TGFβ1 Induces Senescence and Attenuated VEGF Production in Retinal Pericytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents
2.3. Senescence Assay
2.4. MTT Assay
2.5. LDH Assay
2.6. Luminex Assay
2.7. QPCR
2.8. ELISA
2.9. TGFβ-Injected Mice
2.10. Fixation and Paraffin Embedding for Histology
2.11. ISH Staining
2.12. Statistical Analysis
3. Results
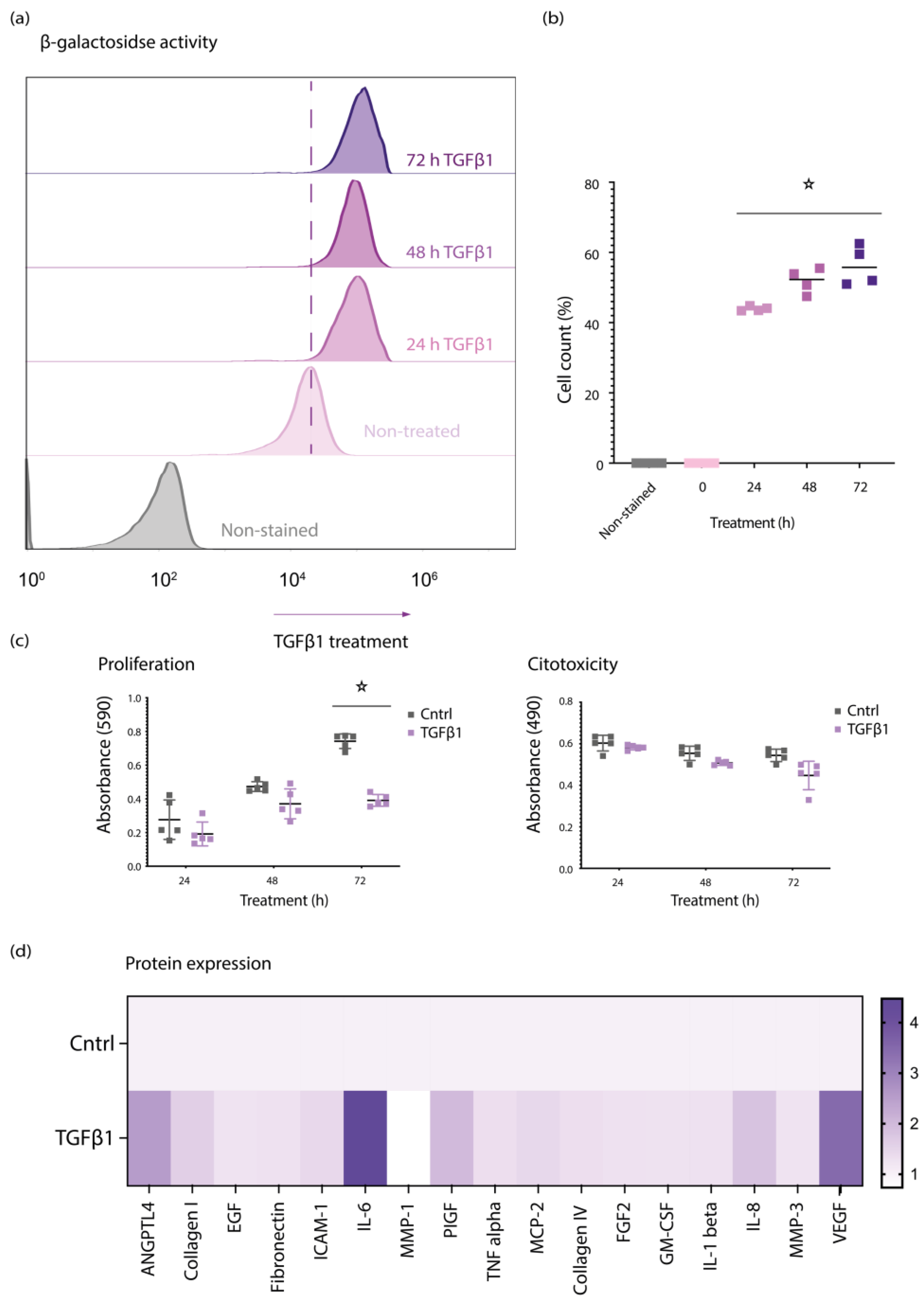
3.1. TGFβ1 Induces Senescent Features in Primary Retinal Pericytes
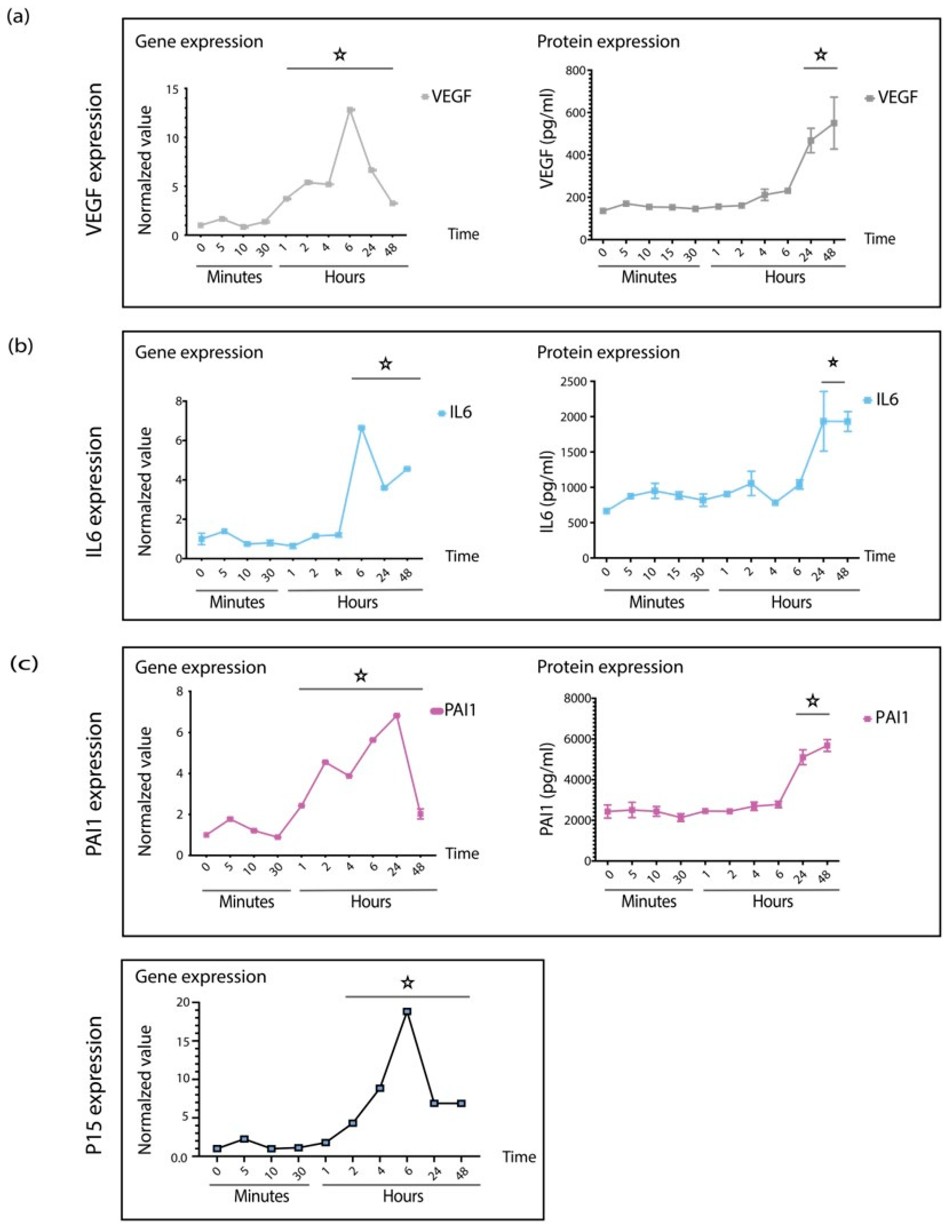
3.2. TGFβ1 Regulates na SASP, p15, and PAI1 in Senescent Retinal Pericytes
3.3. TGFβ1 Induces VEGF Expression in Mice Retina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, S.E.; Klein, R.; Klein, B.E. The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998, 105, 998–1003. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef]
- Anderson, B., Jr.; Saltzman, H.A. Retinal oxygen utilization measured by hyperbaric blackout. Arch. Ophthalmol. 1964, 72, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.; Fruttiger, M. Keeping blood vessels out of sight. eLife 2013, 2, e00948. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Cogan, D.G.; Toussaint, D.; Kuwabara, T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch. Ophthalmol. 1961, 66, 366–378. [Google Scholar] [CrossRef]
- Speiser, P.; Gittelsohn, A.M.; Patz, A. Studies on diabetic retinopathy. 3. Influence of diabetes on intramural pericytes. Arch. Ophthalmol. 1968, 80, 332–337. [Google Scholar] [CrossRef]
- Beltramo, E.; Porta, M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P. Pericytes and the pathogenesis of diabetic retinopathy. Horm. Metab. Res. 2005, 37 (Suppl. S1), 39–43. [Google Scholar] [CrossRef] [PubMed]
- Engerman, R.L. Pathogenesis of diabetic retinopathy. Diabetes 1989, 38, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Wong, J.-S. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int. 2000, 58, S113–S119. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Yao, Y.; Li, R.; Du, J.; Long, L.; Li, X.; Luo, N. Interleukin-6 and Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Curr. Eye Res. 2019, 44, 564–574. [Google Scholar] [CrossRef]
