Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
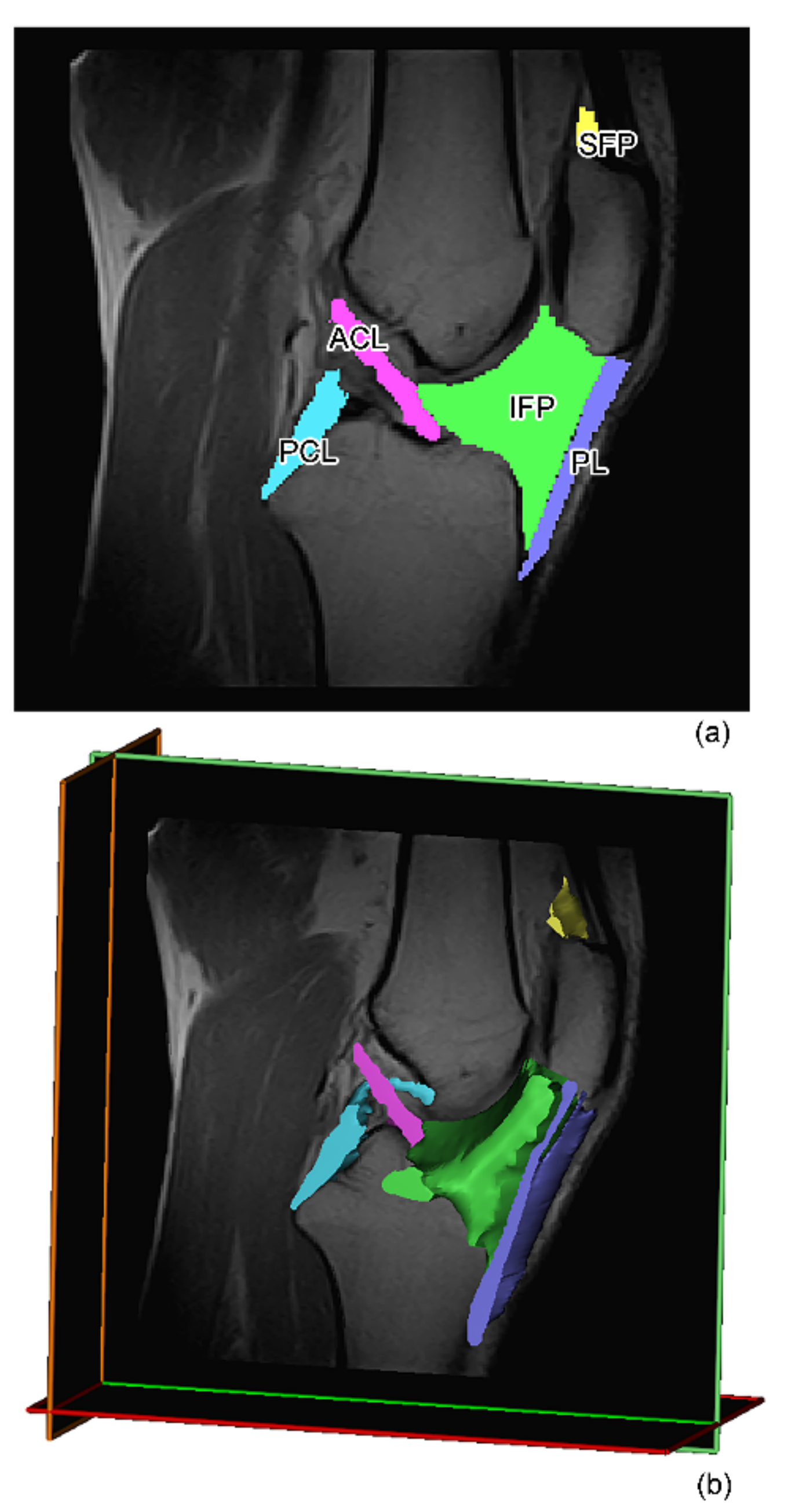
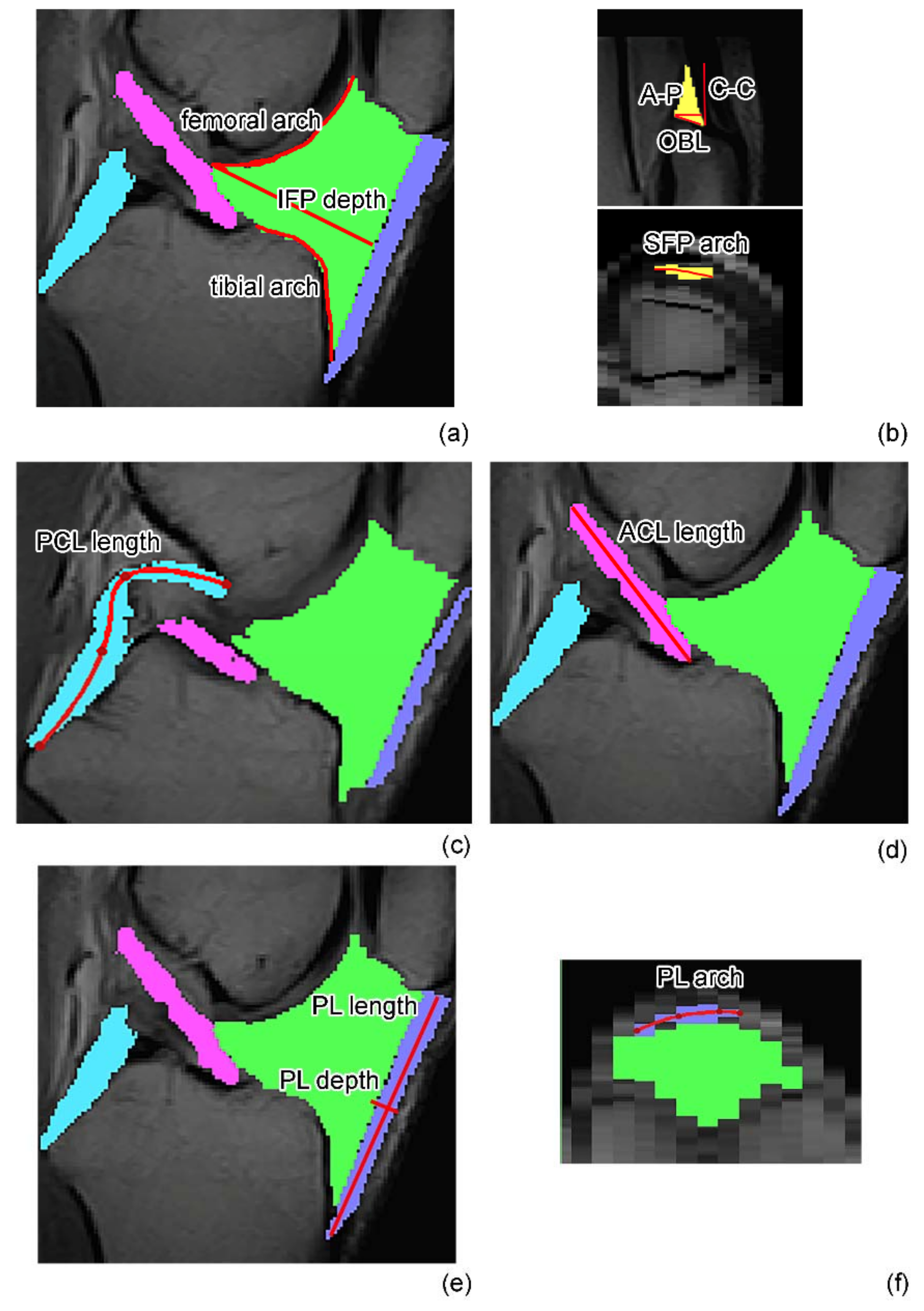
2.2. MR Image Acquisition and Analysis
Volumetric Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. IFP and SFP Morphometric Characteristics
3.3. ACL, PCL, and PL Morphometric Characteristics
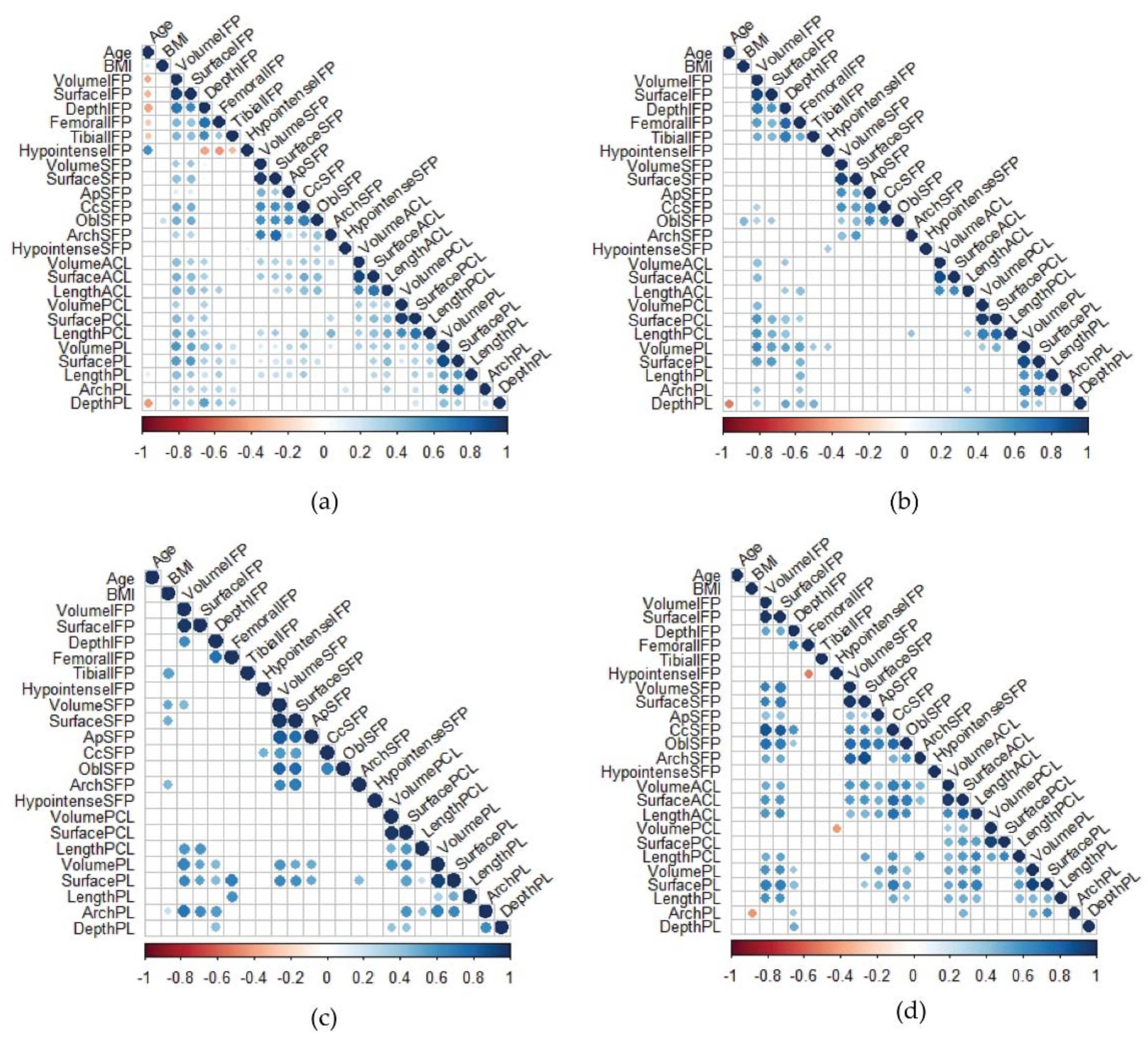
3.4. Correlation Matrices
3.5. Age, BMI, and Gender Influence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callahan, L.F.; Ambrose, K.R.; Albright, A.L.; Altpeter, M.; Golightly, Y.M.; Huffman, K.F.; Nelson, A.E.; Weisner, S.E. Public Health Interventions for Osteoarthritis—Updates on the Osteoarthritis Action Alliance’s efforts to address the 2010 OA Public Health Agenda Recommendations. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 31–39. [Google Scholar] [PubMed]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; El Hadi, H.; Granzotto, M.; Rossato, M.; Ramonda, R.; Macchi, V.; De Caro, R.; Vettor, R.; Favero, M. Systemic and Local Adipose Tissue in Knee Osteoarthritis. J. Cell. Physiol. 2017, 232, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. BioMed Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef] [PubMed]
- Man, G.S.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar] [PubMed]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Belluzzi, E.; Olivotto, E.; Toso, G.; Cigolotti, A.; Pozzuoli, A.; Biz, C.; Trisolino, G.; Ruggieri, P.; Grigolo, B.; Ramonda, R.; et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect. Tissue Res. 2019, 60, 136–145. [Google Scholar] [CrossRef]
- Eymard, F.; Pigenet, A.; Citadelle, D.; Tordjman, J.; Foucher, L.; Rose, C.; Flouzat Lachaniette, C.H.; Rouault, C.; Clement, K.; Berenbaum, F.; et al. Knee and hip intra-articular adipose tissues (IAATs) compared with autologous subcutaneous adipose tissue: A specific phenotype for a central player in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 1142–1148. [Google Scholar] [CrossRef]
- Stocco, E.; Belluzzi, E.; Contran, M.; Boscolo-Berto, R.; Picardi, E.; Guidolin, D.; Fontanella, C.G.; Olivotto, E.; Filardo, G.; Borile, G.; et al. Age-Dependent Remodeling in Infrapatellar Fat Pad Adipocytes and Extracellular Matrix: A Comparative Study. Front. Med. 2021, 8, 661403. [Google Scholar] [CrossRef]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Favero, M.; Ruggieri, P.; Macchi, V.; Carniel, E.L. Mechanical behavior of infrapatellar fat pad of patients affected by osteoarthritis. J. Biomech. 2022, 131, 110931. [Google Scholar] [CrossRef]
