An Integrative Analysis of DNA Methylation Pattern in Myotonic Dystrophy Type 1 Samples Reveals a Distinct DNA Methylation Profile between Tissues and a Novel Muscle-Associated Epigenetic Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Registry
2.2. Tissue and Cell Culture
2.3. DNA Isolation
2.4. CTG Expansion Size Analysis
2.5. Bisulphite Treatment and Sanger Sequencing of Four CpG Islands
2.6. Statistical Analysis
3. Results
3.1. A Study Cohort Encompassing All Clinical Subtypes of DM1
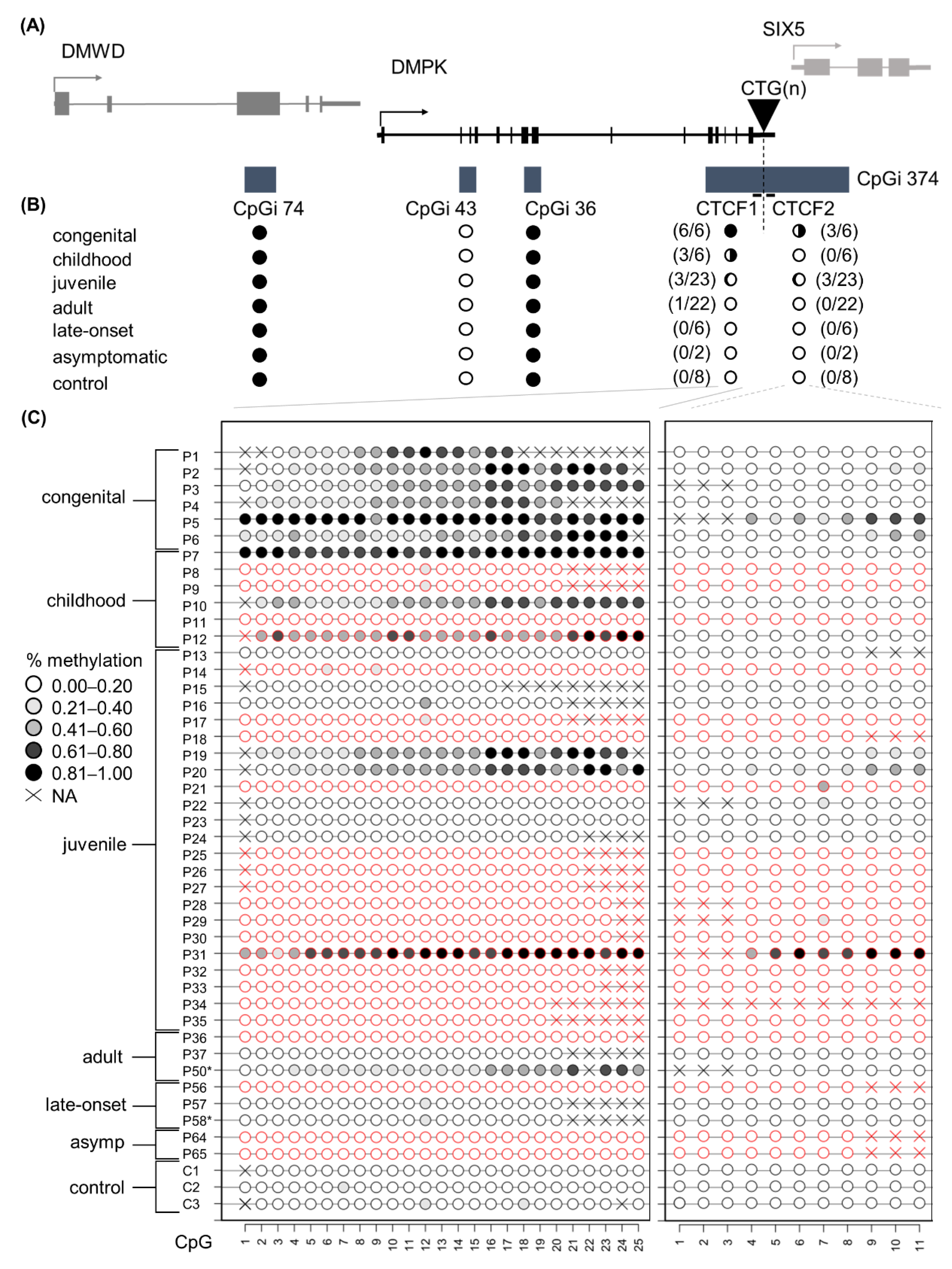
3.2. DNA Methylation Profiles across the DMPK Locus in Blood
3.3. Aberrant DNA Methylation Profiles of CTCF1 Associated with Higher Disease Severity in Childhood Cases
3.4. A Higher Chance of Methylation in CTCF1 with Increasing CTG Expansion Size
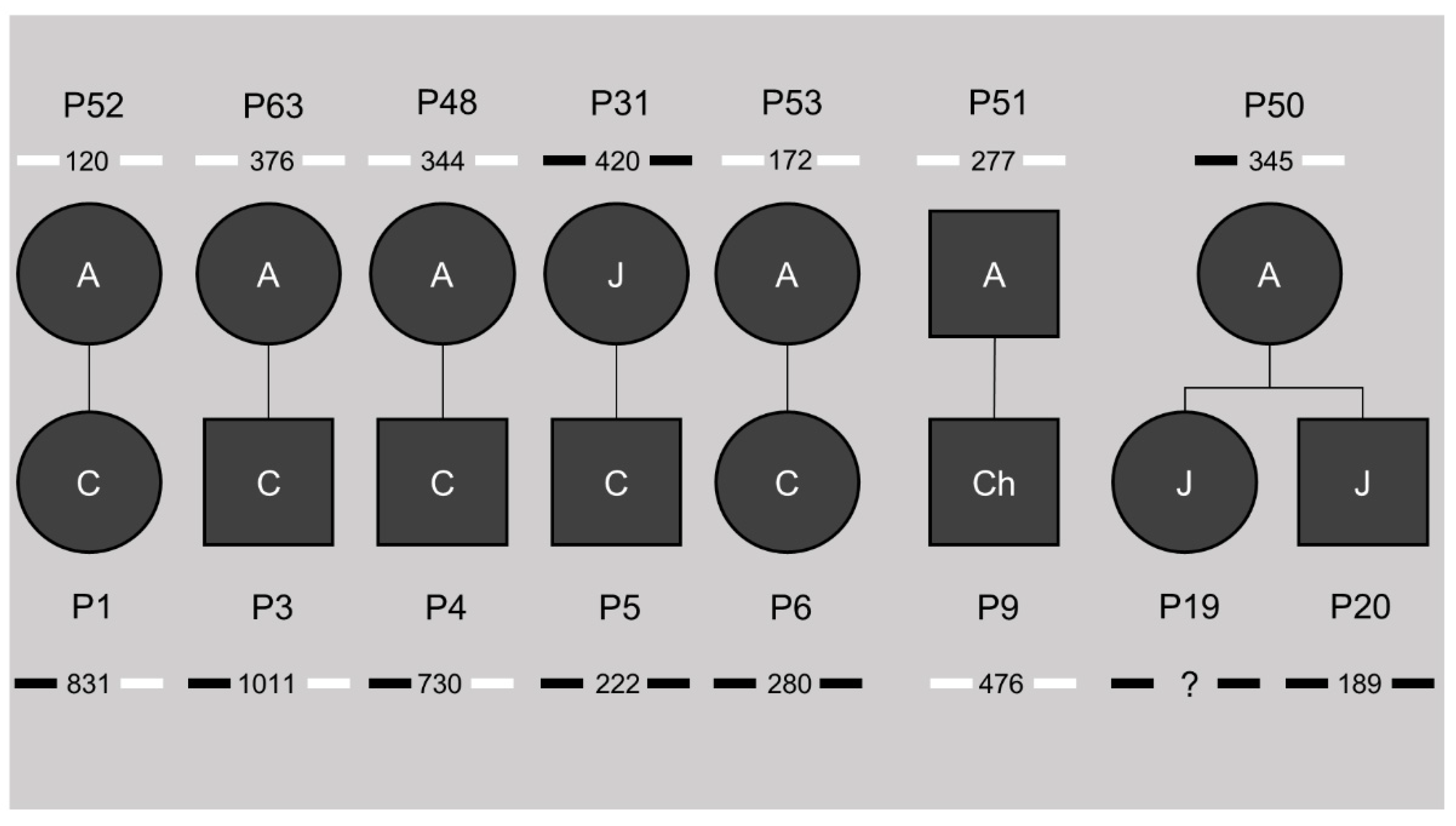
3.5. Increased, but Not Exclusive, Maternal Transmission in CTCF1 Methylated Cases
3.6. Methylation Status Is Not Inheritable and Associated with the Transmission of CTG Repeat Contractions
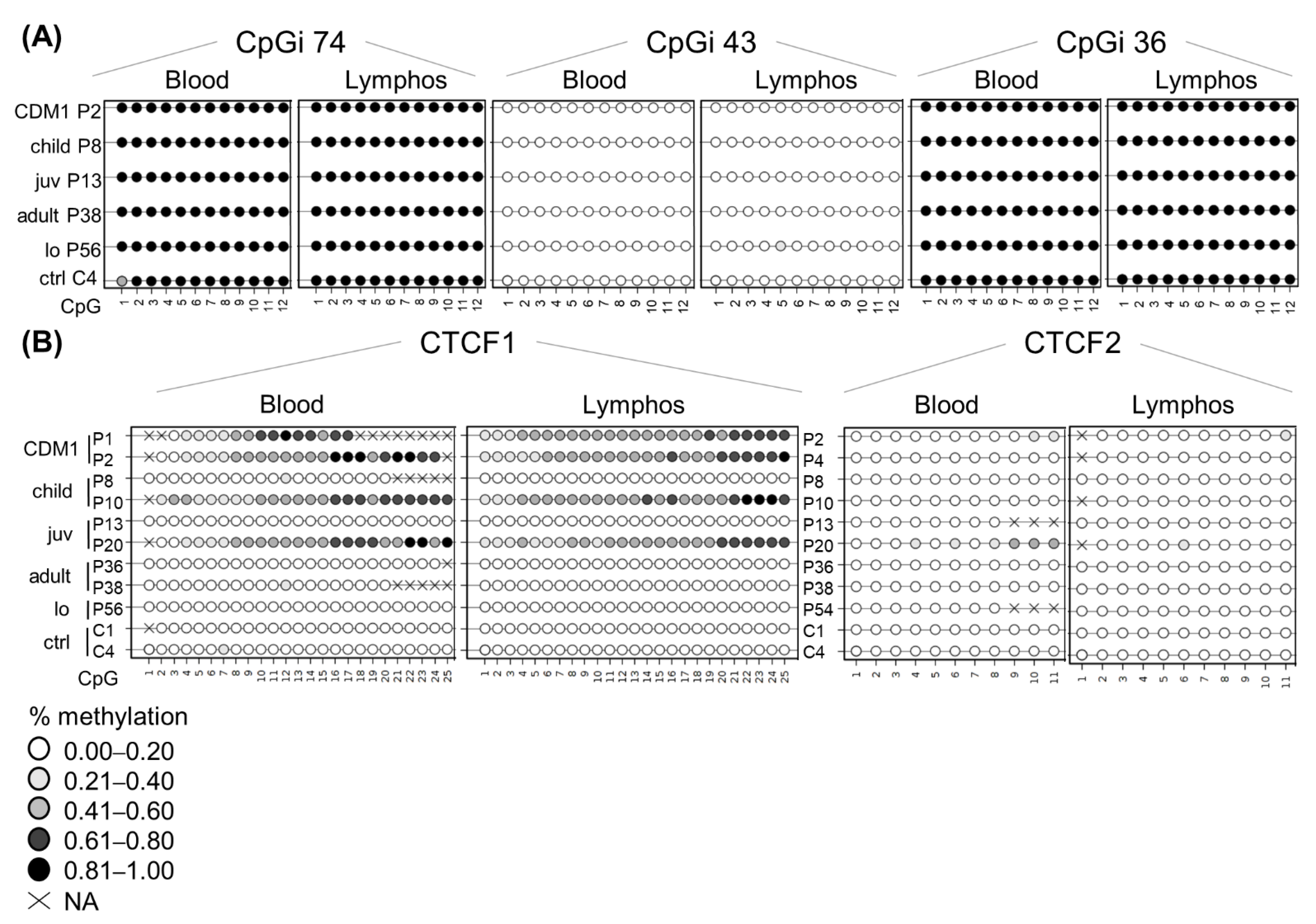
3.7. DNA Methylation Profiles Are Preserved in Blood-Derived Cells
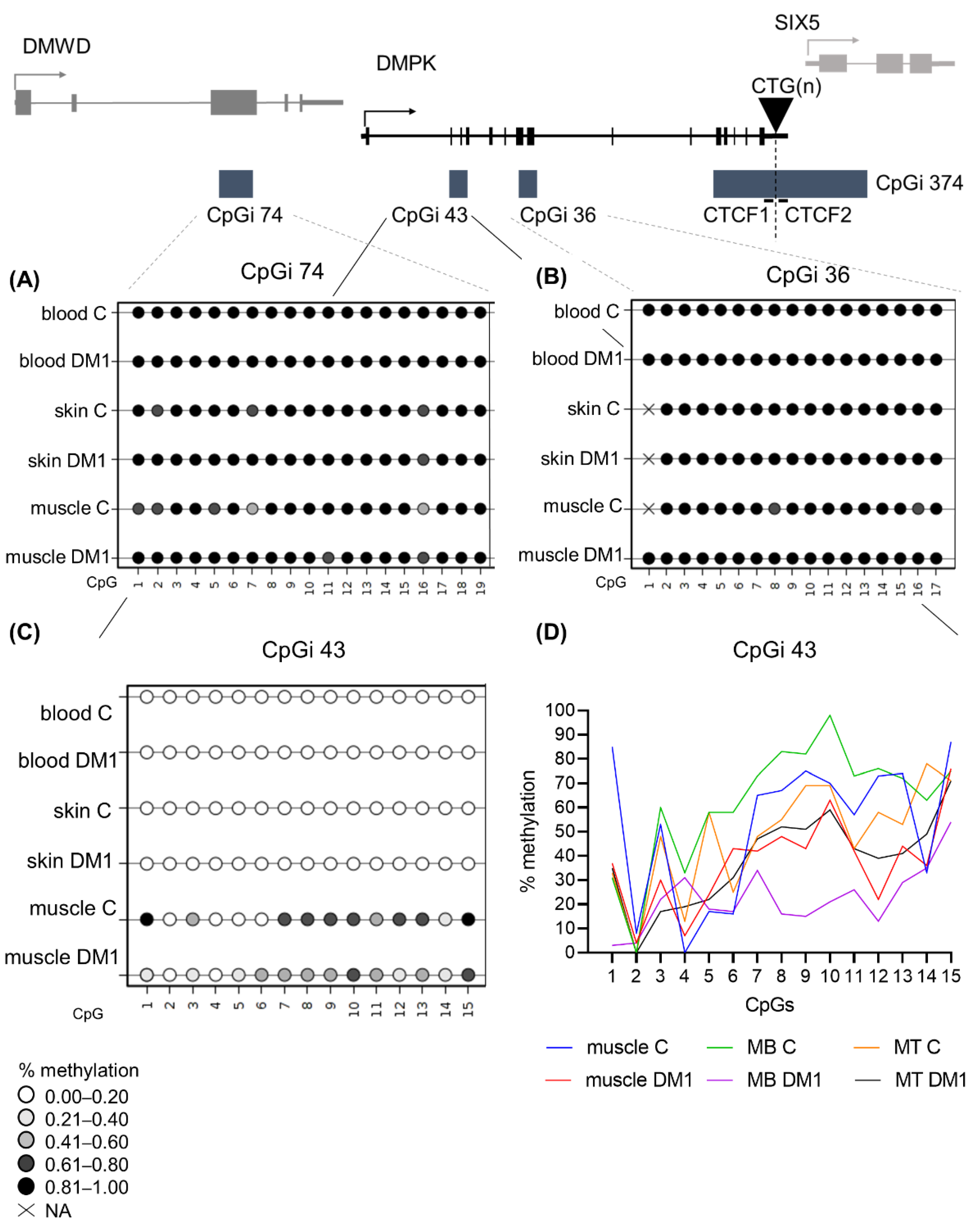
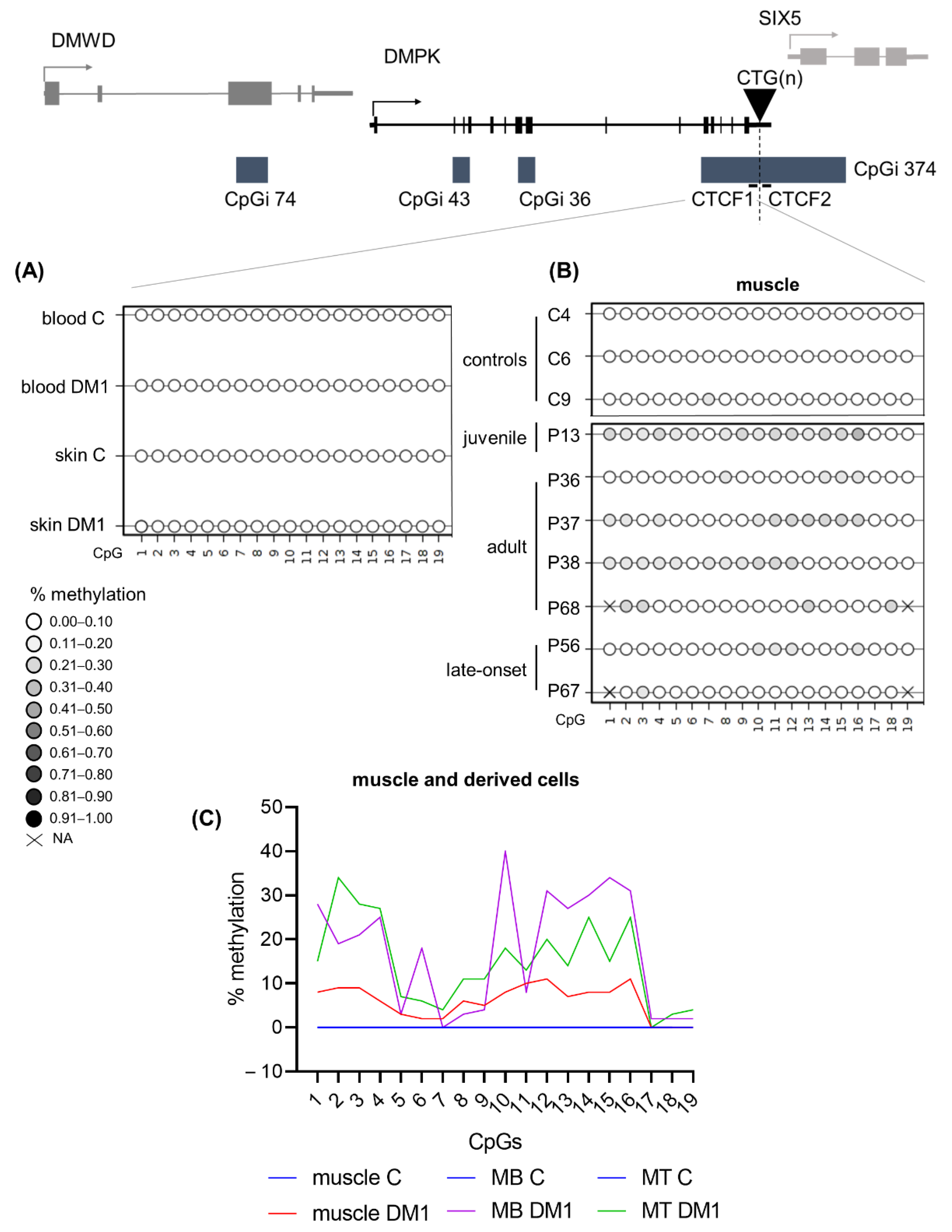
3.8. DM1 Is Associated with Hypomethylation of CpGi 43 in Muscle Tissue and Muscle-Derived Cells
3.9. DM1 Is Associated with Hypermethylation in CTCF1 in Muscle Tissue and Muscle Derived Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Harper, P.S. Major Problems in Neurology: Myotonic Dystrophy, 3rd ed.; WB Saunders: London, UK, 2001. [Google Scholar]
- De Antonio, M.; Dogan, C.; Hamroun, D.; Mati, M.; Zerrouki, S.; Eymard, B.; Katsahian, S.; Bassez, G. French Myotonic Dystrophy Clinical Network Unravelling the myotonic dystrophy type 1 clinical spectrum: A systematic registry-based study with implications for disease classification. Rev. Neurol. 2016, 172, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Lanni, S.; Pearson, C.E. Molecular genetics of congenital myotonic dystrophy. Neurobiol. Dis. 2019, 132, 104533. