Pharmacological Treatments Available for Immune-Checkpoint-Inhibitor-Induced Colitis
Abstract
1. Introduction
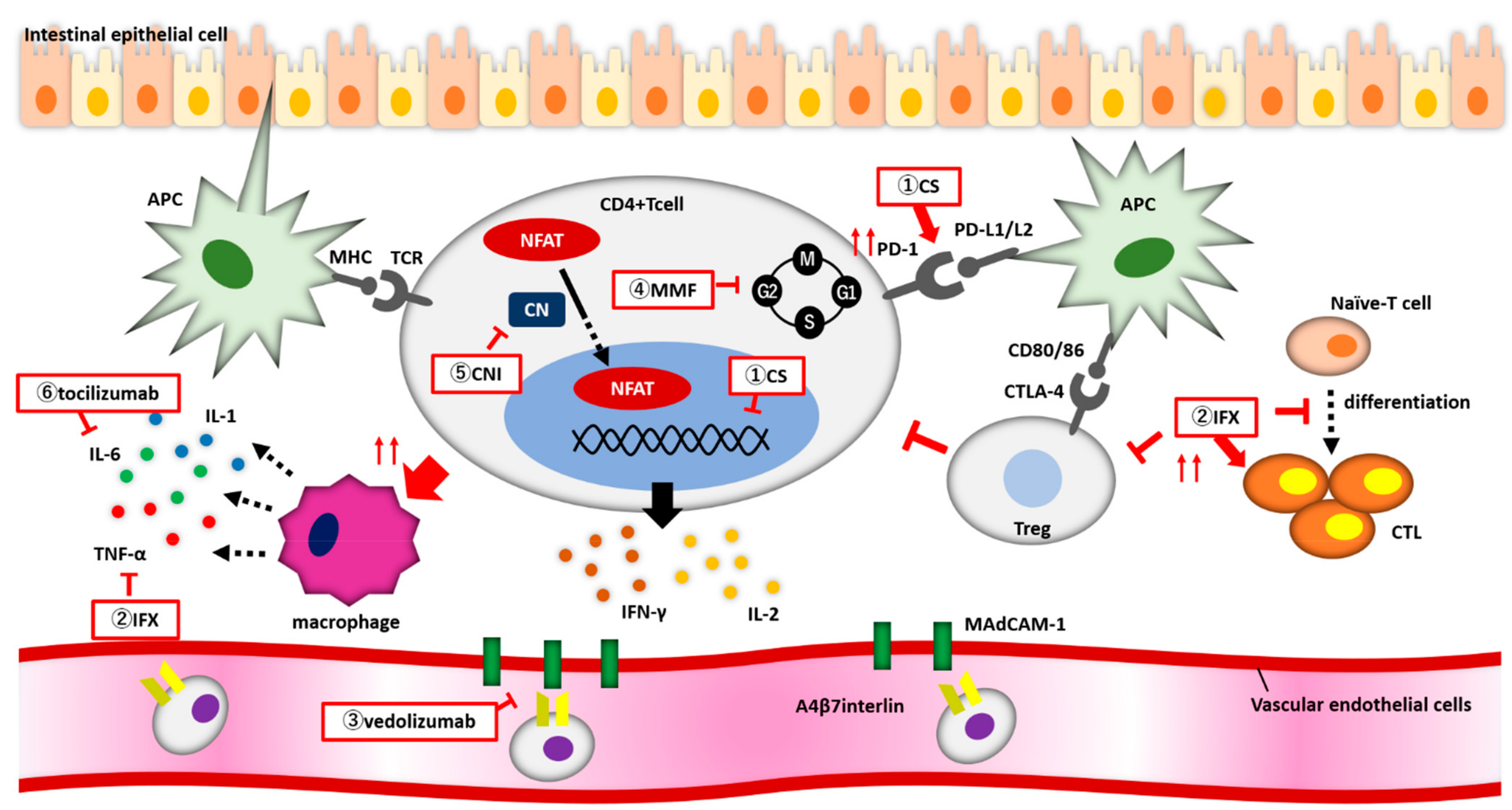
2. Pathophysiology of ICI-Induced Colitis
3. Clinical Characteristics of ICI-Induced Colitis
4. Treatment of irAE Colitis
4.1. Corticosteroids
4.2. Infliximab
4.3. Vedolizumab
4.4. Other Therapeutic Agents
5. Discussions
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Kiyohara, Y.; Uhara, H.; Uehara, J.; Fujimoto, M.; Takenouchi, T.; Otsuka, M.; Uchi, H.; Ihn, H.; Minami, H. Efficacy and Safety of Nivolumab in Japanese Patients with Previously Untreated Advanced Melanoma: A phase II Study. Cancer Sci. 2017, 108, 1223–1230. [Google Scholar] [CrossRef]
- Escudier, B.; Sharma, P.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab versus Everolimus in Advanced Renal Cell Carcinoma. Eur. Urol. 2017, 72, 962–971. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in Patients with Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Cutaneous, Gastrointestinal, Hepatic, Endocrine, and Renal Side-Effects of Anti-PD-1 Therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef]
- Wang, D.Y.; Ye, F.; Zhao, S.; Johnson, D.B. Incidence of Immune Checkpoint Inhibitor-Related Colitis in Solid Tumor Patients: A Systematic Review and Meta-Analysis. Oncoimmunology 2017, 6, e1344805. [Google Scholar] [CrossRef]
- Tandon, P.; Bourassa-Blanchette, S.; Bishay, K.; Parlow, S.; Laurie, S.A.; McCurdy, J.D. The Risk of Diarrhea and Colitis in Patients with Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. J. Immunother. 2018, 41, 101–108. [Google Scholar] [CrossRef]
- Bishay, K.; Tandon, P.; Bourassa-Blanchette, S.; Laurie, S.A.; McCurdy, J.D. The Risk of Diarrhea and Colitis in Patients with Lung Cancer Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Curr. Oncol. 2020, 27, e486–e494. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Weber, J.S.; Gibney, G.; Sullivan, R.J.; Sosman, J.A.; Slingluff, C.L., Jr.; Lawrence, D.P.; Logan, T.F.; Schuchter, L.M.; Nair, S.; Fecher, L.; et al. Sequential Administration of Nivolumab and Ipilimumab with a Planned Switch in Patients with Advanced Melanoma (CheckMate 064): An Open-Label, Randomised, phase 2 Trial. Lancet Oncol. 2016, 17, 943–955. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Faleck, D.M.; Ricciuti, B.; Mendelsohn, R.B.; Naqash, A.R.; Cohen, J.V.; Sellers, M.C.; Balaji, A.; Ben-Betzalel, G.; Hajir, I.; et al. Immune Checkpoint Inhibitor Therapy in Patients with Preexisting Inflammatory Bowel Disease. J. Clin. Oncol. 2020, 38, 576–583. [Google Scholar] [CrossRef]
- Kähler, K.C.; Eigentler, T.K.; Gesierich, A.; Heinzerling, L.; Loquai, C.; Meier, F.; Meiss, F.; Pföhler, C.; Schlaak, M.; Terheyden, P.; et al. Ipilimumab in Metastatic Melanoma Patients with Pre-Existing Autoimmune Disorders. Cancer Immunol. Immunother. 2018, 67, 825–834. [Google Scholar] [CrossRef]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Ali, F.S.; Qiao, W.; Lum, P.; Raju, G.; Shuttlesworth, G.; Stroehlein, J.; et al. Immune-Checkpoint Inhibitor-Induced Diarrhea and Colitis in Patients with Advanced Malignancies: Retrospective Review at MD Anderson. J. Immunother. Cancer 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef]
- Portenkirchner, C.; Kienle, P.; Horisberger, K. Checkpoint Inhibitor-Induced Colitis-A Clinical Overview of Incidence, Prognostic Implications and Extension of Current Treatment Options. Pharmaceuticals 2021, 14, 367. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Qiao, W.; Lu, Y.; Patel, S.; Diab, A.; Wang, Y. Immune Checkpoint Inhibitor-Induced Colitis as a Predictor of Survival in Metastatic Melanoma. Cancer Immunol. Immunother. 2019, 68, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Shoji, H.; Nagashima, K.; Yamamoto, S.; Ishikawa, M.; Imazeki, H.; Aoki, M.; Miyamoto, T.; Hirano, H.; Honma, Y.; et al. Correlation between Immune-Related Adverse Events and Prognosis in Patients with Gastric Cancer Treated with Nivolumab. BMC Cancer 2019, 19, 974. [Google Scholar] [CrossRef]
