 10, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucherenko, Y.V.; Bhavsar, S.K.; Grischenko, V.I.; Fischer, U.R.; Huber, S.M.; Lang, F. Increased cation conductance in human erythrocytes artificially aged by glycation. J. Membr. Biol. 2010, 235, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Salinas, J.V.; Munoz-Reyes, E.G.; Guerrero-Romero, J.F.; Rodriguez-Moran, M.; Bracho-Riquelme, R.L.; Carrera-Gracia, M.A.; Quintanar-Escorza, M.A. Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol. Cell Biochem. 2011, 357, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Maellaro, E.; Leoncini, S.; Moretti, D.; Del Bello, B.; Tanganelli, I.; De Felice, C.; Ciccoli, L. Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and in plasma of type 2 diabetic patients. Acta Diabetol. 2013, 50, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Borst, O.; Abed, M.; Alesutan, I.; Towhid, S.T.; Qadri, S.M.; Foller, M.; Gawaz, M.; Lang, F. Dynamic adhesion of eryptotic erythrocytes to endothelial cells via CXCL16/SR-PSOX. Am. J. Physiol. Cell Physiol. 2012, 302, C644–C651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, J.E.; Steenbergen, R. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 2005, 44, 207–234. [Google Scholar] [CrossRef]
- Tong, D.; Yu, M.; Guo, L.; Li, T.; Li, J.; Novakovic, V.A.; Dong, Z.; Tian, Y.; Kou, J.; Bi, Y.; et al. Phosphatidylserine-exposing blood and endothelial cells contribute to the hypercoagulable state in essential thrombocythemia patients. Ann. Hematol. 2018, 97, 605–616. [Google Scholar] [CrossRef]
- Nicolay, J.P.; Liebig, G.; Niemoeller, O.M.; Koka, S.; Ghashghaeinia, M.; Wieder, T.; Haendeler, J.; Busse, R.; Lang, F. Inhibition of suicidal erythrocyte death by nitric oxide. Pflug. Arch. 2008, 456, 293–305. [Google Scholar] [CrossRef]
- Vota, D.M.; Crisp, R.L.; Nesse, A.B.; Vittori, D.C. Oxidative stress due to aluminum exposure induces eryptosis which is prevented by erythropoietin. J. Cell Biochem. 2012, 113, 1581–1589. [Google Scholar] [CrossRef]
- Wang, L.; Guo, L.; Zhang, L.; Zhou, Y.; He, Q.; Zhang, Z.; Wang, M. Effects of glucose load and nateglinide intervention on endothelial function and oxidative stress. J. Diabetes Res. 2013, 2013, 849295. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Oba, K.; Suzuki, T.; Ouchi, M.; Suzuki, K.; Futami-Suda, S.; Sekimizu, K.; Yamamoto, N.; Nakano, H. Oral glucose loading attenuates endothelial function in normal individual. Eur. J. Clin. Investig. 2011, 41, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Ozuyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eligini, S.; Porro, B.; Lualdi, A.; Squellerio, I.; Veglia, F.; Chiorino, E.; Crisci, M.; Garlasche, A.; Giovannardi, M.; Werba, J.P.; et al. Nitric oxide synthetic pathway in red blood cells is impaired in coronary artery disease. PLoS ONE 2013, 8, e66945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese-Krott, M.M.; Kelm, M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Bermudez-Pirela, V.J.; Cano, C.; Medina, M.T.; Souki, A.; Lemus, M.A.; Leal, E.M.; Seyfi, H.A.; Cano, R.; Ciscek, A.; Bermudez-Arias, F.; et al. Metformin plus low-dose glimeperide significantly improves Homeostasis Model Assessment for insulin resistance (HOMA(IR)) and beta-cell function (HOMA(beta-cell)) without hyperinsulinemia in patients with type 2 diabetes mellitus. Am. J. Ther. 2007, 14, 194–202. [Google Scholar] [CrossRef]