- Favero, M.; El-Hadi, H.; Belluzzi, E.; Granzotto, M.; Porzionato, A.; Sarasin, G.; Rambaldo, A.; Iacobellis, C.; Cigolotti, A.; Fontanella, C.G.; et al. Infrapatellar fat pad features in osteoarthritis: A histopathological and molecular study. Rheumatology 2017, 56, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Macchi, V.; Fontanella, C.G.; Carniel, E.L.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Macchi, V.; Carniel, E.L.; Frigo, A.; Porzionato, A.; Picardi, E.E.E.; Favero, M.; Ruggieri, P.; de Caro, R.; Natali, A.N. Biomechanical behavior of Hoffa’s fat pad in healthy and osteoarthritic conditions: Histological and mechanical investigations. Australas. Phys. Eng. Sci. Med. 2018, 41, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Felson, D.; Gold, G.; Lohmander, S.; Totterman, S.; Altman, R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthr. Cartil. 2006, 14, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Roemer, F.W.; Eckstein, F.; Samuels, J.; Guermazi, A. Imaging of OA—From disease modification to clinical utility. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101588. [Google Scholar] [CrossRef]
- Cowan, S.M.; Hart, H.F.; Warden, S.J.; Crossley, K.M. Infrapatellar fat pad volume is greater in individuals with patellofemoral joint osteoarthritis and associated with pain. Rheumatol. Int. 2015, 35, 1439–1442. [Google Scholar] [CrossRef]
- Ruhdorfer, A.; Haniel, F.; Petersohn, T.; Dorrenberg, J.; Wirth, W.; Dannhauer, T.; Hunter, D.J.; Eckstein, F. Between-group differences in infra-patellar fat pad size and signal in symptomatic and radiographic progression of knee osteoarthritis vs non-progressive controls and healthy knees—Data from the FNIH Biomarkers Consortium Study and the Osteoarthritis Initiative. Osteoarthr. Cartil. 2017, 25, 1114–1121. [Google Scholar] [CrossRef]
- Chuckpaiwong, B.; Charles, H.C.; Kraus, V.B.; Guilak, F.; Nunley, J.A. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 1149–1154. [Google Scholar] [CrossRef]
- Cai, J.; Xu, J.; Wang, K.; Zheng, S.; He, F.; Huan, S.; Xu, S.; Zhang, H.; Laslett, L.; Ding, C. Association Between Infrapatellar Fat Pad Volume and Knee Structural Changes in Patients with Knee Osteoarthritis. J. Rheumatol. 2015, 42, 1878. [Google Scholar] [CrossRef]