- Yamada, K.; Sawada, T.; Nakamura, M.; Yamamura, T.; Maeda, K.; Ishikawa, E.; Iida, T.; Mizutani, Y.; Kakushima, N.; Ishikawa, T.; et al. Clinical Characteristics of Gastrointestinal Immune-Related Adverse Events of Immune Checkpoint Inhibitors and Their Association with Survival. World J. Gastroenterol. 2021, 27, 7190–7206. [Google Scholar] [CrossRef]
- Zou, F.; Abu-Sbeih, H.; Ma, W.; Peng, Y.; Qiao, W.; Wang, J.; Shah, A.Y.; Glitza Oliva, I.C.; Piha-Paul, S.A.; Thompson, J.A.; et al. Association of Chronic Immune-Mediated Diarrhea and Colitis with Favorable Cancer Response. J. Natl. Compr. Cancer Netw. 2020, 19, 700–708. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced Expression of PD-1, a Novel Member of the Immunoglobulin Gene Superfamily, Upon Programmed Cell Death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.Y.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-DC, a New Dendritic Cell Molecule with Potent costimulatory Properties for T Cells. J. Exp. Med. 2001, 193, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of Programmed Death 1 Ligands by Murine T Cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef]
- Zhong, X.; Tumang, J.R.; Gao, W.; Bai, C.; Rothstein, T.L. PD-L2 Expression Extends beyond Dendritic Cells/Macrophages to B1 Cells Enriched for V(H)11/V(H)12 and Phosphatidylcholine Binding. Eur. J. Immunol. 2007, 37, 2405–2410. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and Type-II-Interferon-Mediated Signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Quandt, D.; Jasinski-Bergner, S.; Müller, U.; Schulze, B.; Seliger, B. Synergistic Effects of IL-4 and TNFα on the Induction of B7-H1 in Renal Cell Carcinoma Cells Inhibiting Allogeneic T Cell Proliferation. J. Transl. Med. 2014, 12, 151. [Google Scholar] [CrossRef]
- Karakhanova, S.; Meisel, S.; Ring, S.; Mahnke, K.; Enk, A.H. ERK/p38 MAP-Kinases and PI3K Are Involved in the Differential Regulation of B7-H1 Expression in DC Subsets. Eur. J. Immunol. 2010, 40, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 Improves Myeloid Dendritic Cell-Mediated Antitumor Immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiao, X.; Wu, Y.; Wei, Y.; Zhu, L.Y.; Zhou, J.; Kuang, D.M. Interleukin-17-Educated Monocytes Suppress Cytotoxic T-Cell Function through B7-H1 in Hepatocellular Carcinoma Patients. Eur. J. Immunol. 2011, 41, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Mazanet, M.M.; Hughes, C.C. B7-H1 Is Expressed by Human Endothelial Cells and Suppresses T Cell Cytokine Synthesis. J. Immunol. 2002, 169, 3581–3588. [Google Scholar] [CrossRef]
- Hofmeyer, K.A.; Jeon, H.; Zang, X. The PD-1/PD-L1 (B7-H1) Pathway in Chronic Infection-Induced Cytotoxic T Lymphocyte Exhaustion. J. Biomed. Biotechnol. 2011, 2011, 451694. [Google Scholar] [CrossRef]
- Cassol, C.; Satoskar, A.; Lozanski, G.; Rovin, B.; Hebert, L.; Nadasdy, T.; Brodsky, S.V. Anti-PD-1 Immunotherapy May Induce Interstitial Nephritis with Increased Tubular Epithelial Expression of PD-L1. Kidney Int. Rep. 2019, 4, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Chikuma, S.; Bluestone, J.A. CTLA-4 and Tolerance: The Biochemical Point of View. Immunol. Res. 2003, 28, 241–253. [Google Scholar] [CrossRef]
- Rudd, C.E. CTLA-4 Co-Receptor Impacts on the Function of Treg and CD8+ T-Cell Subsets. Eur. J. Immunol. 2009, 39, 687–690. [Google Scholar] [CrossRef]
- Liakou, C.I.; Kamat, A.; Tang, D.N.; Chen, H.; Sun, J.; Troncoso, P.; Logothetis, C.; Sharma, P. CTLA-4 Blockade Increases IFNgamma-Producing CD4+ICOShi Cells to Shift the Ratio of Effector to Regulatory T Cells in Cancer Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 14987–14992. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells within B16 Melanoma Tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef]