- Squellerio, I.; Tremoli, E.; Cavalca, V. Quantification of arginine and its metabolites in human erythrocytes using liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2011, 412, 108–110. [Google Scholar] [CrossRef]
- Morris, C.R.; Kato, G.J.; Poljakovic, M.; Wang, X.; Blackwelder, W.C.; Sachdev, V.; Hazen, S.L.; Vichinsky, E.P.; Morris, S.M., Jr.; Gladwin, M.T. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 2005, 294, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Sourij, H.; Meinitzer, A.; Pilz, S.; Grammer, T.B.; Winkelmann, B.R.; Boehm, B.O.; Marz, W. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 2011, 218, 220–225. [Google Scholar] [CrossRef]
- Jung, C.; Gonon, A.T.; Sjoquist, P.O.; Lundberg, J.O.; Pernow, J. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc. Res. 2010, 85, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Cavalca, V.; Minardi, F.; Scurati, S.; Guidugli, F.; Squellerio, I.; Veglia, F.; Dainese, L.; Guarino, A.; Tremoli, E.; Caruso, D. Simultaneous quantification of 8-iso-prostaglandin-F(2alpha) and 11-dehydro thromboxane B(2) in human urine by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2010, 397, 168–174. [Google Scholar] [CrossRef]
- Squellerio, I.; Caruso, D.; Porro, B.; Veglia, F.; Tremoli, E.; Cavalca, V. Direct glutathione quantification in human blood by LC-MS/MS: Comparison with HPLC with electrochemical detection. J. Pharm. Biomed. Anal. 2012, 71, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.; Mehlhorn, U.; Krahwinkel, A.; Reiner, M.; Dittrich, M.; Schmidt, A.; Addicks, K. Ischemia increases detectable endothelial nitric oxide synthase in rat and human myocardium. Nitric Oxide 2001, 5, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.M. Red cell distribution width in type 2 diabetic patients. Diabetes Metab. Syndr. Obes. 2015, 8, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalot, F.; Petrelli, A.; Traversa, M.; Bonomo, K.; Fiora, E.; Conti, M.; Anfossi, G.; Costa, G.; Trovati, M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga Diabetes Study. J. Clin. Endocrinol. Metab. 2006, 91, 813–819. [Google Scholar] [CrossRef] [Green Version]
- Siervo, M.; Corander, M.; Stranges, S.; Bluck, L. Post-challenge hyperglycaemia, nitric oxide production and endothelial dysfunction: The putative role of asymmetric dimethylarginine (ADMA). Nutr. Metab. Cardiovasc. Dis. 2011, 21, 1–10. [Google Scholar] [CrossRef]
- Diwadkar, V.A.; Anderson, J.W.; Bridges, S.R.; Gowri, M.S.; Oelgten, P.R. Postprandial low-density lipoproteins in type 2 diabetes are oxidized more extensively than fasting diabetes and control samples. Proc. Soc. Exp. Biol. Med. 1999, 222, 178–184. [Google Scholar] [CrossRef]
- Lemkes, B.A.; Hermanides, J.; Devries, J.H.; Holleman, F.; Meijers, J.C.; Hoekstra, J.B. Hyperglycemia: A prothrombotic factor? J. Thromb. Haemost. 2010, 8, 1663–1669. [Google Scholar] [CrossRef]
- Konukoglu, D.; Firtina, S.; Serin, O. The relationship between plasma asymmetrical dimethyl-L-arginine and inflammation and adhesion molecule levels in subjects with normal, impaired, and diabetic glucose tolerance. Metabolism 2008, 57, 110–115. [Google Scholar] [CrossRef]