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Sherlock, R.; Jacob, P.; Blayney, M. Congenital myotonic dystrophy: Assisted ventilation duration and outcome. Pediatrics 2004, 113, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Meola, G.; Cardani, R. Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Douniol, M.; Jacquette, A.; Cohen, D.; Bodeau, N.; Rachidi, L.; Angeard, N.; Cuisset, J.-M.; Vallée, L.; Eymard, B.; Plaza, M.; et al. Psychiatric and cognitive phenotype of childhood myotonic dystrophy type 1. Dev. Med. Child Neurol. 2012, 54, 905–911. [Google Scholar] [CrossRef]
- Echenne, B.; Bassez, G. Congenital and infantile myotonic dystrophy. Handb. Clin. Neurol. 2013, 113, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Carey, K.A.; Cardamone, M.; Farrar, M.A. Myotonic dystrophy type 1: Clinical manifestations in children and adolescents. Arch. Dis. Child. 2019, 104, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Allard, P.; Potvin, L.; Prévost, C.; Bégin, P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology 1999, 52, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- De Die-Smulders, C.E.; Höweler, C.J.; Thijs, C.; Mirandolle, J.F.; Anten, H.B.; Smeets, H.J.; Chandler, K.E.; Geraedts, J.P. Age and causes of death in adult-onset myotonic dystrophy. Brain 1998, 121, 1557–1563. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H. Myotonic Dystrophy Type 1. In Atlas of Genetic Diagnosis and Counseling; Springer: Cham, Switzerland, 2017; pp. 1999–2011. [Google Scholar]
- Groh, W.J.; Groh, M.R.; Saha, C.; Kincaid, J.C.; Simmons, Z.; Ciafaloni, E.; Pourmand, R.; Otten, R.F.; Bhakta, D.; Nair, G.V.; et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N. Engl. J. Med. 2008, 358, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Carrió, E.; Díez-Villanueva, A.; Lois, S.; Mallona, I.; Cases, I.; Forn, M.; Peinado, M.A.; Suelves, M. Deconstruction of DNA methylation patterns during myogenesis reveals specific epigenetic events in the establishment of the skeletal muscle lineage. Stem Cells 2015, 33, 2025–2036. [Google Scholar] [CrossRef]