- Sury, K.; Perazella, M.A.; Shirali, A.C. Cardiorenal Complications of Immune Checkpoint Inhibitors. Nat. Rev. Nephrol. 2018, 14, 571–588. [Google Scholar] [CrossRef]
- Cassol, C.A.; Owen, D.; Kendra, K.; Braga, J.R.; Frankel, W.L.; Arnold, C.A. Programmed Cell death-1 (PD-1) and Programmed Death-Ligand 1 (PD-L1) Expression in PD-1 Inhibitor-Associated Colitis and Its Mimics. Histopathology 2020, 77, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.J.; Griseri, T.; Johnson, A.M.; Young, W.; Powrie, F.; Izcue, A. CTLA-4 Promotes Foxp3 Induction and Regulatory T Cell Accumulation in the Intestinal Lamina Propria. Mucosal Immunol. 2013, 6, 324–334. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Herrera, L.N.; Tang, T.; Altan, M.; Chaftari, A.M.; Okhuysen, P.C.; Jenq, R.R.; Wang, Y. Impact of Antibiotic Therapy on the Development and Response to Treatment of Immune Checkpoint Inhibitor-Mediated Diarrhea and Colitis. J. Immunother. Cancer 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, N.; Luo, Q.; Jiang, L.; He, B.; Yuan, X.; Shen, L. Probiotics Lactobacillus Reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front. Immunol. 2019, 10, 1235. [Google Scholar] [CrossRef]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium Alters the Gut Microbiota and Modulates the Functional Metabolism of T Regulatory Cells in the Context of Immune Checkpoint Blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lee, A.; Huang, S.; Gao, J.; Spence, J.R.; Owyang, C. Lactobacillus Rhamnosus GG Prevents Epithelial Barrier Dysfunction Induced by Interferon-Gamma and Fecal Supernatants from Irritable Bowel Syndrome Patients in Human Intestinal Enteroids and Colonoids. Gut Microbes 2019, 10, 59–76. [Google Scholar] [CrossRef]
- Mendoza, T.R.; Dueck, A.C.; Bennett, A.V.; Mitchell, S.A.; Reeve, B.B.; Atkinson, T.M.; Li, Y.; Castro, K.M.; Denicoff, A.; Rogak, L.J.; et al. Evaluation of Different Recall Periods for the US National Cancer Institute’s PRO-CTCAE. Clin. Trials 2017, 14, 255–263. [Google Scholar] [CrossRef]
- Beck, K.E.; Blansfield, J.A.; Tran, K.Q.; Feldman, A.L.; Hughes, M.S.; Royal, R.E.; Kammula, U.S.; Topalian, S.L.; Sherry, R.M.; Kleiner, D.; et al. Enterocolitis in Patients with Cancer after Antibody Blockade of Cytotoxic T-Lymphocyte-Associated Antigen 4. J. Clin. Oncol. 2006, 24, 2283–2289. [Google Scholar] [CrossRef]
- Geukes Foppen, M.H.; Rozeman, E.A.; van Wilpe, S.; Postma, C.; Snaebjornsson, P.; van Thienen, J.V.; van Leerdam, M.E.; van den Heuvel, M.; Blank, C.U.; van Dieren, J.; et al. Immune Checkpoint Inhibition-Related Colitis: Symptoms, Endoscopic Features, Histology and Response to Management. ESMO Open 2018, 3, e000278. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Mooradian, M.J.; Kim, D.; Shah, N.J.; Fenton, S.E.; Conry, R.M.; Mehta, R.; Silk, A.W.; Zhou, A.; Compton, M.L.; et al. Clinical Characterization of Colitis Arising from Anti-PD-1 Based Therapy. Oncoimmunology 2019, 8, e1524695. [Google Scholar] [CrossRef]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Qiao, W.; Trinh, V.A.; Zobniw, C.; Johnson, D.H.; Samdani, R.; Lum, P.; et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Prieux-Klotz, C.; Dior, M.; Damotte, D.; Dreanic, J.; Brieau, B.; Brezault, C.; Abitbol, V.; Chaussade, S.; Coriat, R. Immune Checkpoint Inhibitor-Induced Colitis: Diagnosis and Management. Target. Oncol. 2017, 12, 301–308. [Google Scholar] [CrossRef]
- Yanai, S.; Nakamura, S.; Matsumoto, T. Nivolumab-Induced Colitis Treated by Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, e80–e81. [Google Scholar] [CrossRef][Green Version]