- Sharma, S. Interleukin-6 Trans-signaling: A Pathway with Therapeutic Potential for Diabetic Retinopathy. Front. Physiol. 2021, 12, 689429. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alibrandi, S.; Vadalà, M.; Giglia, G.; Sidoti, A.; D’Angelo, R. N-retinylidene-N-retinylethanolamine adduct induces expression of chronic inflammation cytokines in retinal pigment epithelium cells. Exp. Eye Res. 2021, 209, 108641. [Google Scholar] [CrossRef]
- Rinaldi, C.; Donato, L.; Alibrandi, S.; Scimone, C.; D’Angelo, R.; Sidoti, A. Oxidative Stress and the Neurovascular Unit. Life 2021, 11, 767. [Google Scholar] [CrossRef]
- Yun, J.H.; Park, S.W.; Kim, K.J.; Bae, J.S.; Lee, E.H.; Paek, S.H.; Kim, S.U.; Ye, S.; Kim, J.H.; Cho, C.H. Endothelial STAT3 Activation Increases Vascular Leakage Through Downregulating Tight Junction Proteins: Implications for Diabetic Retinopathy. J. Cell. Physiol. 2017, 232, 1123–1134. [Google Scholar] [CrossRef]
- Yener, S.; Comlekci, A.; Akinci, B.; Akan, P.; Demir, T.; Bayraktar, F.; Yesil, S. Serum transforming growth factor-beta 1 levels in normoalbuminuric and normotensive patients with type 2 diabetes. Effect of metformin and rosiglitazone. Hormones 2008, 7, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, V.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Pizzo, C.; Reibaldi, M.; Russo, A.; Fallico, M.; Ortisi, E.; Pignatelli, F.; et al. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. Int. J. Mol. Sci. 2020, 21, 9558. [Google Scholar] [CrossRef] [PubMed]
- McGowan, T.A.; Dunn, S.R.; Falkner, B.; Sharma, K. Stimulation of urinary TGF-beta and isoprostanes in response to hyperglycemia in humans. Clin. J. Am. Soc. Nephrol. 2006, 1, 263–268. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakamura, T.; Noble, N.A.; Ruoslahti, E.; Border, W.A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1993, 90, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.B.; Sharma, K.; Zhu, Y.; Ziyadeh, F.N. Transcriptional activation of transforming growth factor-beta1 in mesangial cell culture by high glucose concentration. Kidney Int. 1998, 54, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.B.; Andreoli, T.E. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2000, 97, 7667–7669. [Google Scholar] [CrossRef]
- Mou, X.; Zhou, D.Y.; Zhou, D.Y.; Ma, J.R.; Liu, Y.H.; Chen, H.P.; Hu, Y.B.; Shou, C.M.; Chen, J.W.; Liu, W.H.; et al. Serum TGF-β1 as a Biomarker for Type 2 Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0149513. [Google Scholar] [CrossRef]