- Reik, W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 2007, 447, 425–432. [Google Scholar] [CrossRef]
- Gardiner-Garden, M.; Frommer, M. CpG islands in vertebrate genomes. J. Mol. Biol. 1987, 196, 261–282. [Google Scholar] [CrossRef]
- Bird, A.P. CpG-rich islands and the function of DNA methylation. Nature 1986, 321, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.M.; Bird, A. CpG islands and the regulation of transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef]
- Filippova, G.N.; Thienes, C.P.; Penn, B.H.; Cho, D.H.; Hu, Y.J.; Moore, J.M.; Klesert, T.R.; Lobanenkov, V.V.; Tapscott, S.J. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat. Genet. 2001, 28, 335–343. [Google Scholar] [CrossRef]
- Barbé, L.; Lanni, S.; López-Castel, A.; Franck, S.; Spits, C.; Keymolen, K.; Seneca, S.; Tomé, S.; Miron, I.; Letourneau, J.; et al. CpG Methylation, a Parent-of-Origin Effect for Maternal-Biased Transmission of Congenital Myotonic Dystrophy. Am. J. Hum. Genet. 2017, 100, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.R.; Huguet, A.; Nicole, A.; Munnich, A.; Gourdon, G. Transcriptionally Repressive Chromatin Remodelling and CpG Methylation in the Presence of Expanded CTG-Repeats at the DM1 Locus. J. Nucleic Acids 2013, 2013, 567435. [Google Scholar] [CrossRef]
- Spits, C.; Seneca, S.; Hilven, P.; Liebaers, I.; Sermon, K. Methylation of the CpG sites in the myotonic dystrophy locus does not correlate with CTG expansion size or with the congenital form of the disease. J. Med. Genet. 