- Chen, J.H.; Pezhouh, M.K.; Lauwers, G.Y.; Masia, R. Histopathologic Features of Colitis Due to Immunotherapy with Anti-PD-1 Antibodies. Am. J. Surg. Pathol. 2017, 41, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lipson, E.J.; Sharfman, W.H.; Brant, S.R.; Lazarev, M.G. Colonic Ulcerations May Predict Steroid-Refractory Course in Patients with Ipilimumab-Mediated Enterocolitis. World J. Gastroenterol. 2017, 23, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Beattie, J.A.; Fuentes, P.; Rizvi, H.; Egger, J.V.; Kern, J.A.; Leung, D.Y.M.; Lacouture, M.E.; Kris, M.G.; Gambarin, M.; et al. Beyond Steroids: Immunosuppressants in Steroid-Refractory or Resistant Immune-Related Adverse Events. J. Thorac. Oncol. 2021, 16, 1759–1764. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Suarez-Almazor, M.E. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS ONE 2016, 11, e0160221. [Google Scholar] [CrossRef]
- Herold, M.J.; McPherson, K.G.; Reichardt, H.M. Glucocorticoids in T Cell Apoptosis and Function. Cell. Mol. Life Sci. 2006, 63, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Hutchinson, M.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-Induced Immunosuppression: Mechanisms and Implications for Immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef]
- Almawi, W.Y.; Beyhum, H.N.; Rahme, A.A.; Rieder, M.J. Regulation of Cytokine and Cytokine Receptor Expression by Glucocorticoids. J. Leukoc. Biol. 1996, 60, 563–572. [Google Scholar] [CrossRef]
- Xing, K.; Gu, B.; Zhang, P.; Wu, X. Dexamethasone Enhances Programmed Cell Death 1 (PD-1) Expression during T Cell Activation: An Insight into the Optimum Application of Glucocorticoids in Anti-Cancer Therapy. BMC Immunol. 2015, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Burla, J.; Bluemel, S.; Biedermann, L.; Barysch, M.J.; Dummer, R.; Levesque, M.P.; Gubler, C.; Morell, B.; Rogler, G.; Scharl, M. Retrospective Analysis of Treatment and Complications of Immune Checkpoint Inhibitor-Associated Colitis: Histological Ulcerations as Potential Predictor for a Steroid-Refractory Disease Course. Inflamm. Intest. Dis. 2020, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Adam, J.; Soularue, E.; Collins, M.; Racine, A.; Mussini, C.; Boselli, L.; Kamsukom, N.; Mateus, C.; Charrier, M.; et al. Colon Immune-Related Adverse Events: Anti-CTLA-4 and Anti-PD-1 Blockade Induce Distinct Immunopathological Entities. J. Crohn’s Colitis 2017, 11, 1238–1246. [Google Scholar] [CrossRef]
- Yoshino, K.; Nakayama, T.; Ito, A.; Sato, E.; Kitano, S. Severe Colitis After PD-1 Blockade with Nivolumab in Advanced Melanoma Patients: Potential Role of Th1-Dominant Immune Response in Immune-Related Adverse Events: Two Case Reports. BMC Cancer 2019, 19, 1019. [Google Scholar] [CrossRef]
- Correale, P.; Saladino, R.E.; Giannarelli, D.; Sergi, A.; Mazzei, M.A.; Bianco, G.; Giannicola, R.; Iuliano, E.; Forte, I.M.; Calandruccio, N.D.; et al. HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis. Cells 2020, 9, 1964. [Google Scholar] [CrossRef]
- d’Apolito, M.; Spagnuolo, R.; Siciliano, M.A.; Barbieri, V.; Cosco, C.; Fiorillo, L.; Cuomo, O.; Zuccalà, V.; Correale, P.; Pensabene, L.; et al. Autoimmune Colitis and Neutropenia in Adjuvant Anti-PD-1 Therapy for Malignant Melanoma: Efficacy of Vedolizumab, a Case Report. Ther. Adv. Chronic Dis. 2022, 13, 20406223211063024. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; De Velasco, M.A.; Sakai, K.; Nagai, T.; Nishiyama, H.; Hashimoto, K.; Uemura, H.; Kawakami, H.; Nakagawa, K.; Ogata, H.; et al. Integrative Analysis of Gut Microbiome and Host Transcriptomes Reveals Associations between Treatment Outcomes and Immunotherapy-Induced Colitis. Mol. Oncol. 2021, 16, 1493–1507. [Google Scholar] [CrossRef]
- Stone, M.L.; Forster, E.M. Use of Vedolizumab in Immune Checkpoint Inhibitor-Associated Enterocolitis. Inflamm. Bowel Dis. 2021, 27, e147. [Google Scholar] [CrossRef]