- Akai, Y.; Sato, H.; Ozaki, H.; Iwano, M.; Dohi, Y.; Kanauchi, M. Association of transforming growth factor-beta1 T29C polymorphism with the progression of diabetic nephropathy. Am. J. Kidney Dis. 2001, 38 (Suppl. S1), S182–S185. [Google Scholar] [CrossRef]
- Akhurst, R.J. TGF beta signaling in health and disease. Nat. Genet. 2004, 36, 790–792. [Google Scholar] [CrossRef]
- Massagué, J.; Chen, Y.G. Controlling TGF-beta signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar] [CrossRef]
- Wrighton, K.H.; Lin, X.; Feng, X.-H. Phospho-control of TGF-β superfamily signaling. Cell Res. 2009, 19, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Saharinen, J.; Keski-Oja, J. Extracellular Matrix-Associated Transforming Growth Factor-β: Role in Cancer Cell Growth and Invasion. In Advances in Cancer Research; Vande Woude, G.F., Klein, G., Eds.; Academic Press: Cambridge, MA, USA, 1998; Volume 75, pp. 87–134. [Google Scholar]
- Wang, H.-L.; Wang, L.; Zhao, C.-Y.; Lan, H.-Y. Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes. Biomolecules 2022, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Prud’homme, G.J. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Invest. 2007, 87, 1077–1091. [Google Scholar] [CrossRef]
- Massagué, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef]
- Senturk, S.; Mumcuoglu, M.; Gursoy-Yuzugullu, O.; Cingoz, B.; Akcali, K.C.; Ozturk, M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 2010, 52, 966–974. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Kumar, S.; Sharife, H.; Volinsky, E.; Gileles-Hillel, A.; Licht, T.; Permyakova, A.; Hinden, L.; Azar, S.; Friedmann, Y.; et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 2021, 373, eabc8479. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C. Obesity, insulin resistance and diabetes—A worldwide epidemic. Br. J. Nutr. 2000, 83 (Suppl. S1), S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.V.; Chen, Y.; Goldfarb, S.; Ziyadeh, F.N. Elevated glucose stimulates TGF-β gene expression and bioactivity in proximal tubule. Kidney Int. 1992, 41, 107–114. [Google Scholar] [CrossRef]
- Sharma, K.; Jin, Y.; Guo, J.; Ziyadeh, F.N. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996, 45, 522–530. [Google Scholar] [CrossRef]