2010, 47, 700–703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breton, É.; Légaré, C.; Overend, G.; Guay, S.P.; Monckton, D.; Mathieu, J.; Gagnon, C.; Richer, L.; Gallais, B.; Bouchard, L. DNA methylation at the DMPK gene locus is associated with cognitive functions in myotonic dystrophy type 1. Epigenomics 2020, 12, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Hildonen, M.; Knak, K.L.; Dunø, M.; Vissing, J.; Tümer, Z. Stable Longitudinal Methylation Levels at the CpG Sites Flanking the CTG Repeat of DMPK in Patients with Myotonic Dystrophy Type 1. Genes 2020, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Vásquez, M.; Corrales, E.; Vindas-Smith, R.; Santamaría-Ulloa, C.; Zhang, B.; Sirito, M.; Estecio, M.R.; Krahe, R.; Monckton, D.G. Longitudinal increases in somatic mosaicism of the expanded CTG repeat in myotonic dystrophy type 1 are associated with variation in age-at-onset. Hum. Mol. Genet. 2020, 29, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.; Corrales, E.; Zhang, B.; Vásquez, M.; Santamaría-Ulloa, C.; Quesada, H.; Sirito, M.; Estecio, M.R.; Monckton, D.G.; Krahe, R. Myotonic dystrophy type 1 (DM1) clinical sub-types and CTCF site methylation status flanking the CTG expansion are mutant allele length-dependent. Hum. Mol. Genet. 2021, 31, 262–274. [Google Scholar] [CrossRef]
- Santoro, M.; Fontana, L.; Masciullo, M.; Bianchi, M.L.E.; Rossi, S.; Leoncini, E.; Novelli, G.; Botta, A.; Silvestri, G. Expansion size and presence of CCG/CTC/CGG sequence interruptions in the expanded CTG array are independently associated to hypermethylation at the DMPK locus in myotonic dystrophy type 1 (DM1). Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 2645–2652. [Google Scholar] [CrossRef]
- Steinbach, P.; Gläser, D.; Vogel, W.; Wolf, M.; Schwemmle, S. The DMPK gene of severely affected myotonic dystrophy patients is hypermethylated proximal to the largely expanded CTG repeat. Am. J. Hum. Genet. 1998, 62, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky-Dagan, S.; Avitzour, M.; Altarescu, G.; Renbaum, P.; Eldar-Geva, T.; Schonberger, O.; Mitrani-Rosenbaum, S.; Levy-Lahad, E.; Birnbaum, R.Y.; Gepstein, L.; et al. Uncovering the Role of Hypermethylation by CTG Expansion in Myotonic Dystrophy Type 1 Using Mutant Human