- Pan, F.; Han, W.; Wang, X.; Liu, Z.; Jin, X.; Antony, B.; Cicuttini, F.; Jones, G.; Ding, C. A longitudinal study of the association between infrapatellar fat pad maximal area and changes in knee symptoms and structure in older adults. Ann. Rheum. Dis. 2015, 74, 1818–1824. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wulidasari, E.; Brady, S.R.E.; Wang, Y.; Wluka, A.E.; Ding, C.; Giles, G.G.; Cicuttini, F.M. A large infrapatellar fat pad protects against knee pain and lateral tibial cartilage volume loss. Arthritis Res. Ther. 2015, 17, 318. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Belluzzi, E.; Rossato, M.; Olivotto, E.; Trisolino, G.; Ruggieri, P.; Rubini, A.; Porzionato, A.; Natali, A.; De Caro, R.; et al. Quantitative MRI analysis of infrapatellar and suprapatellar fat pads in normal controls, moderate and end-stage osteoarthritis. Ann. Anat. 2019, 221, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Steidle-Kloc, E.; Culvenor, A.G.; Dorrenberg, J.; Wirth, W.; Ruhdorfer, A.; Eckstein, F. Relationship Between Knee Pain and Infrapatellar Fat Pad Morphology: A Within- and Between-Person Analysis from the Osteoarthritis Initiative. Arthritis Care Res. 2018, 70, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Aitken, D.; Zhu, Z.; Halliday, A.; Wang, X.; Antony, B.; Cicuttini, F.; Jones, G.; Ding, C. Hypointense signals in the infrapatellar fat pad assessed by magnetic resonance imaging are associated with knee symptoms and structure in older adults: A cohort study. Arthritis Res. Ther. 2016, 18, 234. [Google Scholar] [CrossRef]
- De Vries, B.A.; van der Heijden, R.A.; Poot, D.H.J.; van Middelkoop, M.; Meuffels, D.E.; Krestin, G.P.; Oei, E.H.G. Quantitative DCE-MRI demonstrates increased blood perfusion in Hoffa’s fat pad signal abnormalities in knee osteoarthritis, but not in patellofemoral pain. Eur. Radiol. 2020, 30, 3401–3408. [Google Scholar] [CrossRef]
- Tsavalas, N.; Karantanas, A.H. Suprapatellar fat-pad mass effect: MRI findings and correlation with anterior knee pain. AJR Am. J. Roentgenol. 2013, 200, W291–W296. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.; Wang, X.; Pan, F.; Liu, Z.; Halliday, A.; Jin, X.; Antony, B.; Cicuttini, F.; Jones, G.; et al. Mass effect and signal intensity alteration in the suprapatellar fat pad: Associations with knee symptoms and structure. Osteoarthr. Cartil. 2014, 22, 1619–1626. [Google Scholar] [CrossRef]