- Kolm-Litty, V.; Sauer, U.; Nerlich, A.; Lehmann, R.; Schleicher, E.D. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Invest. 1998, 101, 160–169. [Google Scholar] [CrossRef]
- Hong, S.W.; Isono, M.; Chen, S.; Iglesias-De La Cruz, M.C.; Han, D.C.; Ziyadeh, F.N. Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am. J. Pathol. 2001, 158, 1653–1663. [Google Scholar] [CrossRef]
- Gao, C.; Lin, X.; Fan, F.; Liu, X.; Wan, H.; Yuan, T.; Zhao, X.; Luo, Y. Status of higher TGF-β1 and TGF-β2 levels in the aqueous humour of patients with diabetes and cataracts. BMC Ophthalmol. 2022, 22, 156. [Google Scholar] [CrossRef]
- Khuu, L.-A.; Tayyari, F.; Sivak, J.M.; Flanagan, J.G.; Singer, S.; Brent, M.H.; Huang, D.; Tan, O.; Hudson, C. Aqueous humour concentrations of TGF-β, PLGF and FGF-1 and total retinal blood flow in patients with early non-proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, e206–e211. [Google Scholar] [CrossRef]
- Saucedo, L.; Pfister, I.B.; Zandi, S.; Gerhardt, C.; Garweg, J.G. Ocular TGF-β, Matrix Metalloproteinases, and TIMP-1 Increase with the Development and Progression of Diabetic Retinopathy in Type 2 Diabetes Mellitus. Mediators Inflamm. 2021, 2021, 9811361. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Malinowska, E.; Raczyńska, D.; Myśliwiec, M.; Raczyńska, K. Serum concentrations of transforming growth factor-Beta 1 in predicting the occurrence of diabetic retinopathy in juvenile patients with type 1 diabetes mellitus. J. Diabetes Res. 2013, 2013, 614908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braunger, B.M.; Leimbeck, S.V.; Schlecht, A.; Volz, C.; Jägle, H.; Tamm, E.R. Deletion of Ocular Transforming Growth Factor β Signaling Mimics Essential Characteristics of Diabetic Retinopathy. Am. J. Pathol. 2015, 185, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Neri, G.; Caldi, E.; Fusco, F.; Bacci, T.; Tarantello, A.; Nuti, E.; Marigliani, D.; Baiocchi, S.; Traversi, C.; et al. TGF-β concentrations and activity are down-regulated in the aqueous humor of patients with neovascular age-related macular degeneration. Sci. Rep. 2018, 8, 8053. [Google Scholar] [CrossRef] [PubMed]
- Kvanta, A. Expression and secretion of transforming growth factor-beta in transformed and nontransformed retinal pigment epithelial cells. Ophthalmic. Res. 1994, 26, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Yoshida, M.; Matsumoto, M.; Yoshimura, N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1993, 34, 413–419. [Google Scholar]