Embryonic Stem Cells. Stem Cell Rep. 2015, 5, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.; Lacey, M.; Ehrlich, M. Epigenetics of the myotonic dystrophy-associated DMPK gene neighborhood. Epigenomics 2016, 8, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Lopez, A.; Linares-Pardo, I.; Koehorst, E.; Núñez-Manchón, J.; Pintos-Morell, G.; Coll-Cantí, J.; Almendrote, M.; Lucente, G.; Arbex, A.; Magaña, J.J.; et al. The need for establishing a universal CTG sizing method in myotonic dystrophy type 1. Genes 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Koehorst, E.; Núñez-manchón, J.; Ballester-lópez, A.; Almendrote, M.; Lucente, G.; Arbex, A.; Chojnacki, J.; Vázquez-manrique, R.P.; Gómez-escribano, A.P.; Pintos-morell, G.; et al. Characterization of RAN Translation and Antisense Transcription in Primary Cell Cultures of Patients with Myotonic Dystrophy Type 1. J. Clin. Med. 2021, 10, 5520. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Carrió, E.; Magli, A.; Muñoz, M.; Peinado, M.A.; Perlingeiro, R.; Suelves, M. Muscle cell identity requires Pax7-mediated lineage-specific DNA demethylation. BMC Biol. 2016, 14, 30. [Google Scholar] [CrossRef]
- Mallona, I.; Díez-Villanueva, A.; Peinado, M.A. Methylation plotter: A web tool for dynamic visualization of DNA methylation data. Source Code Biol. Med. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Légaré, C.; Overend, G.; Guay, S.-P.; Monckton, D.G.; Mathieu, J.; Gagnon, C.; Bouchard, L. DMPK gene DNA methylation levels are associated with muscular and respiratory profiles in DM1. Neurol. Genet. 2019, 5, e338. [Google Scholar] [CrossRef]
- Groh, W.J.; Groh, M.R.; Shen, C.; Monckton, D.G.; Bodkin, C.L.; Pascuzzi, R.M. Survival and CTG repeat expansion in adults with myotonic dystrophy type 1. Muscle Nerve 2011, 43, 648–651. [Google Scholar] [CrossRef]