- Schwaiger, B.J.; Mbapte Wamba, J.; Gersing, A.S.; Nevitt, M.C.; Facchetti, L.; McCulloch, C.E.; Link, T.M. Hyperintense signal alteration in the suprapatellar fat pad on MRI is associated with degeneration of the patellofemoral joint over 48 months: Data from the Osteoarthritis Initiative. Skelet. Radiol. 2018, 47, 329–339. [Google Scholar] [CrossRef]
- Trisolino, G.; Favero, M.; Lazzaro, A.; Martucci, E.; Strazzari, A.; Belluzzi, E.; Goldring, S.R.; Goldring, M.B.; Punzi, L.; Grigolo, B.; et al. Is arthroscopic videotape a reliable tool for describing early joint tissue pathology of the knee? Knee 2017, 24, 1374–1382. [Google Scholar] [CrossRef]
- Steidle-Kloc, E.; Wirth, W.; Ruhdorfer, A.; Dannhauer, T.; Eckstein, F. Intra- and inter-observer reliability of quantitative analysis of the infra-patellar fat pad and comparison between fat- and non-fat-suppressed imaging—Data from the osteoarthritis initiative. Ann. Anat. Anat. Anz. Off. J. Anat. Ges. 2016, 204, 29–35. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Cheruvu, B.; Tsatalis, J.; Laughlin, R.; Goswami, T. Methods to determine the volume of infrapatellar fat pad as an indicator of anterior cruciate ligament tear. Biomater. Biomech. Bioeng. 2016, 3, 27–35. [Google Scholar] [CrossRef][Green Version]
- Abreu, M.R.; Chung, C.B.; Trudell, D.; Resnick, D. Hoffa’s fat pad injuries and their relationship with anterior cruciate ligament tears: New observations based on MR imaging in patients and MR imaging and anatomic correlation in cadavers. Skelet. Radiol. 2008, 37, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Klass, D.; Toms, A.P.; Greenwood, R.; Hopgood, P. MR imaging of acute anterior cruciate ligament injuries. Knee 2007, 14, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Diepold, J.; Ruhdorfer, A.; Dannhauer, T.; Wirth, W.; Steidle, E.; Eckstein, F. Sex-differences of the healthy infra-patellar (Hoffa) fat pad in relation to intermuscular and subcutaneous fat content—Data from the Osteoarthritis Initiative. Ann. Anat. Anat. Anz. Off. J. Anat. Ges. 2015, 200, 30–36. [Google Scholar] [CrossRef]