- Favara, D.M.; Spain, L.; Au, L.; Clark, J.; Daniels, E.; Diem, S.; Chauhan, D.; Turajlic, S.; Powell, N.; Larkin, J.M.; et al. Five-Year Review of Corticosteroid Duration and Complications in the Management of Immune Checkpoint Inhibitor-Related Diarrhoea and Colitis in Advanced Melanoma. ESMO Open 2020, 5, e000585. [Google Scholar] [CrossRef] [PubMed]
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients with Melanoma Treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Skribek, M.; Rounis, K.; Afshar, S.; Grundberg, O.; Friesland, S.; Tsakonas, G.; Ekman, S.; De Petris, L. Effect of Corticosteroids on the Outcome of Patients with Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Eur. J. Cancer 2021, 145, 245–254. [Google Scholar] [CrossRef]
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Freedman, C.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-Dose Glucocorticoids for the Treatment of Ipilimumab-Induced Hypophysitis Is Associated with Reduced Survival in Patients with Melanoma. Cancer 2018, 124, 3706–3714. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Collins, M.; Michot, J.M.; Danlos, F.X.; Mussini, C.; Soularue, E.; Mateus, C.; Loirat, D.; Buisson, A.; Rosa, I.; Lambotte, O.; et al. Inflammatory Gastrointestinal Diseases Associated with PD-1 Blockade Antibodies. Ann. Oncol. 2017, 28, 2860–2865. [Google Scholar] [CrossRef]
- Alexander, J.L.; Ibraheim, H.; Sheth, B.; Little, J.; Khan, M.S.; Richards, C.; Hunter, N.; Chauhan, D.; Ratnakumaran, R.; McHugh, K.; et al. Clinical Outcomes of Patients with Corticosteroid Refractory Immune Checkpoint Inhibitor-Induced Enterocolitis Treated with Infliximab. J. Immunother. Cancer 2021, 9, e002742. [Google Scholar] [CrossRef] [PubMed]
- Lesage, C.; Longvert, C.; Prey, S.; Maanaoui, S.; Dréno, B.; Machet, L.; Zehou, O.; Kramkimel, N.; Jeudy, G.; Skowron, F.; et al. Incidence and Clinical Impact of Anti-TNFα Treatment of Severe Immune Checkpoint Inhibitor-Induced Colitis in Advanced Melanoma: The Mecolit Survey. J. Immunother. 2019, 42, 175–179. [Google Scholar] [CrossRef]
- Miyahara, K.; Noda, T.; Ito, Y.; Hidaka, H.; Fujimoto, S.; Takedomi, H.; Akutagawa, T.; Sakata, Y.; Shimamura, T.; Tominaga, N.; et al. An Investigation of Nine Patients with Gastrointestinal Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors. Digestion 2020, 101, 60–65. [Google Scholar] [CrossRef]
- Hillock, N.T.; Heard, S.; Kichenadasse, G.; Hill, C.L.; Andrews, J. Infliximab for Ipilimumab-Induced Colitis: A Series of 13 Patients. Asia Pac. J. Clin. Oncol. 2017, 13, e284–e290. [Google Scholar] [CrossRef]
- Lankes, K.; Hundorfean, G.; Harrer, T.; Pommer, A.J.; Agaimy, A.; Angelovska, I.; Tajmir-Riahi, A.; Göhl, J.; Schuler, G.; Neurath, M.F.; et al. Anti-TNF-Refractory Colitis after Checkpoint Inhibitor Therapy: Possible Role of CMV-Mediated Immunopathogenesis. Oncoimmunology 2016, 5, e1128611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Luo, W.; Wang, Y. Acute Liver Injury in the Context of Immune Checkpoint Inhibitor-Related Colitis Treated with Infliximab. J. Immunother. Cancer 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Callens, R.; Tamsin, A.; van Zandweghe, L. Nivolumab-Induced Fulminant Immune-Related Colitis Despite Infliximab in a Patient with NSCLC. J. Thorac. Oncol. 2019, 14, e49–e50. [Google Scholar] [CrossRef] [PubMed]
- Paparoupa, M.; Stupperich, S.; Goerg-Reifenberg, L.; Wittig, A.; Schuppert, F. Successful Treatment of an Immune-Mediated Colitis Induced by Checkpoint Inhibitor Therapy in a Patient with Advanced Melanoma. Case Rep. Gastroenterol. 2020, 14, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Minor, D.R.; Chin, K.; Kashani-Sabet, M. Infliximab in the Treatment of Anti-CTLA4 Antibody (Ipilimumab) Induced Immune-Related Colitis. Cancer Biother. Radiopharm. 2009, 24, 321–325. [Google Scholar] [CrossRef]