- Logigian, E.L.; Moxley, R.T.; Blood, C.L.; Barbieri, C.A.; Martens, W.B.; Wiegner, A.W.; Thornton, C.A.; Moxley, R.T. Leukocyte CTG repeat length correlates with severity of myotonia in myotonic dystrophy type 1. Neurology 2004, 62, 1081–1089. [Google Scholar] [CrossRef]
- Nichol, K.; Pearson, C.E. CpG methylation modifies the genetic stability of cloned repeat sequences. Genome Res. 2002, 12, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky-Dagan, S.; Cohen, E.; Megalli, P.; Altarescu, G.; Schonberger, O.; Eldar-Geva, T.; Epsztejn-Litman, S.; Eiges, R. DMPK hypermethylation in sperm cells of myotonic dystrophy type 1 patients. Eur. J. Hum. Genet. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Antequera, F.; Boyes, J.; Bird, A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell 1990, 62, 503–514. [Google Scholar] [CrossRef]
- Jones, P.A.; Wolkowicz, M.J.; Rideout, W.M.; Gonzales, F.A.; Marziasz, C.M.; Coetzee, G.A.; Tapscott, S.J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc. Natl. Acad. Sci. USA 1990, 87, 6117–6121. [Google Scholar] [CrossRef] [PubMed]

| Clinical Subtype | Age of Onset | Main Symptoms |
|---|---|---|
| Congenital | <1 year | Poor fetal movement Hypotonia Feeding difficulties Clubfoot deformities Respiratory failure Learning disability Cardiorespiratory complications |
| Childhood | 1–10 years | Cognitive and learning disabilities Facial weakness Myotonia Conduction defects |
| Juvenile | 11–20 years | Skeletal muscle weakness Myotonia Cognitive and learning disabilities Conduction defects |
| Adult | 21–40 years | Progressive muscle weakness Myotonia Early-onset cataracts Conduction defects Endocrine dysfunction |
| Late-onset | >40 years | Low-grade muscle weakness Early-onset cataracts alopecia |
| Clinical Subtype | n Patients | Age of Onset (Years) | Age at Sampling (Years) | Inheritance Maternal | Gender (Male) | ePAL (CTGs) | Myotonia | Biceps MRC Scale | MIRS | Cardiac Involvement | NVM | Cataracts | mRS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Congenital | 6 | At birth | 12.83 ± 5.43 | (6/6) | (4/6) | 610 (222–1011) | (2/6) | 4.25 (3–5) | 3.80 (3–5) | (1/6) | (2/6) | (0/6) | 3.60 (2–5) |