- Heilmeier, U.; Mamoto, K.; Amano, K.; Eck, B.; Tanaka, M.; Bullen, J.A.; Schwaiger, B.J.; Huebner, J.L.; Stabler, T.V.; Kraus, V.B.; et al. Infrapatellar fat pad abnormalities are associated with a higher inflammatory synovial fluid cytokine profile in young adults following ACL tear. Osteoarthr. Cartil. 2020, 28, 82–91. [Google Scholar] [CrossRef]
- Zhong, Y.-c.; Wang, S.-c.; Han, Y.-h.; Wen, Y. Recent Advance in Source, Property, Differentiation, and Applications of Infrapatellar Fat Pad Adipose-Derived Stem Cells. Stem Cells Int. 2020, 2020, 2560174. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Piccione, M.; Belluzzi, E.; Petrelli, L.; Pozzuoli, A.; Ramonda, R.; Rossato, M.; Favero, M.; Ruggieri, P.; et al. Infrapatellar Fat Pad Stem Cells Responsiveness to Microenvironment in Osteoarthritis: From Morphology to Function. Front. Cell Dev. Biol. 2019, 7, 323. [Google Scholar] [CrossRef]
- Muñoz-Criado, I.; Meseguer-Ripolles, J.; Mellado-López, M.; Alastrue-Agudo, A.; Griffeth, R.J.; Forteza-Vila, J.; Cugat, R.; García, M.; Moreno-Manzano, V. Human Suprapatellar Fat Pad-Derived Mesenchymal Stem Cells Induce Chondrogenesis and Cartilage Repair in a Model of Severe Osteoarthritis. Stem Cells Int. 2017, 2017, 4758930. [Google Scholar] [CrossRef]
- Taneja, A.K.; Miranda, F.C.; Demange, M.K.; Prado, M.P.; Santos, D.C.B.; Rosemberg, L.A.; Baroni, R.H. Evaluation of Posterior Cruciate Ligament and Intercondylar Notch in Subjects With Anterior Cruciate Ligament Tear: A Comparative Flexed-Knee 3D Magnetic Resonance Imaging Study. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2018, 34, 557–565. [Google Scholar] [CrossRef]
- Jamison, S.T.; Flanigan, D.C.; Nagaraja, H.N.; Chaudhari, A.M.W. Side-to-side differences in anterior cruciate ligament volume in healthy control subjects. J. Biomech. 2010, 43, 576–578. [Google Scholar] [CrossRef]
- Fayad, L.M.; Rosenthal, E.H.; Morrison, W.B.; Carrino, J.A. Anterior cruciate ligament volume: Analysis of gender differences. J. Magn. Reson. Imaging JMRI 2008, 27, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.W.H.; Lee, R.K.L.; Ho, E.P.Y.; Law, B.K.Y.; Griffith, J.F. Anterior cruciate ligament bundle measurement by MRI. Skelet. Radiol. 2013, 42, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, R.; Van Schoor, A.