- O’Connor, A.; Marples, M.; Mulatero, C.; Hamlin, J.; Ford, A.C. Ipilimumab-Induced Colitis: Experience from a Tertiary Referral Center. Ther. Adv. Gastroenterol. 2016, 9, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Wang, X.; Mallepally, N.; Chen, E.; Altan, M.; Bresalier, R.S.; Charabaty, A.; Dadu, R.; Jazaeri, A.; et al. Early Introduction of Selective Immunosuppressive Therapy Associated with Favorable Clinical Outcomes in Patients with Immune Checkpoint Inhibitor-Induced Colitis. J. Immunother. Cancer 2019, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Zobniw, C.M.; Trinh, V.A.; Ma, J.; Bassett, R.L., Jr.; Abdel-Wahab, N.; Anderson, J.; Davis, J.E.; Joseph, J.; Uemura, M.; et al. Infliximab Associated with Faster Symptom Resolution Compared with Corticosteroids Alone for the Management of Immune-Related Enterocolitis. J. Immunother. Cancer 2018, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Merrill, S.P.; Reynolds, P.; Kalra, A.; Biehl, J.; Vandivier, R.W.; Mueller, S.W. Early Administration of Infliximab for Severe Ipilimumab-Related Diarrhea in a Critically Ill Patient. Ann. Pharmacother. 2014, 48, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Badran, Y.R.; Cohen, J.V.; Brastianos, P.K.; Parikh, A.R.; Hong, T.S.; Dougan, M. Concurrent Therapy with Immune Checkpoint Inhibitors and TNFα Blockade in Patients with Gastrointestinal Immune-Related Adverse Events. J. Immunother. Cancer 2019, 7, 226. [Google Scholar] [CrossRef]
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274. [Google Scholar] [CrossRef]
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the Era of Immune Checkpoint Inhibitors: Friend or Foe? Nat. Rev. Rheumatol. 2021, 17, 213–223. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Hsieh, A.H.; Ferman, M.; Brown, M.P.; Andrews, J.M. Vedolizumab: A Novel Treatment for Ipilimumab-Induced Colitis. BMJ Case Rep. 2016, 2016, bcr2016216641. [Google Scholar] [CrossRef]
- Diana, P.; Mankongpaisarnrung, C.; Atkins, M.B.; Zeck, J.C.; Charabaty, A. Emerging Role of Vedolizumab in Managing Refractory Immune Checkpoint Inhibitor-Induced Enteritis. ACG Case Rep. J. 2018, 5, e17. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.; Gaughran, G.; Archer, C.; Pavli, P.; Morey, A.; Ali, S.; Yip, D. Vedolizumab in Combined Immune Checkpoint Therapy-Induced Infliximab-Refractory Colitis in a Patient with Metastatic Melanoma: A Case Report. World J. Clin. Oncol. 2019, 10, 350–357. [Google Scholar] [CrossRef]
- Bergqvist, V.; Hertervig, E.; Gedeon, P.; Kopljar, M.; Griph, H.; Kinhult, S.; Carneiro, A.; Marsal, J. Vedolizumab Treatment for Immune Checkpoint Inhibitor-Induced Enterocolitis. Cancer Immunol. Immunother. 2017, 66, 581–592. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Alsaadi, D.; Jennings, J.; Luo, W.; Gong, Z.; Richards, D.M.; Charabaty, A.; Wang, Y. Outcomes of Vedolizumab Therapy in Patients with Immune Checkpoint Inhibitor-Induced Colitis: A Multi-Center Study. J. Immunother. Cancer 2018, 6, 142. [Google Scholar] [CrossRef]
- Zou, F.; Faleck, D.; Thomas, A.; Harris, J.; Satish, D.; Wang, X.; Charabaty, A.; Ernstoff, M.S.; Glitza Oliva, I.C.; Hanauer, S.; et al. Efficacy and Safety of Vedolizumab and Infliximab Treatment for Immune-Mediated Diarrhea and Colitis in Patients with Cancer: A Two-Center Observational Study. J. Immunother. Cancer 2021, 9, e003277. [Google Scholar] [CrossRef]
- Mir, R.; Shaw, H.M.; Nathan, P.D. Mycophenolate Mofetil Alongside High-Dose Corticosteroids: Optimizing the Management of Combination Immune Checkpoint Inhibitor-Induced Colitis. Melanoma Res. 2019, 29, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kunogi, Y.; Tominaga, K.; Abe, K.; Kanazawa, M.; Tanaka, T.; Watanabe, S.; Kondo, M.; Kanamori, A.; Iijima, M.; Goda, K.; et al. Refractory Immune Checkpoint Inhibitor-Induced Colitis Improved by Tacrolimus: A Case Report. Healthcare 2021, 9, 418. [Google Scholar] [CrossRef]