| Childhood | 6 | 6.83 ± 2.99 | 41.83 ± 12.04 | (2/6) | (5/6) | 549 (296–796) | (6/6) | 4.17 (3–5) | 3.33 (2–4) | (4/6) | (4/6) | (3/6) | 3.33 (2–4) |
| Juvenile | 23 | 15.05 ± 2.50 | 27.09 ± 12.54 | (7/23) | (11/22) | 317 (189–642) | (23/23) | 4.87 (4–5) | 2.30 (1–4) | (6/23) | (6/23) | (3/23) | 1.48 (1–3) |
| Adult | 22 | 31.50 ± 4.50 | 49.00 ± 9.33 | (4/13) | (6/22) | 290 (115–628) | (21/21) | 4.86 (4–5) | 2.57 (1–4) | (7/21) | (7/21) | (8/21) | 1.62 (0–4) |
| Late Onset | 6 | 52.17 ±7.99 | 61.33 ± 10.23 | (0/4) | (5/6) | 332 (131–911) | (3/5) | 5.00 (5–5) | 2.40 (1–4) | (5/5) | (1/5) | (5/5) | 2.00 (0–4) |
| Asymptomatic | 2 | N/A | 30.00 ± 22.63 | (0/2) | (2/2) | 238 a | (0/2) | 5.00 (5–5) | 1 (1–1) | (0/2) | (0/2) | (0/2) | 0 (0–0) |
| Patient | Methyl CTCF1/2 | Age of Onset (Years) | Age at Sampling (Years) | Gender | ePAL (CTGs) | Myotonia | Facial Weakness | Axial Weakness | Limb Weakness | MIRS | Cardiac Involvement | NVM | CNS Involvement | Hypersomnolence | Cataracts | mRS | DM1-ACTIV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P7 | yes/no | 6 | 56 | male | 756 | yes | moderate | moderate | severe distal | 4 | pacemaker | yes | Moderate learning disability | yes | yes | 4 | 9 |
| P8 | no/no | 7 | 43 | male | 296 | yes | mild | mild | mild distal | 3 | no | yes | no | no | no | 2 | 25 |
| P9 | no/no | 2 | 20 | male | 476 | yes | moderate | mild | mild distal | 2 | 1°AV block | no | moderate learning disability | no | no | 4 | 22 |
| P10 | yes/no | 6 | 40 | female | 324 | yes | severe | moderate | mild distal | 3 | no | no | severe cognitive delay | yes | yes | 3 | 14 |
| P11 | no/no | 10 | 44 | male | 644 | yes | mild | moderate | mild proximal + distal | 4 | 1°AV block | yes | no | no | yes | 3 | 38 |