-N.; Du Toit, P.J.; Suleman, F.E.; Velleman, M.D.; Glatt, V.; Tetsworth, K.; Hohmann, E. The Association Between Anterior Cruciate Ligament Length and Femoral Epicondylar Width Measured on Preoperative Magnetic Resonance Imaging or Radiograph. Arthrosc. Sports Med. Rehabil. 2020, 2, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, C.M.; Civitarese, D.M.; Rasmussen, M.T.; LaPrade, R.F. Emerging Updates on the Posterior Cruciate Ligament: A Review of the Current Literature. Am. J. Sports Med. 2015, 43, 3077–3092. [Google Scholar] [CrossRef] [PubMed]
- Ranmuthu, C.D.S.; MacKay, J.W.; Crowe, V.A.; Kaggie, J.D.; Kessler, D.A.; McDonnell, S.M. Quantitative analysis of the ACL and PCL using T1rho and T2 relaxation time mapping: An exploratory, cross-sectional comparison between OA and healthy control knees. BMC Musculoskelet. Disord. 2021, 22, 916. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakasa, T.; Matsuoka, Y.; Akiyama, Y.; Ishikawa, M.; Nakamae, A.; Awai, K.; Adachi, N. Evaluation of the degenerative pattern of PCL in osteoarthritis patients using UTE-T2 mapping. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2021, 24, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Navali, A.M.; Jafarabadi, M.A. Is There Any Correlation Between Patient Height and Patellar Tendon Length? Arch. Bone Jt. Surg. 2015, 3, 99–103. [Google Scholar] [PubMed]
- Yoo, J.H.; Yi, S.R.; Kim, J.H. The geometry of patella and patellar tendon measured on knee MRI. Surg. Radiol. Anat. SRA 2007, 29, 623–628. [Google Scholar] [CrossRef]
- Lemon, M.; Packham, I.; Narang, K.; Craig, D.M. Patellar tendon length after knee arthroplasty with and without preservation of the infrapatellar fat pad. J. Arthroplast. 2007, 22, 574–580. [Google Scholar] [CrossRef]
- Wang, H.; Hua, C.; Cui, H.; Li, Y.; Qin, H.; Han, D.; Yue, J.; Liang, C.; Yang, R. Measurement of normal patellar ligament and anterior cruciate ligament by MRI and data analysis. Exp. Ther. Med. 2013, 5, 917–921. [Google Scholar] [CrossRef]
- Kang, H.; Fu, K.; Dong, C.; Wang, F. The patellar tendon wavy sign as a new secondary sign of ACL tear on MRI. Acta Orthop. Traumatol. Turc. 2018, 52, 372–375. [Google Scholar] [CrossRef] [PubMed]

| Meniscal Tear | ACLR | End-Stage OA | p-Value | |
|---|---|---|---|---|
| Number of patients | 32 | 29 | 28 | |
| Sex, male number (%) | 20 (62.5) | 21 (72.4) | 7 (25) | <0.001 |
| Age, years, median (IQR) | 48.0 (58.0–35.0) | 34.0 (43.5–24.5) | 73.5 (78.0–66.5) | a 0.016 b <0.001 c <0.001 |