- Zhang, E.; Kiely, C.; Sandanayake, N.; Tattersall, S. Calcineurin Inhibitors in Steroid and Anti-TNF-alpha Refractory Immune Checkpoint Inhibitor Colitis. JGH Open 2021, 5, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, T.; Kurita, N.; Takada, A.; Watanabe, H.; Ando, M. Resolution of Infliximab-Refractory Nivolumab-Induced Acute Severe Enterocolitis after Cyclosporine Treatment in a Patient with Non-Small Cell Lung Cancer. Am. J. Case Rep. 2018, 19, 360–364. [Google Scholar] [CrossRef]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T. Refractory Immune Checkpoint Inhibitor-Induced Colitis Improved by Tacrolimus: A Case Report; Hardin, J.; Walker, P. Tocilizumab for the Management of Immune Mediated Adverse Events Secondary to PD-1 Blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-Based Clinical Practice Guidelines for Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef]
- Powell, N.; Ibraheim, H.; Raine, T.; Speight, R.A.; Papa, S.; Brain, O.; Green, M.; Samaan, M.A.; Spain, L.; Yousaf, N.; et al. British Society of Gastroenterology Endorsed Guidance for the Management of Immune Checkpoint Inhibitor-Induced Enterocolitis. Lancet Gastroenterol. Hepatol. 2020, 5, 679–697. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Daly, L.; Bromberg, J. The IL-6 Feed-Forward Loop: A Driver of Tumorigenesis. Semin. Immunol. 2014, 26, 48–53. [Google Scholar] [CrossRef]
- Gout, T.; Ostör, A.J.; Nisar, M.K. Lower Gastrointestinal Perforation in Rheumatoid Arthritis Patients Treated with Conventional DMARDs or Tocilizumab: A Systematic Literature Review. Clin. Rheumatol. 2011, 30, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, G.C.; Yan, F.; Polk, D.B. Mesalamine Blocks tumor necrosis factor growth inhibition and nuclear factor kB activation in mouse colonocytes. Gastroenterology 1999, 116, 602–609. [Google Scholar] [CrossRef]
- Criscuoli, V.; Modesto, I.; Orland, A.; Cottone, M. Mesalazine for the treatment of inflammatory bowel disease. Expert Opin. Pharmacother. 2013, 14, 1669–1678. [Google Scholar] [CrossRef]
- Iwamoto, M.; Kato, K.; Moriyama, M.; Yamaguchi, K.; Takahashi, S. Remission of ulcerative colitis flare-up induced by nivolmab. Int. J. Colorectal Dis. 2020, 35, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
| Colitis Grade | Symptoms | Management |
|---|---|---|
| Grade 1 | Asymptomatic (clinical or laboratory findings only) | Consider applying closely monitored immunotherapy with loperamide or diphenoxylate/atropine |
| Grade 2 | Abdominal pain, mucus, blood in stool | Consider systemic corticosteroids (1–2 mg/kg/day); if no response in 2–3 days, continue corticosteroids and consider adding infliximab or vedolizumab within two weeks |
| Grade 3 | Severe abdominal pain, peritoneal signs | Consider inpatient supportive care; intravenous corticosteroids (1–2 mg/kg/day); if no response in 2 days, continue corticosteroids and strongly consider adding infliximab or vedolizumab within two weeks |
| Grade 4 | Severe and persistent abdominal pain, fever, ileus, life-threatening complications such as perforation and peritonitis | |
| Grade 5 | Death |
| Authors | Year | Original Disease | No. of Cases (CS-Naïve: Needed CS) | Impact of CS on Response Rate or Survival |
|---|---|---|---|---|
| Horvat et al. [75] | 2015 | melanoma | 195:103 | Systemic CS was not associated with OS or TTF |
| Weber et al. [76] | 2017 | melanoma | 462:114 | ORR was 31.8% in CS-naïve group and 29.8% in CS-needed group (p = 0.736); median duration of response was 22.0 months in CS-naïve group and not reached in CS-needed group |
| Skribek et al. [77] | 2020 | lung cancer | 104:31 | OS was 14.43 months in CS-naïve group and not reached in CS-needed group (p = 0.38) |
| Author | Year | No. of Cases | Age [Range] | Gender (Male: Female) | Original Disease | Therapeutic Drugs | CS Treatment Period | Number of IFX Doses until Remission Number of Doses [Range] | Patients Achieving Remission | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Lankes et al. [85] | 2016 | 1 | 32 | 1:0 | melanoma | combination | 30 w | 4 | 0% (0/1) | CMV colitis |