| P12 | yes/no | 10 | 48 | male | 796 | yes | moderate | moderate | severe proximal + distal | 4 | pacemaker | yes | severe cognitive delay | yes | no | 4 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koehorst, E.; Odria, R.; Capó, J.; Núñez-Manchón, J.; Arbex, A.; Almendrote, M.; Linares-Pardo, I.; Natera-de Benito, D.; Saez, V.; Nascimento, A.; et al. An Integrative Analysis of DNA Methylation Pattern in Myotonic Dystrophy Type 1 Samples Reveals a Distinct DNA Methylation Profile between Tissues and a Novel Muscle-Associated Epigenetic Dysregulation. Biomedicines 2022, 10, 1372. https://doi.org/10.3390/biomedicines10061372
Koehorst E, Odria R, Capó J, Núñez-Manchón J, Arbex A, Almendrote M, Linares-Pardo I, Natera-de Benito D, Saez V, Nascimento A, et al. An Integrative Analysis of DNA Methylation Pattern in Myotonic Dystrophy Type 1 Samples Reveals a Distinct DNA Methylation Profile between Tissues and a Novel Muscle-Associated Epigenetic Dysregulation. Biomedicines. 2022; 10(6):1372. https://doi.org/10.3390/biomedicines10061372
Chicago/Turabian StyleKoehorst, Emma, Renato Odria, Júlia Capó, Judit Núñez-Manchón, Andrea Arbex, Miriam Almendrote, Ian Linares-Pardo, Daniel Natera-de Benito, Verónica Saez, Andrés Nascimento, and et al. 2022. "An Integrative Analysis of DNA Methylation Pattern in Myotonic Dystrophy Type 1 Samples Reveals a Distinct DNA Methylation Profile between Tissues and a Novel Muscle-Associated Epigenetic Dysregulation" Biomedicines 10, no. 6: 1372. https://doi.org/10.3390/biomedicines10061372
APA StyleKoehorst, E., Odria, R., Capó, J., Núñez-Manchón, J., Arbex, A., Almendrote, M., Linares-Pardo, I., Natera-de Benito, D., Saez, V., Nascimento, A., Ortez, C., Rubio, M. Á., Díaz-Manera, J., Alonso-Pérez, J., Lucente, G., Rodriguez-Palmero, A., Ramos-Fransi, A., Martínez-Piñeiro, A., Nogales-Gadea, G., & Suelves, M. (2022). An Integrative Analysis of DNA Methylation Pattern in Myotonic Dystrophy Type 1 Samples Reveals a Distinct DNA Methylation Profile between Tissues and a Novel Muscle-Associated Epigenetic Dysregulation. Biomedicines, 10(6), 1372. https://doi.org/10.3390/biomedicines10061372