| BMI, Kg/m2, median (IQR) | 25.1 (29.9–23.8) | 25.2 (26.3–22.2) | 28.2 (31.6–24.8) | c <0.001 |
| Meniscal Tear | ACLR | End-Stage OA | p-Value | |
|---|---|---|---|---|
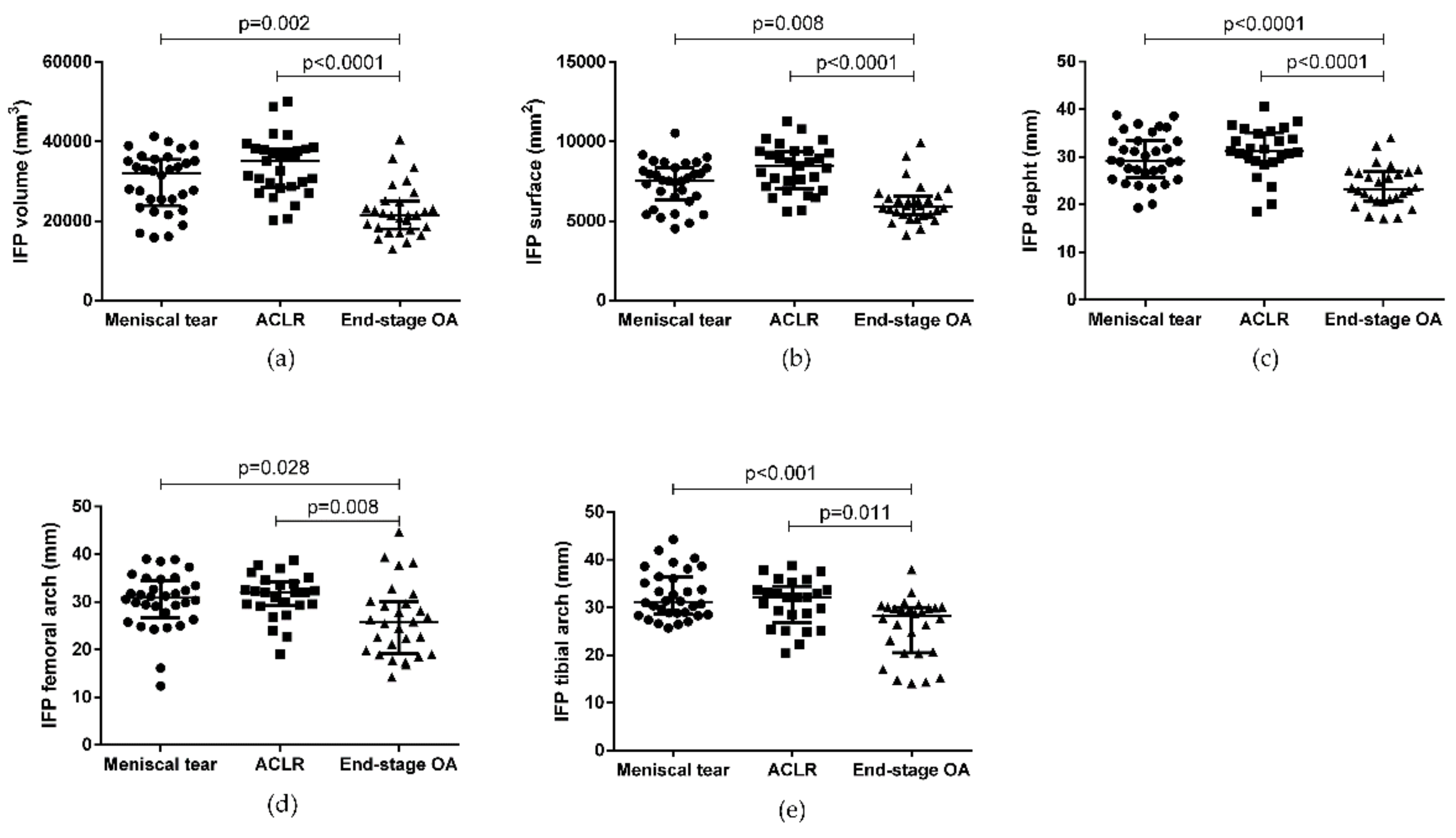
| IFP volume (mm3) | 32113 (35460–23943) | 35073 (38249–28536) | 21552 (25111–18017) | a 0.250 b 0.002 c <0.0001 |
| IFP surface (mm2) | 7550 (8366–6319) | 8495 (9393–7062) | 5906 (6549–5405) | a 0.074 b 0.008 c <0.0001 |
| IFP depth (mm) | 29.2 (33.4–25.7) | 31.3 (35.1–29.0) | 23.2 (26.8–20.8) | a 0.508 b <0.0001 c <0.0001 |
| IFP Femoral arch length (mm) | 30.9 (34.4–26.7) | 32.0 (34.2–29.2) | 25.7 (30.1–19.2) | a 0.999 b 0.028 c 0.008 |
| IFP Tibial arch length (mm) | 31.2 (36.4–28.6) | 32.1 (34.4–26.9) | 28.3 (30.0–20.5) | a 0.999 b <0.001 c 0.011 |
| SFP volume (mm3) | 1264.2 (1454.0–842.2) | 1979.3 (2401.5–1203.9) | 1021.6 (1480.0–784.0) | a 0.001 b 0.999 c <0.0001 |
| SFP surface (mm2) | 903.3 (1021.3–754.8) | 1236.3 (1395.1–890.1) | 782.0 (1066.1–608.8) | a 0.001 b 0.999 c <0.0001 |
| SFP OBL (mm) | 10.1 (11.8–9.3) | 9.2 (10.5–8.4) | 9.5 (11.0–9.1) | 0.113 |
| SFP C–C (mm) | 16.3 (18.4–13.9) | 16.7 (18.6–14.4) | 15.6 (18.0–14.3) | 0.837 |
| SFP A–P (mm) | 8.4 (9.3–6.9) | 8.3 (9.1–6.8) | 8.5 (9.2–7.6) | 0.689 |
| SFP arch (mm) | 27.4 (30.4–27.4) | 27.7 (30.4–23.1) | 24.6 (30.4–21.9) | 0.380 |
| Meniscal Tear (n = 32) | ACLR (n = 29) | End-Stage OA (n = 28) | p-Value | |
|---|---|---|---|---|
| IFP | <0.0001 | |||
| Grade 0 | 19 (59.4%) | 15 (51.7%) | 1 (3.6%) | a 0.101 |
| Grade 1 | 12 (37.5%) | 7 (24.1%) | 8 (28.6%) | b <0.0001 |
| Grade 2 | 1 (3.1%) | 6 (20.7%) | 10 (20.7%) | c <0.0001 |
| Grade 3 | 0 | 1 (3.4%) | 9 (32.1%) | |
| Meniscal tear (n = 32) | ACLR (n = 26) | End-stage OA (n = 27) | p-value | |
| SFP | <0.012 | |||
| Grade 0 | 8 (25%) | 13 (50%) | 8 (28.6%) | a 0.002 |
| Grade 1 | 12 (37.5%) | 13 (50%) | 10 (37%) | b 0.910 |
| Grade 2 | 12 (37.5%) | 0 | 9 (32.1%) | c 0.005 |
| Grade 3 | 0 | 0 | 0 | |
| Meniscal tear (n = 32) | ACLR (n = 24) | End-stage OA (n = 26) | p-value | |
| SFP edema | 0.385 | |||
| Yes | 12 (37.5%) | 5 (20.8%) | 9 (34.6%) | |
| No | 20 (62.5%) | 19 (79.2%) | 17 (65.4%) |
| Meniscal Tear | ACLR | End-Stage OA | p-Value | |
|---|---|---|---|---|
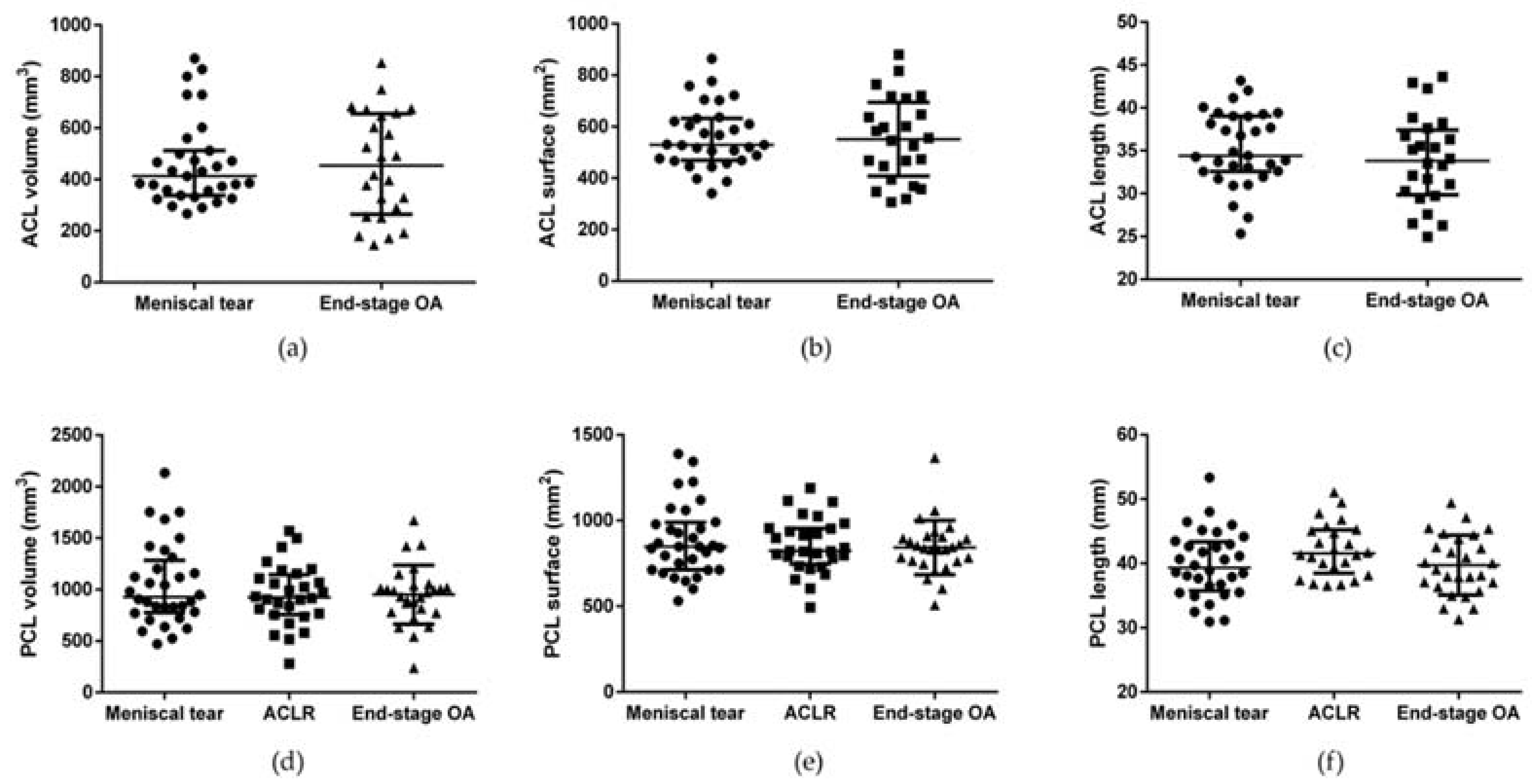
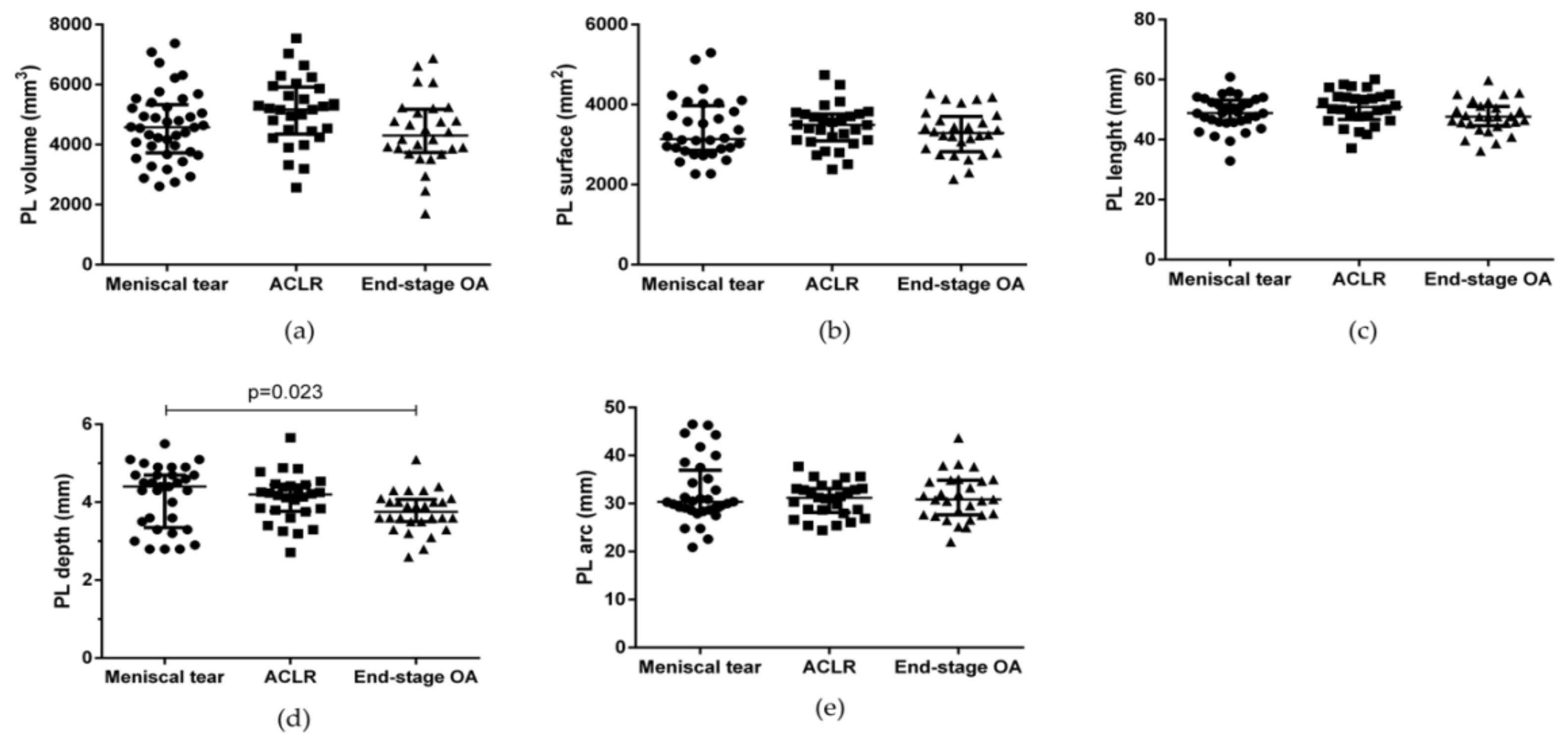