- Baird, A.; Durkin, T. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: Interactions with acidic and basic fibroblast growth factors. Biochem. Biophys Res. Commun. 1986, 138, 476–482. [Google Scholar] [CrossRef]
- Nakagawa, T.; Li, J.H.; Garcia, G.; Mu, W.; Piek, E.; Böttinger, E.P.; Chen, Y.; Zhu, H.J.; Kang, D.H.; Schreiner, G.F.; et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004, 66, 605–613. [Google Scholar] [CrossRef]
- Donovan, J.; Slingerland, J. Transforming growth factor-beta and breast cancer: Cell cycle arrest by transforming growth factor-beta and its disruption in cancer. Breast Cancer Res. BCR 2000, 2, 116–124. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, P.; Chen, W.; Lu, L.; Zheng, Z. ANGPTL-4 induces diabetic retinal inflammation by activating Profilin-1. Exp. Eye Res. 2018, 166, 140–150. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; De Falco, S.; Behar-Cohen, F.; Lam, W.C.; Li, X.; Reichhart, N.; Ricci, F.; Pluim, J.; Li, W.W. Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol. 2018, 96, e1–e9. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramovic, D.; Archaimbault, S.A.; Kemble, A.M.; Gruener, S.; Lazendic, M.; Westenskow, P.D. TGFβ1 Induces Senescence and Attenuated VEGF Production in Retinal Pericytes. Biomedicines 2022, 10, 1404. https://doi.org/10.3390/biomedicines10061404
Avramovic D, Archaimbault SA, Kemble AM, Gruener S, Lazendic M, Westenskow PD. TGFβ1 Induces Senescence and Attenuated VEGF Production in Retinal Pericytes. Biomedicines. 2022; 10(6):1404. https://doi.org/10.3390/biomedicines10061404
Chicago/Turabian StyleAvramovic, Dragana, Sébastien A. Archaimbault, Alicia M. Kemble, Sabine Gruener, Mirjana Lazendic, and Peter D. Westenskow. 2022. "TGFβ1 Induces Senescence and Attenuated VEGF Production in Retinal Pericytes" Biomedicines 10, no. 6: 1404. https://doi.org/10.3390/biomedicines10061404
APA StyleAvramovic, D., Archaimbault, S. A., Kemble, A. M., Gruener, S., Lazendic, M., & Westenskow, P. D. (2022). TGFβ1 Induces Senescence and Attenuated VEGF Production in Retinal Pericytes. Biomedicines, 10(6), 1404. https://doi.org/10.3390/biomedicines10061404