- Giugliano, D.; Marfella, R.; Coppola, L.; Verrazzo, G.; Acampora, R.; Giunta, R.; Nappo, F.; Lucarelli, C.; D’Onofrio, F. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation 1997, 95, 1783–1790. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.W.; Tomlinson, B.; Benzie, I.F. A study of the effect of oral glucose loading on plasma oxidant:antioxidant balance in normal subjects. Eur. J. Nutr. 2005, 44, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Chakarova, N.; Grozeva, G.; Kirilov, G.; Tankova, T. The relationship between glucose variability and insulin sensitivity and oxidative stress in subjects with prediabetes. Diabetes Res. Clin. Pract. 2019, 158, 107911. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Xiang, G.D.; Sun, H.L.; Hou, J.; Yue, L.; Xu, L. Acute hyperglycemia rapidly suppresses endothelium-dependent arterial dilation in first-degree relatives of type 2 diabetic patients. Exp. Clin. Endocrinol. Diabetes 2008, 116, 112–117. [Google Scholar] [CrossRef]
- Wieczor, R.; Wieczor, A.M.; Kulwas, A.; Rosc, D. ADMA (asymmetric dimethylarginine) and angiogenic potential in patients with type 2 diabetes and prediabetes. Exp. Biol. Med. 2021, 246, 153–162. [Google Scholar] [CrossRef]
- Lin, K.Y.; Ito, A.; Asagami, T.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Tsuji, H.; Reaven, G.M.; Cooke, J.P. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002, 106, 987–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, M.; Guillemette, J.G.; Dieckmann, T. Dynamics of nitric oxide synthase-calmodulin interactions at physiological calcium concentrations. Biochemistry 2015, 54, 1989–2000. [Google Scholar] [CrossRef]
- Bor-Kucukatay, M.; Wenby, R.B.; Meiselman, H.J.; Baskurt, O.K. Effects of nitric oxide on red blood cell deformability. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1577–H1584. [Google Scholar] [CrossRef] [Green Version]
- Horn, P.; Cortese-Krott, M.M.; Keymel, S.; Kumara, I.; Burghoff, S.; Schrader, J.; Kelm, M.; Kleinbongard, P. Nitric oxide influences red blood cell velocity independently of changes in the vascular tone. Free Radic. Res. 2011, 45, 653–661. [Google Scholar] [CrossRef]
- Kanias, T.; Wang, L.; Lippert, A.; Kim-Shapiro, D.B.; Gladwin, M.T. Red blood cell endothelial nitric oxide synthase does not modulate red blood cell storage hemolysis. Transfusion 2013, 53, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Arese, P.; Turrini, F.; Schwarzer, E. Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes. Cell Physiol. Biochem. 2005, 16, 133–146. [Google Scholar] [CrossRef]
- Bosman, G.J.; Willekens, F.L.; Werre, J.M. Erythrocyte aging: A more than superficial resemblance to apoptosis? Cell Physiol. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, E.; Qadri, S.M.; Lang, F. Killing me softly—Suicidal erythrocyte death. Int. J. Biochem Cell Biol 2012, 44, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Romana, M.; Guillot, N.; Fort, R.; Stauffer, E.; Lemonne, N.; Garnier, Y.; Skinner, S.C.; Etienne-Julan, M.; Robert, M.; et al. Association Between Nitric Oxide, Oxidative Stress, Eryptosis, Red Blood Cell Microparticles, and Vascular Function in Sickle Cell Anemia. Front. Immunol. 2020, 11, 551441. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Nikolaidou, B.; Gavriilaki, E.; Lazaridis, A.; Yiannaki, E.; Anyfanti, P.; Zografou, I.; Markala, D.; Douma, S. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2019, 16, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zalawadiya, S.K.; Veeranna, V.; Niraj, A.; Pradhan, J.; Afonso, L. Red cell distribution width and risk of coronary heart disease events. Am. J. Cardiol. 2010, 106, 988–993. [Google Scholar] [CrossRef]