| Yanai et al. [56] | 2017 | 1 | 51 | 1:0 | melanoma | PD-1/L1 | 17 d | 1 | 100% (1/1) | Without |
| Zhang et al. [86] | 2019 | 1 | 79 | 1:0 | prostate cancer | combination | 25 d | 1 | 100% (1/1) | Liver disorders |
| Callens et al. [87] | 2019 | 1 | 63 | 0:1 | lung cancer | PD-1/L1 | 3 | 1 | 0% (0/1) | Perforation of the large intestine |
| Miyahara et al. [83] | 2020 | 1 | 72 | 1:0 | melanoma | combination | ND | 1 | 100% (1/1) | Without |
| Paparoupa et al. [88] | 2020 | 1 | 54 | 0:1 | melanoma | combination | 2 m | 17 | 100% (1/1) | Without |
| Minor et al. [89] | 2009 | 3 | 57 [47,48,49,50,51,52,53,54,55,56,57,58] | 3:0 | melanoma | CTLA-4 | ND | 2 [1,2] | 100% (3/3) | Without |
| O’Connor et al. [90] | 2016 | 4 | ND | ND | melanoma | ND | ND | ND | 100% (4/4) | Without |
| Jain et al. [60] | 2017 | 9 | ND | ND | melanoma | CTLA-4 | ND | 1 [1,2] | 100% (9/9) | Without |
| Hillock et al. [84] | 2017 | 13 | 64 [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86] | 6:7 | melanoma: 13 | CTLA-4 | ND | 1 [1,2,3] | 54% (7/13) | Without |
| Alexander et al. [81] | 2021 | 127 | 59 [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] | 73:54 | melanoma: 90, kidney cancer: 15, lung cancer: 7, urinary tract cancer: 8, others: 7 | PD-1/L1:40, CTLA-4:21 combination: 66 | ND | ND | 71.4% (75/105) | Without |
| Author | Year | No. of Cases | Age [Range] | Gender (Male: Female) | Original Disease | Therapeutic Drugs | Duration of CS Treatment | Administration of IFX | Number of Vedolizumab Doses until Remission [Range] | Patients Achieving Remission |
|---|---|---|---|---|---|---|---|---|---|---|
| Hsieh et al. [99] | 2016 | 1 | 69 | 1:0 | melanoma | CTLA-4 | 33 w | no | 3 | 100% |
| Diana et al. [100] | 2018 | 1 | 62 | 0:1 | melanoma | combination | ND | yes | 2 | 100% |
| Randhawa et al. [101] | 2019 | 1 | 27 | 0:1 | melanoma | combination | 55 d | yes | 3 | 100% |
| Stone et al. [73] | 2021 | 1 | 68 | 0:1 | lung cancer | PD-L1 | 6 w | no | ND | 100% |
| d’Apolito et al. [71] | 2022 | 1 | 44 | 0:1 | melanoma | PD-1/L1 | 15 d | no | 3 | 100% |
| Bergqvist et al. [102] | 2017 | 7 | 55 [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] | 4:3 | melanoma: 6, lung cancer: 1 | CTLA-4: 6, PD-1/L1: 1 | 57 d (52–92) | no: 6, yes: 1 | 2 [2,3,4] | 100% |
| Abu-S et al. [103] | 2018 | 28 | 63 | 20:8 | melanoma: 7, urinary tract cancer: 7, prostate cancer: 4, others: 10 | CTLA-4: 8, PD-1/L1: 12, combination:8 | 96 d | yes: 9, no: 19 | 3 [1,2,3,4] | 86% |
| Zou et al. [104] | 2021 | 62 | 63 [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] | 41:21 | melanoma: 10, urinary tract cancer: 23, lung cancer: 10, others: 19 | CTLA-4: 6, PD-1/L1: 38, combination:18 | 35 d (27–43) | no | 3 | 89% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohwada, S.; Ishigami, K.; Akutsu, N.; Nakase, H. Pharmacological Treatments Available for Immune-Checkpoint-Inhibitor-Induced Colitis. Biomedicines 2022, 10, 1334. https://doi.org/10.3390/biomedicines10061334
Ohwada S, Ishigami K, Akutsu N, Nakase H. Pharmacological Treatments Available for Immune-Checkpoint-Inhibitor-Induced Colitis. Biomedicines. 2022; 10(6):1334. https://doi.org/10.3390/biomedicines10061334
Chicago/Turabian StyleOhwada, Sae, Keisuke Ishigami, Noriyuki Akutsu, and Hiroshi Nakase. 2022. "Pharmacological Treatments Available for Immune-Checkpoint-Inhibitor-Induced Colitis" Biomedicines 10, no. 6: 1334. https://doi.org/10.3390/biomedicines10061334
APA StyleOhwada, S., Ishigami, K., Akutsu, N., & Nakase, H. (2022). Pharmacological Treatments Available for Immune-Checkpoint-Inhibitor-Induced Colitis. Biomedicines, 10(6), 1334. https://doi.org/10.3390/biomedicines10061334