| PL volume (mm3) | 4476 (5242–3659) | 5172 (5913–4351) | 4302 (5175–3735) | 0.076 |
| PL surface (mm2) | 3139 (3973–2856) | 3494 (3764–3093) | 3287 (3704–2821) | 0.716 |
| PL length (mm) | 48.8 (53.1–45.8) | 50.8 (54.4–46.7) | 47.6 (51.0–44.6) | 0.108 |
| PL depth (mm) | 4.4 (4.7–3.3) | 4.2 (4.4–3.8) | 3.7 (4.1–3.5) | a 0.999 b 0.023 c 0.153 |
| PL arch (mm) | 30.3 (36.9–28.6) | 31.2 (33.1–28.2) | 30.8 (34.8–27.6) | 0.962 |
| Age (p-Value) | Gender (p-Value) | BMI (p-Value) | ACLR vs. Meniscal Tear (p-Value) | End-Stage OA vs. Meniscal Tear (p-Value) | |
|---|---|---|---|---|---|
| IFP volume | 0.579 | <0.0001 | 0.066 | 0.010 | 0.018 |
| IFP surface | 0.549 | <0.0001 | 0.028 | 0.002 | 0.045 |
| IFP depth | 0.413 | <0.0001 | 0.907 | 0.322 | 0.004 |
| IFP Femoral arch length | 0.145 | 0.0012 | 0.540 | 0.457 | 0.059 |
| IFP Tibial arch length | 0.217 | <0.001 | 0.621 | 0.341 | <0.001 |
| SFP volume | 0.243 | 0.025 | 0.025 | <0.001 | 0.288 |
| SFP surface | 0.279 | 0.018 | 0.035 | <0.001 | 0.196 |
| IFP hypotense signal | 0.662 | 0.011 | 0.590 | 0.225 | <0.001 |
| SFP hypotense signal | 0.319 | 0.609 | 0.832 | <0.005 | 0.598 |
| PL depth | 0.082 | <0.001 | 0.229 | 0.137 | 0.659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Scioni, M.; Olivotto, E.; Reale, D.; Ruggieri, P.; De Caro, R.; Ramonda, R.; Carniel, E.L.; et al. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines 2022, 10, 1369. https://doi.org/10.3390/biomedicines10061369
Fontanella CG, Belluzzi E, Pozzuoli A, Scioni M, Olivotto E, Reale D, Ruggieri P, De Caro R, Ramonda R, Carniel EL, et al. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines. 2022; 10(6):1369. https://doi.org/10.3390/biomedicines10061369
Chicago/Turabian StyleFontanella, Chiara Giulia, Elisa Belluzzi, Assunta Pozzuoli, Manuela Scioni, Eleonora Olivotto, Davide Reale, Pietro Ruggieri, Raffaele De Caro, Roberta Ramonda, Emanuele Luigi Carniel, and et al. 2022. "Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions" Biomedicines 10, no. 6: 1369. https://doi.org/10.3390/biomedicines10061369
APA StyleFontanella, C. G., Belluzzi, E., Pozzuoli, A., Scioni, M., Olivotto, E., Reale, D., Ruggieri, P., De Caro, R., Ramonda, R., Carniel, E. L., Favero, M., & Macchi, V. (2022). Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines, 10(6), 1369. https://doi.org/10.3390/biomedicines10061369