- Chen, P.C.; Sung, F.C.; Chien, K.L.; Hsu, H.C.; Su, T.C.; Lee, Y.T. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am. J. Epidemiol. 2010, 171, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Sacks, F.; Arnold, M.; Moye, L.; Davis, B.; Pfeffer, M.; for the Cholesterol Recurrent Events Trial Investigators. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People with Coronary Disease. Circulation 2008, 117, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Tripolino, C.; Irace, C.; Carallo, C.; De Franceschi, M.S.; Scavelli, F.B.; Gnasso, A. Red blood cell distribution width predicts two-hours plasma glucose levels during OGTT. Clin. Hemorheol. Microcirc. 2016, 62, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Hazegh, K.; Fang, F.; Bravo, M.D.; Tran, J.Q.; Muench, M.O.; Jackman, R.P.; Roubinian, N.; Bertolone, L.; D’Alessandro, A.; Dumont, L.; et al. Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion 2021, 61, 435–448. [Google Scholar] [CrossRef] [PubMed]
| Variables | Prediabetic Patients (n = 18) |
|---|---|
| Age (years) | 64.1 ± 5.6 |
| Male sex, n (%) | 18 (100) |
| BMI (kg/m2) | 26.3 ± 2.6 |
| Abdominal circumference (cm) | 93.6 ± 9.81 |
| Central obesity, n (%) | 3 (17) |
| Metabolic syndrome, n (%) | 11 (61) |
| Systolic blood pressure (mmHg) | 129 ± 10 |
| Diastolic blood pressure (mmHg) | 75 ± 8 |
| Total cholesterol (mmol/L) | 4.3 ± 1.0 |
| LDL cholesterol (mmol/L) | 2.7 ± 0.9 |
| HDL cholesterol (mmol/L) | 1.1 ± 0.3 |
| Triglycerides (mmol/L) | 1.1 ± 0.5 |
| Creatinine (μmol/L) | 77 ± 14 |
| HOMA-IR | 2.68 ± 1.00 |
| HOMA-β-cell | 66.69 (47.6; 94.14) |
| RDW-SD (fL) | 40.9 ± 2.9 |
| Pharmacological treatments | |
| Aspirin, n (%) | 11 (61) |
| Beta-blockers, n (%) | 2 (11) |
| Calcium blockers, n (%) | 8 (44) |
| Angiotensin converting enzyme inhibitors, n (%) | 2 (11) |
| Angiotensin II receptor antagonists, n (%) | 4 (22) |
| Statins, n (%) | 11 (61) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eligini, S.; Porro, B.; Werba, J.P.; Capra, N.; Genovese, S.; Greco, A.; Cavalca, V.; Banfi, C. Oxidative Stress and Arginine/Nitric Oxide Pathway in Red Blood Cells Derived from Patients with Prediabetes. Biomedicines 2022, 10, 1407. https://doi.org/10.3390/biomedicines10061407
Eligini S, Porro B, Werba JP, Capra N, Genovese S, Greco A, Cavalca V, Banfi C. Oxidative Stress and Arginine/Nitric Oxide Pathway in Red Blood Cells Derived from Patients with Prediabetes. Biomedicines. 2022; 10(6):1407. https://doi.org/10.3390/biomedicines10061407
Chicago/Turabian StyleEligini, Sonia, Benedetta Porro, José Pablo Werba, Nicolò Capra, Stefano Genovese, Arianna Greco, Viviana Cavalca, and Cristina Banfi. 2022. "Oxidative Stress and Arginine/Nitric Oxide Pathway in Red Blood Cells Derived from Patients with Prediabetes" Biomedicines 10, no. 6: 1407. https://doi.org/10.3390/biomedicines10061407
APA StyleEligini, S., Porro, B., Werba, J. P., Capra, N., Genovese, S., Greco, A., Cavalca, V., & Banfi, C. (2022). Oxidative Stress and Arginine/Nitric Oxide Pathway in Red Blood Cells Derived from Patients with Prediabetes. Biomedicines, 10(6), 1407. https://doi.org/10.3390/biomedicines10061407








